Reovirus Low-Density Particles Package Cellular RNA
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Viruses
2.3. Reovirus Particle Enrichment
2.4. Virus Particle Normalization
2.5. Bioanalyzer Analysis
2.6. Library Preparation and Next-Generation RNA-Sequencing
2.7. Sequence Analysis
2.8. RT-qPCR
2.9. Fluorescent Focus Assay
2.10. Negative-Stain Electron Microscopy
2.11. Statistical Analysis
3. Results
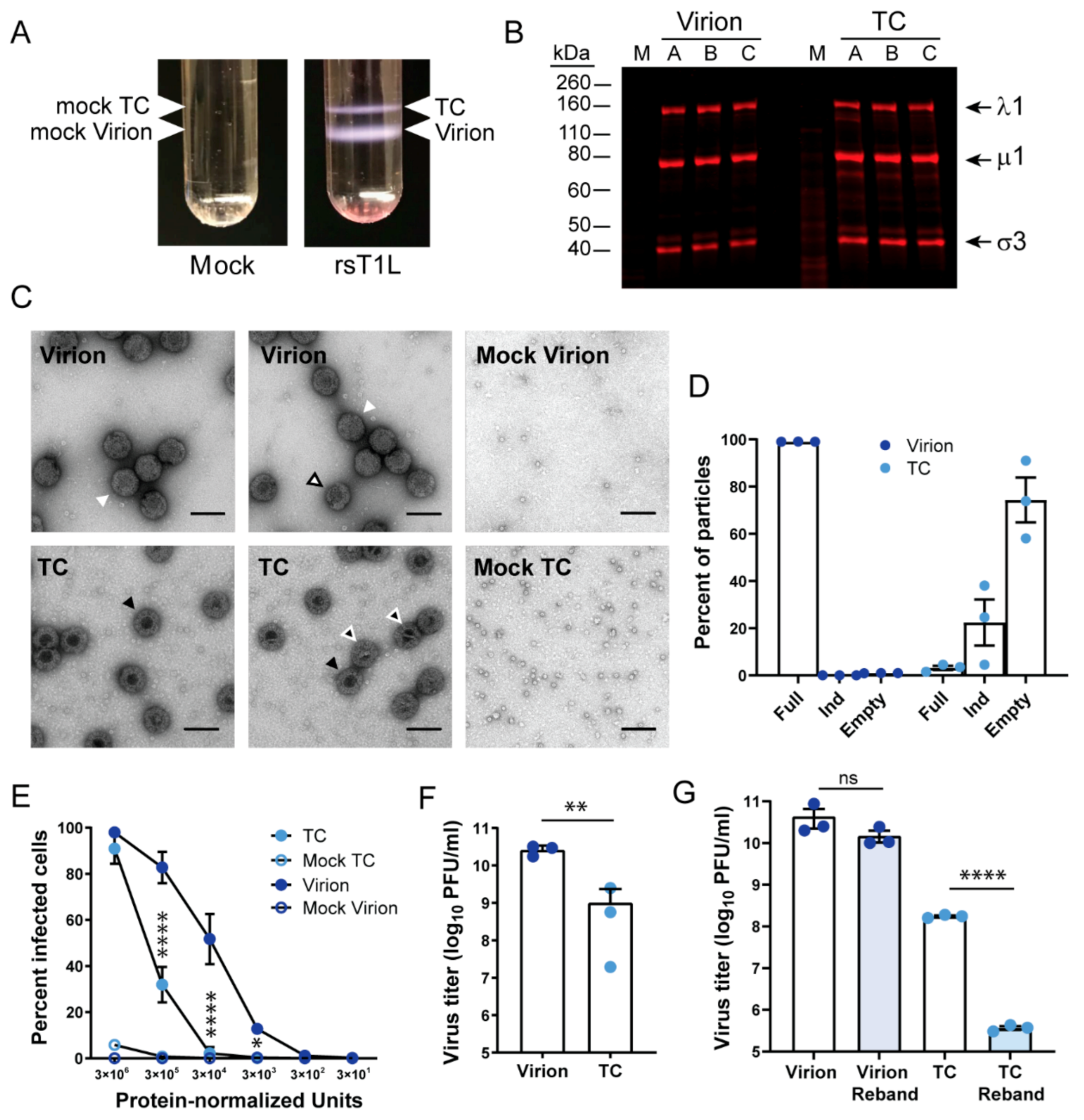
3.1. Reovirus Top Component Particles Are Less Infectious Than Virions
3.2. Reovirus Particles Contain Viral Double-Stranded RNA
3.3. Top Component Particles Contain Host RNA
3.4. The Viral Polymerase Fails to Confer Complete Host RNA Packaging Specificity
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borodavka, A.; Desselberger, U.; Patton, J.T. Genome packaging in multi-segmented dsRNA viruses: Distinct mechanisms with similar outcomes. Curr. Opin. Virol. 2018, 33, 106–112. [Google Scholar] [CrossRef] [PubMed]
- McDonald, S.M.; Patton, J.T. Assortment and packaging of the segmented rotavirus genome. Trends Microbiol. 2011, 19, 136–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, P. Bluetongue virus structure and assembly. Curr. Opin. Virol. 2017, 24, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Taraporewala, Z.F.; Patton, J.T. Nonstructural proteins involved in genome packaging and replication of rotaviruses and other members of the Reoviridae. Virus Res. 2004, 101, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Borodavka, A.; Ault, J.; Stockley, P.G.; Tuma, R. Evidence that avian reovirus σNS is an RNA chaperone: Implications for genome segment assortment. Nucleic Acids Res. 2015, 43, 7044–7057. [Google Scholar] [CrossRef]
- Bravo, J.P.K.; Borodavka, A.; Barth, A.; Calabrese, A.N.; Mojzes, P.; Cockburn, J.J.B.; Lamb, D.C.; Tuma, R. Stability of local secondary structure determines selectivity of viral RNA chaperones. Nucleic Acids Res. 2018, 46, 7924–7937. [Google Scholar] [CrossRef]
- Bouziat, R.; Hinterleitner, R.; Brown, J.J.; Stencel-Baerenwald, J.E.; Ikizler, M.; Mayassi, T.; Meisel, M.; Kim, S.M.; Discepolo, V.; Pruijssers, A.J.; et al. Reovirus infection triggers inflammatory responses to dietary antigens and development of celiac disease. Science 2017, 356, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Dermody, T.S.; Parker, J.S.; Sherry, B. Orthoreoviruses. In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; Volume 2, pp. 1304–1346. [Google Scholar]
- Kobayashi, T.; Antar, A.A.; Boehme, K.W.; Danthi, P.; Eby, E.A.; Guglielmi, K.M.; Holm, G.H.; Johnson, E.M.; Maginnis, M.S.; Naik, S.; et al. A plasmid-based reverse genetics system for animal double-stranded RNA viruses. Cell Host Microbe 2007, 1, 147–157. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Ooms, L.S.; Ikizler, M.; Chappell, J.D.; Dermody, T.S. An improved reverse genetics system for mammalian orthoreoviruses. Virology 2010, 398, 194–200. [Google Scholar] [CrossRef] [Green Version]
- Shatkin, A.J.; Sipe, J.D.; Loh, P. Separation of ten reovirus genome segments by polyacrylamide gel electrophoresis. J. Virol. 1968, 2, 986–991. [Google Scholar] [CrossRef] [Green Version]
- Coombs, K.M. Stoichiometry of reovirus structural proteins in virus, ISVP, and core particles. Virology 1998, 243, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.L.; Arnold, M.M.; Broering, T.J.; Hastings, C.E.; Nibert, M.L. Localization of mammalian orthoreovirus proteins to cytoplasmic factory-like structures via nonoverlapping regions of microNS. J. Virol. 2010, 84, 867–882. [Google Scholar] [CrossRef] [Green Version]
