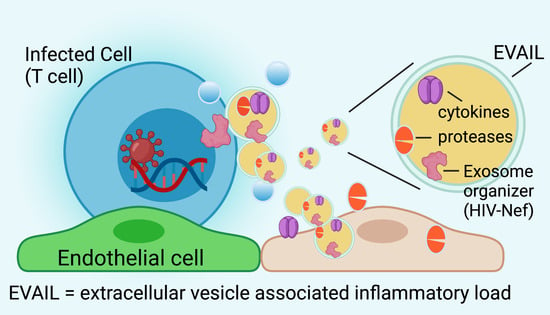
Viral Bad News Sent by EVAIL
Abstract
1. Introduction
2. HIV Mystery: Heightened CVD Risk in HIV-Infected Individuals on ART
2.1. Persistent CVD Risk in HIV-Infected Individuals Despite ART
2.2. Factors Contributing to CVD Risk in HIV-Infected Individuals on ART
3. Increased EV in HIV-Infected Individuals Despite ART
3.1. EVs Are Enriched in the Plasma of HIV Patients on ART
3.2. Nef Protein Persists in EVs from Body Fluids
3.3. Nef Protein in EVs Transfers Rapidly to Blood and Tissue Cells
4. HIV-Nef Induces Extracellular Vesicle-Assisted Inflammatory Load (EVAIL)
4.1. Specific Surface Proteins in EVs Define Proinflammatory Cargo Associated with HIV Proteins
4.2. Nef Initiates Endosomal Routing Leading to Recruitment of ADAM-17/TACE and Secretion of TNF
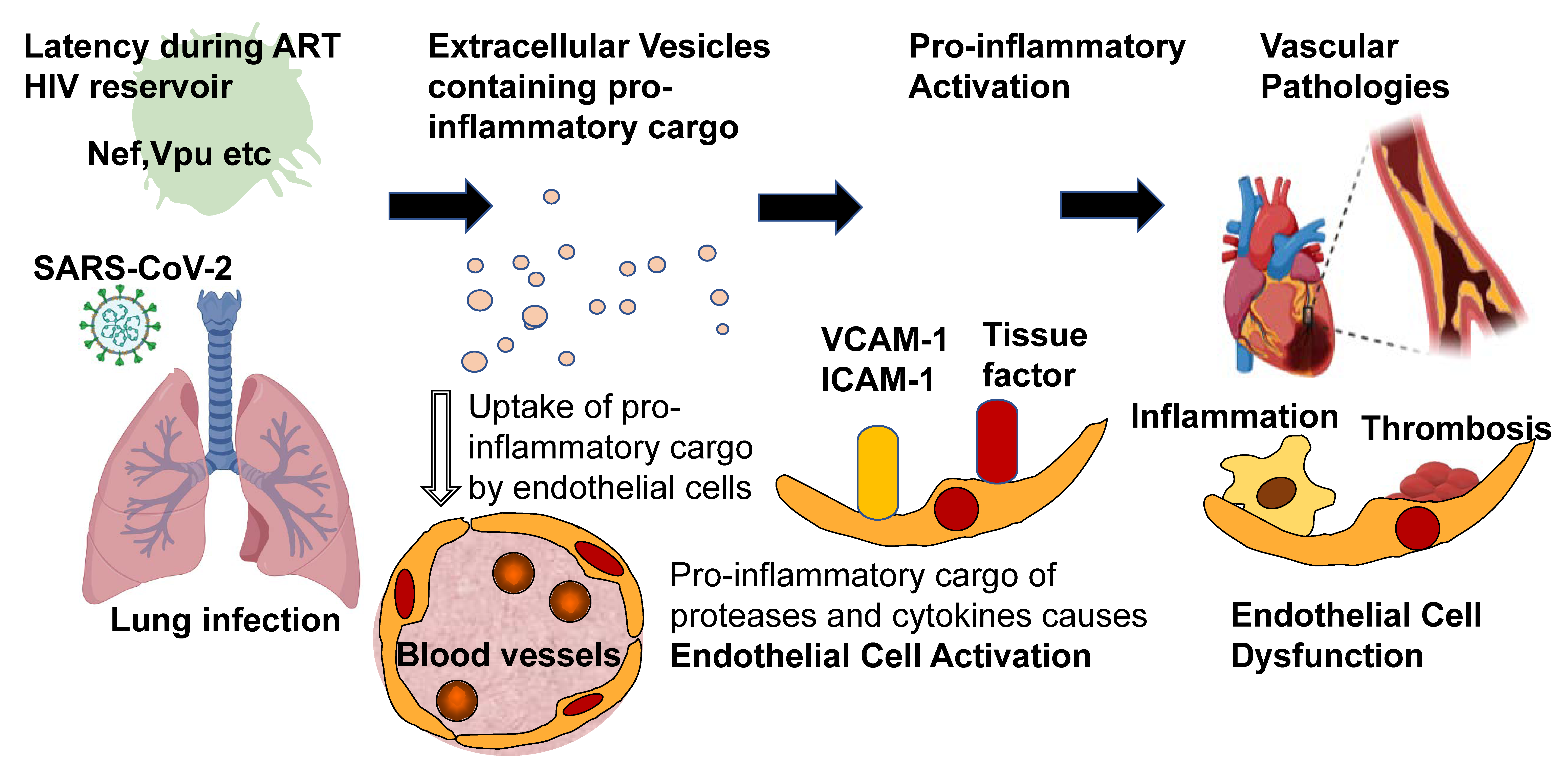
5. Pathophysiology of the HIV-Nef-Associated EVAIL
6. Does the EVAIL Concept also Hold True for Other Viruses? What about Pandemic SARS CoV-2?
7. Conclusions and Potential for Therapy
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, H.; Peng, G.; Bai, J.; Kecheng, H.; Huang, K.; Hu, X.; Liu, D. Cytomegalovirus Infection and Relative Risk of Cardiovascular Disease (Ischemic Heart Disease, Stroke, and Cardiovascular Death): A Meta-Analysis of Prospective Studies Up to 2016. J. Am. Hear. Assoc. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Sackoff, J.E.; Hanna, D.B.; Pfeiffer, M.R.; Torian, L.V. Causes of Death among Persons with Aids in the Era of Highly Active Antiretroviral Therapy: New York City. Ann. Intern. Med. 2006, 145, 397–406. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.-C.H.; Skanderson, M.; McGinnis, K.; Kuller, L.H.; Kraemer, K.L.; Rimland, D.; Goetz, M.B.; Butt, A.A.; Barradas, M.C.R.; et al. The Risk of Incident Coronary Heart Disease Among Veterans With and Without HIV and Hepatitis C. Circ. Cardiovasc. Qual. Outcomes 2011, 4, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Obel, N.; Thomsen, H.F.; Kronborg, G.; Larsen, C.S.; Hildebrandt, P.R.; Sørensen, H.T.; Gerstoft, J. Ischemic Heart Disease in HIV-Infected and HIV-Uninfected Individuals: A Population-Based Cohort Study. Clin. Infect. Dis. 2007, 44, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Triant, V.A.; Lee, H.; Hadigan, C.; Grinspoon, S.K. Increased Acute Myocardial Infarction Rates and Cardiovascular Risk Factors among Patients with Human Immunodeficiency Virus Disease. J. Clin. Endocrinol. Metab. 2007, 92, 2506–2512. [Google Scholar] [CrossRef] [PubMed]