- Fernández de Castro, I.; Zamora, P.F.; Ooms, L.; Fernández, J.J.; Lai, C.M.; Mainou, B.A.; Dermody, T.S.; Risco, C. Reovirus forms neo-organelles for progeny particle assembly within reorganized cell membranes. mBio 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Demidenko, A.A.; Blattman, J.N.; Blattman, N.N.; Greenberg, P.D.; Nibert, M.L. Engineering recombinant reoviruses with tandem repeats and a tetravirus 2A-like element for exogenous polypeptide expression. Proc. Natl. Acad. Sci. USA 2013, 110, E1867–E1876. [Google Scholar] [CrossRef] [Green Version]
- Roner, M.R.; Bassett, K.; Roehr, J. Identification of the 5’ sequences required for incorporation of an engineered ssRNA into the Reovirus genome. Virology 2004, 329, 348–360. [Google Scholar] [CrossRef] [Green Version]
- Roner, M.R.; Roehr, J. The 3’ sequences required for incorporation of an engineered ssRNA into the Reovirus genome. Virol. J. 2006, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Roner, M.R.; Steele, B.G. Features of the mammalian orthoreovirus 3 Dearing l1 single-stranded RNA that direct packaging and serotype restriction. J. Gen. Virol. 2007, 88, 3401–3412. [Google Scholar] [CrossRef]
- Roner, M.R.; Steele, B.G. Localizing the reovirus packaging signals using an engineered m1 and s2 ssRNA. Virology 2007, 358, 89–97. [Google Scholar] [CrossRef] [Green Version]
- Zou, S.; Brown, E.G. Identification of sequence elements containing signals for replication and encapsidation of the reovirus M1 genome segment. Virology 1992, 186, 377–388. [Google Scholar] [CrossRef]
- Borodavka, A.; Dykeman, E.C.; Schrimpf, W.; Lamb, D.C. Protein-mediated RNA folding governs sequence-specific interactions between rotavirus genome segments. eLife 2017, 6. [Google Scholar] [CrossRef]
- Antczak, J.B.; Joklik, W.K. Reovirus genome segment assortment into progeny genomes studied by the use of monoclonal antibodies directed against reovirus proteins. Virology 1992, 187, 760–776. [Google Scholar] [CrossRef]
- McDonald, S.M.; Tao, Y.J.; Patton, J.T. The ins and outs of four-tunneled Reoviridae RNA-dependent RNA polymerases. Curr. Opin. Struct. Biol. 2009, 19, 775–782. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Farsetta, D.L.; Nibert, M.L.; Harrison, S.C. RNA synthesis in a cage—Structural studies of reovirus polymerase lambda3. Cell 2002, 111, 733–745. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Walker, S.B.; Chipman, P.R.; Nibert, M.L.; Baker, T.S. Reovirus polymerase lambda 3 localized by cryo-electron microscopy of virions at a resolution of 7.6 A. Nat. Struct. Biol. 2003, 10, 1011–1018. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.E.; Zweerink, H.J.; Joklik, W.K. Polypeptide components of virions, top component and cores of reovirus type 3. Virology 1969, 39, 791–810. [Google Scholar] [CrossRef]
- Dryden, K.A.; Farsetta, D.L.; Wang, G.; Keegan, J.M.; Fields, B.N.; Baker, T.S.; Nibert, M.L. Internal/structures containing transcriptase-related proteins in top component particles of mammalian orthoreovirus. Virology 1998, 245, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Lai, M.H.; Wérenne, J.J.; Joklik, W.K. The preparation of reovirus top component and its effect on host DNA and protein synthesis. Virology 1973, 54, 237–244. [Google Scholar] [CrossRef]
- Bishop, J.M.; Levinson, W.E.; Sullivan, D.; Fanshier, L.; Quintrell, N.; Jackson, J. The low molecular weight RNAs of Rous sarcoma virus. II. The 7 S RNA. Virology 1970, 42, 927–937. [Google Scholar] [CrossRef]
- Eckwahl, M.J.; Sim, S.; Smith, D.; Telesnitsky, A.; Wolin, S.L. A retrovirus packages nascent host noncoding RNAs from a novel surveillance pathway. Genes Dev. 2015, 29, 646–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Mak, J.; Cao, Q.; Li, Z.; Wainberg, M.A.; Kleiman, L. Incorporation of excess wild-type and mutant tRNA(3Lys) into human immunodeficiency virus type 1. J. Virol. 1994, 68, 7676–7683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Routh, A.; Domitrovic, T.; Johnson, J.E. Host RNAs, including transposons, are encapsidated by a eukaryotic single-stranded RNA virus. Proc. Natl. Acad. Sci. USA 2012, 109, 1907–1912. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, N.; Weber, P.H.; Burke, S.V.; Wysocki, W.P.; Duvall, M.R.; Bujarski, J.J. Next generation sequencing reveals packaging of host RNAs by brome mosaic virus. Virus Res. 2018, 252, 82–90. [Google Scholar] [CrossRef]
- Linial, M.L.; Miller, A.D. Retroviral RNA packaging: Sequence requirements and implications. Curr. Top. Microbiol. Immunol. 1990, 157, 125–152. [Google Scholar] [CrossRef]
- Telesnitsky, A.; Wolin, S.L. The Host RNAs in Retroviral Particles. Viruses 2016, 8, 235. [Google Scholar] [CrossRef] [Green Version]
- AlShaikhahmed, K.; Leonov, G.; Sung, P.Y.; Bingham, R.J.; Twarock, R.; Roy, P. Dynamic network approach for the modelling of genomic sub-complexes in multi-segmented viruses. Nucleic Acids Res. 2018, 46, 12087–12098. [Google Scholar] [CrossRef]
- Sung, P.Y.; Roy, P. Sequential packaging of RNA genomic segments during the assembly of Bluetongue virus. Nucleic Acids Res. 2014, 42, 13824–13838. [Google Scholar] [CrossRef] [Green Version]
- Fajardo, T.; Sung, P.Y.; Celma, C.C.; Roy, P. Rotavirus Genomic RNA Complex Forms via Specific RNA-RNA Interactions: Disruption of RNA Complex Inhibits Virus Infectivity. Viruses 2017, 9, 167. [Google Scholar] [CrossRef] [Green Version]
- Hundley, F.; Biryahwaho, B.; Gow, M.; Desselberger, U. Genome rearrangements of bovine rotavirus after serial passage at high multiplicity of infection. Virology 1985, 143, 88–103. [Google Scholar] [CrossRef]
- Ballard, A.; McCrae, M.A.; Desselberger, U. Nucleotide sequences of normal and rearranged RNA segments 10 of human rotaviruses. J. Gen. Virol. 1992, 73, 633–638. [Google Scholar] [CrossRef]
- Kojima, K.; Taniguchi, K.; Kawagishi-Kobayashi, M.; Matsuno, S.; Urasawa, S. Rearrangement generated in double genes, NSP1 and NSP3, of viable progenies from a human rotavirus strain. Virus Res. 2000, 67, 163–171. [Google Scholar] [CrossRef]
- Komoto, S.; Fukuda, S.; Ide, T.; Ito, N.; Sugiyama, M.; Yoshikawa, T.; Murata, T.; Taniguchi, K. Generation of Recombinant Rotaviruses Expressing Fluorescent Proteins by Using an Optimized Reverse Genetics System. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Philip, A.A.; Perry, J.L.; Eaton, H.E.; Shmulevitz, M.; Hyser, J.M.; Patton, J.T. Generation of Recombinant Rotavirus Expressing NSP3-UnaG Fusion Protein by a Simplified Reverse Genetics System. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Berard, A.; Coombs, K.M. Mammalian reoviruses: Propagation, quantification, and storage. Curr. Protoc. Microbiol. 2009, 15, 15C.1.1–15C.1.18. [Google Scholar] [CrossRef] [PubMed]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: http://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 6 April 2021).
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef] [Green Version]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Liberzon, A.; Birger, C.; Thorvaldsdóttir, H.; Ghandi, M.; Mesirov, J.P.; Tamayo, P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015, 1, 417–425. [Google Scholar] [CrossRef] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [Green Version]
- Robinson, J.T.; Thorvaldsdóttir, H.; Winckler, W.; Guttman, M.; Lander, E.S.; Getz, G.; Mesirov, J.P. Integrative genomics viewer. Nat. Biotechnol. 2011, 29, 24–26. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Vignuzzi, M.; López, C.B. Defective viral genomes are key drivers of the virus-host interaction. Nat. Microbiol. 2019, 4, 1075–1087. [Google Scholar] [CrossRef]
- Sutton, G.; Sun, D.; Fu, X.; Kotecha, A.; Hecksel, C.W.; Clare, D.K.; Zhang, P.; Stuart, D.I.; Boyce, M. Assembly intermediates of orthoreovirus captured in the cell. Nat. Commun. 2020, 11, 4445. [Google Scholar] [CrossRef]
- Broering, T.J.; Parker, J.S.; Joyce, P.L.; Kim, J.; Nibert, M.L. Mammalian reovirus nonstructural protein microNS forms large inclusions and colocalizes with reovirus microtubule-associated protein micro2 in transfected cells. J. Virol. 2002, 76, 8285–8297. [Google Scholar] [CrossRef] [Green Version]
- Eichwald, C.; Kim, J.; Nibert, M.L. Dissection of mammalian orthoreovirus µ2 reveals a self-associative domain required for binding to microtubules but not to factory matrix protein µNS. PLoS ONE 2017, 12, e0184356. [Google Scholar] [CrossRef] [Green Version]
- Parker, J.S.; Broering, T.J.; Kim, J.; Higgins, D.E.; Nibert, M.L. Reovirus core protein mu2 determines the filamentous morphology of viral inclusion bodies by interacting with and stabilizing microtubules. J. Virol. 2002, 76, 4483–4496. [Google Scholar] [CrossRef] [Green Version]
- Shah, P.N.M.; Stanifer, M.L.; Höhn, K.; Engel, U.; Haselmann, U.; Bartenschlager, R.; Kräusslich, H.G.; Krijnse-Locker, J.; Boulant, S. Genome packaging of reovirus is mediated by the scaffolding property of the microtubule network. Cell. Microbiol. 2017, 19. [Google Scholar] [CrossRef] [Green Version]
- Ooms, L.S.; Jerome, W.G.; Dermody, T.S.; Chappell, J.D. Reovirus replication protein μ2 influences cell tropism by promoting particle assembly within viral inclusions. J. Virol. 2012, 86, 10979–10987. [Google Scholar] [CrossRef] [Green Version]
- Ooms, L.S.; Kobayashi, T.; Dermody, T.S.; Chappell, J.D. A post-entry step in the mammalian orthoreovirus replication cycle is a determinant of cell tropism. J. Biol. Chem. 2010, 285, 41604–41613. [Google Scholar] [CrossRef] [Green Version]
- Marzluff, W.F.; Koreski, K.P. Birth and Death of Histone mRNAs. Trends Genet. 2017, 33, 745–759. [Google Scholar] [CrossRef]
- Chapell, J.D.; Goral, M.I.; Rodgers, S.E.; dePamphilis, C.W.; Dermody, T.S. Sequence diversity within the reovirus S2 gene: Reovirus genes reassort in nature, and their termini are predicted to form a panhandle motif. J. Virol. 1994, 68, 750–756. [Google Scholar] [CrossRef] [Green Version]
- Roner, M.R.; Joklik, W.K. Reovirus reverse genetics: Incorporation of the CAT gene into the reovirus genome. Proc. Natl. Acad. Sci. USA 2001, 98, 8036–8041. [Google Scholar] [CrossRef] [Green Version]




| Segment | Number and Percentage of Total Viral Reads | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Inf-A a | Inf-B | % | T1L TC-A | T1L TC-B | T1L TC-C | % | T1L V-A b | T1L V-B | % | |
| T1L L1 | 1,038,877 | 1,063,552 | 10 | 1,129,755 | 177,576 | 1,423,811 | 11 | 3,447,733 | 2,877,184 | 11 |
| T1L L2 | 1,940,530 | 2,058,662 | 18 | 1,259,866 | 217,047 | 1,638,263 | 13 | 4,181,427 | 3,428,793 | 13 |
| T1L L3 | 1,410,996 | 1,569,759 | 14 | 1,243,118 | 211,302 | 1,611,815 | 13 | 3,809,943 | 3,704,721 | 13 |
| T1L M1 | 828,734 | 888,345 | 8 | 860,795 | 457,747 | 754,088 | 12 | 2,229,966 | 2,057,585 | 7 |
| T1L M2 | 880,271 | 987,720 | 9 | 1,079,924 | 386,976 | 1,047,832 | 13 | 3,286,855 | 3,834,136 | 12 |
| T1L M3 | 1,580,719 | 1,574,052 | 14 | 829,202 | 257,314 | 990,199 | 10 | 3,435,740 | 4,379,129 | 13 |
| T1L S1 | 742,227 | 659,561 | 6 | 705,967 | 76,568 | 722,037 | 6 | 2,210,645 | 2,136,583 | 8 |
| T1L S2 | 650,896 | 621,659 | 6 | 663,153 | 211,496 | 695,395 | 8 | 2,020,455 | 2,187,448 | 7 |
| T1L S3 | 922,919 | 900,962 | 8 | 622,472 | 169,088 | 554,903 | 7 | 2,109,575 | 2,326,564 | 8 |
| T1L S4 | 799,830 | 752,994 | 7 | 663,235 | 220,084 | 638,508 | 8 | 2,158,986 | 2,495,732 | 8 |
| T3DI TC-A | T3DI TC-B | T3DI TC-C | % | T3DI V-A | T3DI V-B | % | ||||
| T1L L1 | 765,380 | 755,871 | 841,398 | 6 | 4,508,769 | 2,323,188 | 12 | |||
| T3D L2 | 1,607,504 | 1,377,489 | 1,876,766 | 13 | 3,165,210 | 3,399,842 | 12 | |||
| T3D L3 | 1,902,672 | 2,076,988 | 3,083,154 | 18 | 4,751,091 | 4,271,775 | 17 | |||
| T3D M1 | 825,554 | 652,109 | 620,491 | 6 | 4,088,339 | 1,424,936 | 10 | |||
| T3D M2 | 1,756,670 | 2,928,330 | 2,766,220 | 19 | 4,041,153 | 4,738,918 | 16 | |||
| T3D M3 | 2,218,413 | 2,812,836 | 3,649,313 | 22 | 5,792,592 | 4,661,471 | 19 | |||
| T3D S1 | 526,488 | 684,413 | 619,586 | 5 | 347,908 | 1,347,464 | 3 | |||
| T3D S2 | 378,205 | 403,546 | 313,405 | 3 | 137,275 | 835,851 | 2 | |||
| T3D S3 | 685,219 | 691,058 | 1,147,250 | 6 | 261,883 | 1,439,514 | 3 | |||
| T3D S4 | 378,875 | 527,151 | 514,224 | 4 | 2,156,059 | 1,105,882 | 6 | |||
| Segment | Number and Percentage of Plus-Strand Viral Reads Per Segment c | |||||||||
| Inf-A | Inf-B | % | T1L TC-A | T1L TC-B | T1L TC-C | % | T1L V-A | T1L V-B | % | |
| T1L L1 | 967,418 | 926,730 | 90 | 559,481 | 72,257 | 716,916 | 47 | 1,478,406 | 1,239,329 | 43 |
| T1L L2 | 1,715,514 | 1,682,724 | 85 | 600,385 | 76,108 | 831,884 | 45 | 1,778,263 | 1,542,290 | 44 |
| T1L L3 | 1,318,548 | 1,387,044 | 91 | 643,641 | 136,574 | 824,859 | 56 | 1,947,084 | 2,259,676 | 56 |
| T1L M1 | 776,872 | 756,578 | 90 | 592,363 | 407,332 | 451,155 | 73 | 1,247,124 | 1,246,707 | 58 |
| T1L M2 | 842,077 | 877,752 | 92 | 676,676 | 357,046 | 610,169 | 71 | 2,086,040 | 2,948,204 | 70 |
| T1L M3 | 1,524,971 | 1,463,682 | 95 | 499,057 | 214,596 | 550,533 | 66 | 2,069,244 | 3,280,321 | 68 |
| T1L S1 | 642,149 | 543,860 | 85 | 385,850 | 48,236 | 392,044 | 57 | 1,144,399 | 1,105,627 | 52 |
| T1L S2 | 631,366 | 568,782 | 94 | 413,158 | 190,920 | 379,510 | 69 | 1,099,334 | 1,249,891 | 56 |
| T1L S3 | 884,705 | 829,931 | 94 | 371,082 | 146,717 | 299,734 | 67 | 1,238,926 | 1,588,222 | 64 |
| T1L S4 | 765,558 | 705,075 | 95 | 411,370 | 206,932 | 369,635 | 71 | 1,331,638 | 1,754,691 | 66 |
| T3DI TC-A | T3DI TC-B | T3DI TC-C | % | T3DI V-A | T3DI V-B | % | ||||
| T1L L1 | 420,930 | 444,649 | 462,292 | 56 | 2,284,404 | 1,284,049 | 53 | |||
| T3D L2 | 1,176,817 | 1,101,902 | 1,632,328 | 80 | 1,614,887 | 2,418,628 | 61 | |||
| T3D L3 | 1,625,208 | 1,809,948 | 2,809,072 | 88 | 2,431,490 | 3,306,435 | 64 | |||
| T3D M1 | 496,598 | 436,268 | 424,148 | 65 | 2,036,735 | 878,312 | 56 | |||
| T3D M2 | 1,600,316 | 2,775,413 | 2,623,817 | 93 | 2,195,291 | 4,406,569 | 74 | |||
| T3D M3 | 2,035,177 | 2,626,693 | 3,547,829 | 94 | 3,120,135 | 4,304,327 | 73 | |||
| T3D S1 | 421,810 | 604,230 | 561,397 | 86 | 198,146 | 1,167,135 | 72 | |||
| T3D S2 | 257,660 | 299,371 | 250,122 | 74 | 71,453 | 620,844 | 63 | |||
| T3D S3 | 617,938 | 619,140 | 1,122,183 | 93 | 134,518 | 1,292,697 | 71 | |||
| T3D S4 | 296,978 | 417,187 | 449,194 | 82 | 1,113,725 | 889,520 | 66 | |||
| Sample | Viral CPM | Host CPM | Percent Viral | Percent Host |
|---|---|---|---|---|
| Mock Inf-A a | 413 | 999,587 | <0.1 | >99.9 |
| Mock Inf-B | 332 | 999,668 | <0.1 | >99.9 |
| T1L Inf-A | 762,352 | 237,648 | 76 | 24 |
| T1L Inf-B | 798,981 | 201,019 | 80 | 20 |
| Mock TC | 387 | 999,613 | <0.1 | >99.9 |
| T1L TC-A | 587,065 | 412,935 | 59 | 41 |
| T1L TC-B | 178,652 | 821,348 | 18 | 82 |
| T1L TC-C | 742,350 | 257,650 | 74 | 26 |
| T3DIT1L1 TC-A | 663,806 | 336,194 | 66 | 34 |
| T3DIT1L1 TC-B | 700,570 | 299,430 | 70 | 30 |
| T3DIT1L1 TC-C | 763,056 | 236,944 | 76 | 24 |
| Mock Virion | 697 | 999,303 | <0.1 | >99.9 |
| T1L Virion-A | 999,792 | 208 | >99.9 | <0.1 |
| T1L Virion-B | 999,609 | 391 | >99.9 | <0.1 |
| T3DIT1L1 Virion-A | 999,901 | 99 | >99.9 | <0.1 |
| T3DIT1L1 Virion-B | 998,510 | 1490 | 99.9 | 0.1 |
| Gene Identifier | Log2FC a | p Value | SYMBOL |
|---|---|---|---|
| rsT1L Virions Significant Genes b | |||
| gb|M24734.1| | 16.8 | 7.3 × 10−6 | T1L L1 |
| gb|AF378003.1| | 17.4 | 3.4 × 10−6 | T1L L2 |
| gb|AF129820.1| | 17.2 | 3.7 × 10−6 | T1L L3 |
| gb|AF461682.1| | 17.8 | 2.9 × 10−6 | T1L M1 |
| gb|AF490617.1| | 17.7 | 2.5 × 10−6 | T1L M2 |
| gb|AF174382.1| | 17.8 | 2.5 × 10−6 | T1L M3 |
| gb|EF494445.1| | 17.5 | 2.4 × 10−6 | T1L S1 |
| gb|L19774.1| | 17.6 | 2.3 × 10−6 | T1L S2 |
| gb|M18389.1| | 17.6 | 2.3 × 10−6 | T1L S3 |
| gb|M13139.1| | 17.5 | 2.4 × 10−6 | T1L S4 |
| rsT1L TC Significant Genes | |||
| gb|M24734.1| | 14.3 | 3.9 × 10−5 | T1L L1 |
| gb|AF378003.1| | 15.3 | 1.4 × 10−5 | T1L L2 |
| gb|AF129820.1| | 15.3 | 1.4 × 10−5 | T1L L3 |
| gb|AF461682.1| | 15.4 | 1.1 × 10−5 | T1L M1 |
| gb|AF490617.1| | 15.4 | 1.3 × 10−5 | T1L M2 |
| gb|AF174382.1| | 15.3 | 1.4 × 10−5 | T1L M3 |
| gb|EF494445.1| | 15.2 | 1.2 × 10−5 | T1L S1 |
| gb|L19774.1| | 15.4 | 1.0 × 10−5 | T1L S2 |
| gb|M18389.1| | 14.9 | 1.6 × 10−5 | T1L S3 |
| gb|M13139.1| | 15.1 | 1.3 × 10−5 | T1L S4 |
| ENSMUST00000083211.1 | 7.2 | 1.4 × 10−4 | Vaultrc5 |
| ENSMUST00000062045.3 | 6.0 | 3.5 × 10−4 | Hist1h1e |
| ENSMUST00000147537.5 | 6.5 | 2.8 × 10−5 | Lmna |
| ENSMUST00000098843.2 | 5.9 | 1.4 × 10−4 | Hist2h3b |
| ENSMUST00000079251.7 | 4.9 | 2.8 × 10−4 | Hist1h2bg |
| ENSMUST00000102967.2 | 4.3 | 4.1 × 10−4 | Hist1h4c |
| ENSMUST00000074752.3 | 4.2 | 5.9 × 10−4 | Hist1h2ak |
| ENSMUST00000045301.8 | 4.8 | 6.9 × 10−4 | Hist1h1d |
| ENSMUST00000099703.4 | 4.3 | 4.5 × 10−4 | Hist1h2bb |
| ENSMUST00000102979.1 | 3.8 | 5.0 × 10−4 | Hist1h4n |
| ENSMUST00000102983.1 | 4.3 | 1.1 × 10−4 | Hist1h4k |
| ENSMUST00000070124.4 | 5.5 | 3.2 × 10−6 | Hist1h2ai |
| ENSMUST00000102969.5 | 4.8 | 3.7 × 10−5 | Hist1h2ae |
| ENSMUST00000091752.4 | 4.8 | 2.2 × 10−5 | Hist1h3c |
| ENSMUST00000087714.5 | 4.7 | 1.1 × 10−5 | Hist1h4j |
| ENSMUST00000078369.2 | 5.0 | 4.1 × 10−6 | Hist1h2ab |
| ENSMUST00000091709.2 | 6.0 | 1.2 × 10−8 | Hist1h2bn |
| ENSMUST00000144964.7 | 4.9 | 4.7 × 10−9 | Pex6 |
| ENSMUST00000171127.3 | 3.7 | 2.9 × 10−5 | Hist1h2ac |
| ENSMUST00000091703.2 | 5.6 | 1.1 × 10−7 | Hist1h3b |
| ENSMUST00000091708.5 | 3.8 | 3.7 × 10−4 | Hist1h2al |
| ENSMUST00000105106.1 | 4.8 | 6.5 × 10−7 | Hist1h2bf |
| ENSMUST00000188775.1 | 4.0 | 5.3 × 10−6 | Hist1h3h |
| ENSMUST00000091756.1 | 5.5 | 2.9 × 10−8 | Hist1h2bl |
| ENSMUST00000224651.1 | 5.2 | 1.6 × 10−7 | Hist1h2bm |
| ENSMUST00000224359.1 | 4.5 | 1.8 × 10−8 | Hist1h2bh |
| ENSMUST00000136269.7 | 4.2 | 1.3 × 10−11 | Rpl7a |
| ENSMUST00000149925.7 | 5.1 | 1.6 × 10−12 | Ctu2 |
| ENSMUST00000073261.2 | 10.8 | 1.4 × 10−11 | Hist1h2af |
| ENSMUST00000090776.6 | 4.8 | 2.3 × 10−9 | Hist1h2ad |
| ENSMUST00000181242.1 | 6.5 | 1.7 × 10−6 | Gm26870 lincRNA c |
| ENSMUST00000159697.1 | 7.4 | 1.6 × 10−18 | Acat2 |
| ENSMUST00000107249.7 | 3.3 | 6.9 × 10−9 | Rpl27 |
| ENSMUST00000091751.2 | 3.7 | 5.6 × 10−8 | Hist1h2an |
| rsT3DI-T1L1 Virions Significant Genes | |||
| gb|M24734.1| | 18.1 | 2.9 × 10−6 | T1L L1 |
| gb|EF494436.1| | 17.3 | 4.2 × 10−6 | T3D L2 |
| gb|EF494437.1| | 17.6 | 3.9 × 10−6 | T3D L3 |
| gb|EF494438.1| | 17.9 | 2.7 × 10−6 | T3D M1 |
| gb|EF494439.1| | 18.1 | 2.8 × 10−6 | T3D M2 |
| gb|EF494440.1| | 18.1 | 3.1 × 10−6 | T3D M3 |
| gb|EF494441.1| | 16.6 | 4.2 × 10−6 | T3D S1 |
| gb|EF494442.1| | 15.7 | 6.3 × 10−6 | T3D S2 |
| gb|EF494443.1| | 16.7 | 4.0 × 10−6 | T3D S3 |
| gb|EF494444.1| | 17.7 | 2.6 × 10−6 | T3D S4 |
| rsT3DI-T1L1 TC Significant Genes | |||
| gb|M24734.1| | 14.0 | 4.6 × 10−5 | T1L L1 |
| gb|EF494436.1| | 15.6 | 1.4 × 10−5 | T3D L2 |
| gb|EF494437.1| | 15.8 | 1.3 × 10−5 | T3D L3 |
| gb|EF494438.1| | 14.5 | 3.0 × 10−5 | T3D M1 |
| gb|EF494439.1| | 16.1 | 1.1 × 10−5 | T3D M2 |
| gb|EF494440.1| | 15.7 | 1.5 × 10−5 | T3D M3 |
| gb|EF494441.1| | 15.5 | 8.7 × 10−6 | T3D S1 |
| gb|EF494442.1| | 15.4 | 7.1 × 10−6 | T3D S2 |
| gb|EF494443.1| | 16.2 | 5.2 × 10−6 | T3D S3 |
| gb|EF494444.1| | 14.7 | 2.1 × 10−5 | T3D S4 |
| ENSMUST00000098843.2 | 5.8 | 1.5 × 10−4 | Hist2h3b |
| ENSMUST00000045540.3 | 7.8 | 1.2 × 10−7 | Socs7 |
| ENSMUST00000033930.4 | 4.7 | 1.5 × 10−4 | Dusp4 |
| ENSMUST00000032094.6 | 7.4 | 2.5 × 10−7 | Fbxl14 |
| ENSMUST00000046929.6 | 4.6 | 7.1 × 10−5 | Usp31 |
| ENSMUST00000147545.7 | 5.8 | 3.7 × 10−6 | Ccdc6 |
| ENSMUST00000079869.12 | 3.5 | 4.0 × 10−4 | Znrf2 |
| ENSMUST00000050063.8 | 4.5 | 1.9 × 10−5 | Arf6 |
| ENSMUST00000178344.2 | 4.1 | 2.6 × 10−4 | Itpripl2 |
| ENSMUST00000052838.10 | 5.3 | 5.9 × 10−7 | Mib1 |
| ENSMUST00000007980.6 | 4.0 | 3.7 × 10−4 | Hnrnpa0 |
| ENSMUST00000093962.4 | 3.9 | 4.9 × 10−4 | Ccnd1 |
| ENSMUST00000106113.1 | 3.8 | 5.3 × 10−5 | Foxk2 |
| ENSMUST00000073109.11 | 6.8 | 4.2 × 10−14 | Ctdspl |
| ENSMUST00000058550.14 | 5.7 | 1.9 × 10−8 | Ccni |
| ENSMUST00000035220.11 | 4.9 | 2.1 × 10−7 | Prkar2a |
| ENSMUST00000022875.6 | 4.9 | 1.8 × 10−6 | Ank |
| ENSMUST00000044954.6 | 3.4 | 2.3 × 10−5 | Slc30a1 |
| ENSMUST00000069180.7 | 5.6 | 4.8 × 10−10 | Zcchc24 |
| ENSMUST00000102824.3 | 11.4 | 1.6 × 10−15 | Ifit1 |
| ENSMUST00000085425.4 | 7.9 | 6.8 × 10−12 | Isg15 |
| ENSMUST00000070124.4 | 4.2 | 1.6 × 10−4 | Hist1h2ai |
| ENSMUST00000149978.1 | 5.0 | 3.6 × 10−8 | Inafm2 |
| ENSMUST00000050467.8 | 4.0 | 9.4 × 10−7 | Tob2 |
| ENSMUST00000013807.7 | 3.6 | 2.5 × 10−6 | Pten |
| ENSMUST00000102825.3 | 10.7 | 2.1 × 10−9 | Ifit3 |
| ENSMUST00000078369.2 | 4.0 | 1.2 × 10−4 | Hist1h2ab |
| ENSMUST00000181242.1 | 6.3 | 3.2 × 10−6 | Gm26870 lincRNA |
| ENSMUST00000008537.9 | 4.0 | 2.2 × 10−6 | Carhsp1 |
| ENSMUST00000034832.7 | 5.2 | 1.0 × 10−10 | Ptpn9 |
| ENSMUST00000028648.2 | 4.0 | 9.5 × 10−6 | Syt13 |
| ENSMUST00000224651.1 | 4.4 | 4.4 × 10−6 | Hist1h2bm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thoner, T.W., Jr.; Ye, X.; Karijolich, J.; Ogden, K.M. Reovirus Low-Density Particles Package Cellular RNA. Viruses 2021, 13, 1096. https://doi.org/10.3390/v13061096
Thoner TW Jr., Ye X, Karijolich J, Ogden KM. Reovirus Low-Density Particles Package Cellular RNA. Viruses. 2021; 13(6):1096. https://doi.org/10.3390/v13061096
Chicago/Turabian StyleThoner, Timothy W., Jr., Xiang Ye, John Karijolich, and Kristen M. Ogden. 2021. "Reovirus Low-Density Particles Package Cellular RNA" Viruses 13, no. 6: 1096. https://doi.org/10.3390/v13061096
APA StyleThoner, T. W., Jr., Ye, X., Karijolich, J., & Ogden, K. M. (2021). Reovirus Low-Density Particles Package Cellular RNA. Viruses, 13(6), 1096. https://doi.org/10.3390/v13061096