- Grunfeld, C.; Delaney, J.A.C.; Wanke, C.; Currier, J.S.; Scherzer, R.; Biggs, M.L.; Tien, P.C.; Shlipak, M.G.; Sidney, S.; Polak, J.F.; et al. Preclinical atherosclerosis due to HIV infection: Carotid intima-medial thickness measurements from the FRAM study. AIDS 2009, 23, 1841–1849. [Google Scholar] [CrossRef]
- Mangili, A.; Polak, J.F.; Skinner, S.C.; Gerrior, J.; Sheehan, H.; Harrington, A.; Wanke, C.A. HIV Infection and Progression of Carotid and Coronary Atherosclerosis: The CARE Study. JAIDS J. Acquir. Immune Defic. Syndr. 2011, 58, 148–153. [Google Scholar] [CrossRef]
- D’Ascenzo, F.; Quadri, G.; Cerrato, E.; Calcagno, A.; Omedé, P.; Marra, W.G.; Abbate, A.; Bonora, S.; Zoccai, G.B.; Moretti, C.; et al. A meta-analysis investigating incidence and features of stroke in HIV-infected patients in the highly active antiretroviral therapy era. J. Cardiovasc. Med. 2015, 16, 839–843. [Google Scholar] [CrossRef]
- Speich, R.; Jenni, R.; Opravil, M.; Pfab, M.; Russi, E.W. Primary Pulmonary Hypertension in Hiv Infection. Chest 1991, 100, 1268–1271. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Lascoux-Combe, C.; Delfraissy, J.-F.; Yeni, P.G.; Raffi, F.; De Zuttere, D.; Gressin, V.; Clerson, P.; Sereni, D.; Simonneau, G. Prevalence of HIV-related Pulmonary Arterial Hypertension in the Current Antiretroviral Therapy Era. Am. J. Respir. Crit. Care Med. 2008, 177, 108–113. [Google Scholar] [CrossRef]
- Lang, S.; Mary-Krause, M.; Cotte, L.; Gilquin, J.; Partisani, M.; Simon, A.; Boccara, F.; Bingham, A.; Costagliola, D. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS 2010, 24, 1228–1230. [Google Scholar] [CrossRef]
- Hsue, P.Y.; Hunt, P.W.; Ho, J.E.; Farah, H.H.; Schnell, A.; Hoh, R.; Martin, J.N.; Deeks, S.G.; Bolger, A.F. Impact of HIV Infection on Diastolic Function and Left Ventricular Mass. Circ. Hear. Fail. 2010, 3, 132–139. [Google Scholar] [CrossRef]
- Durand, M.; Sheehy, O.; Baril, J.G.; Lelorier, J.; Tremblay, C.L. Association between Hiv Infection, Antiretroviral Therapy, and Risk of Acute Myocardial Infarction: A Cohort and Nested Case-Control Study Using Quebec’s Public Health Insurance Database. J. Acquir. Immune Defic. Syndr. 2011, 57, 245–253. [Google Scholar] [CrossRef]
- Butt, A.A.; Chang, C.-C.; Kuller, L.; Goetz, M.B.; Leaf, D.; Rimland, D.; Gibert, C.L.; Oursler, K.K.; Rodriguez-Barradas, M.C.; Lim, J.; et al. Risk of Heart Failure With Human Immunodeficiency Virus in the Absence of Prior Diagnosis of Coronary Heart Disease. Arch. Intern. Med. 2011, 171, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Sliwa, K.; Carrington, M.J.; Becker, A.; Thienemann, F.; Ntsekhe, M.; Stewart, S. Contribution of the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic to de novo presentations of heart disease in the Heart of Soweto Study cohort. Eur. Hear. J. 2011, 33, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Lorgis, L.; Cottenet, J.; Molins, G.; Benzenine, E.; Zeller, M.; Aube, H.; Touzery, C.; Hamblin, J.; Gudjoncik, A.; Cottin, Y.; et al. Outcomes after Acute Myocardial Infarction in Hiv-Infected Patients: Analysis of Data from a French Nationwide Hospital Medical Information Database. Circulation 2013, 127, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- Freiberg, M.S.; Chang, C.-C.H.; Kuller, L.H.; Skanderson, M.; Lowy, E.; Kraemer, K.L.; Butt, A.A.; Goetz, M.B.; Leaf, D.; Oursler, K.A.; et al. HIV Infection and the Risk of Acute Myocardial Infarction. JAMA Intern. Med. 2013, 173, 614–622. [Google Scholar] [CrossRef] [PubMed]
- Womack, J.A.; Chang, C.H.; So-Armah, K.; Alcorn, C.; Baker, J.V.; Brown, S.T.; Budoff, M.; Butt, A.A.; Gibert, C.; Goetz, M.B.; et al. HIV Infection and Cardiovascular Disease in Women. J. Am. Hear. Assoc. 2014, 3, e001035. [Google Scholar] [CrossRef]
- Luo, L.; Zeng, Y.; Li, T.; Lv, W.; Wang, H.; Guo, F.; Han, Y.; Xie, J.; Qiu, Z.; Li, Y.; et al. Prospective Echocardiographic Assessment of Cardiac Structure and Function in Chinese Persons Living With HIV. Clin. Infect. Dis. 2014, 58, 1459–1466. [Google Scholar] [CrossRef]
- Carvalho, R.F.; Mancio, J.; Marcos, A.; Sampaio, F.; Mota, M.; Gonçalves, F.R.; Gama, V.; Azevedo, A.; Leite-Moreira, A. HIV Patients Have Impaired Diastolic Function that is Not Aggravated by Anti-Retroviral Treatment. Cardiovasc. Drugs Ther. 2015, 29, 31–39. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Helleberg, M.; May, M.T.; Afzal, S.; Kronborg, G.; Larsen, C.S.; Pedersen, C.; Gerstoft, J.; Nordestgaard, B.G.; Obel, N. Myocardial Infarction Among Danish HIV-Infected Individuals: Population-Attributable Fractions Associated With Smoking. Clin. Infect. Dis. 2015, 60, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Chow, D.; Young, R.; Valcour, N.; Kronmal, R.A.; Lum, C.J.; Parikh, N.I.; Tracy, R.P.; Budoff, M.J.; Shikuma, C.M. HIV and coronary artery calcium score: Comparison of the Hawaii Aging with HIV Cardiovascular Study and Multi-Ethnic Study of Atherosclerosis (MESA) cohorts. HIV Clin. Trials 2015, 16, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Al-Kindi, S.G.; ElAmm, C.; Ginwalla, M.; Mehanna, E.; Zacharias, M.; Benatti, R.; Oliveira, G.H.; Longenecker, C.T. Heart failure in patients with human immunodeficiency virus infection: Epidemiology and management disparities. Int. J. Cardiol. 2016, 218, 43–46. [Google Scholar] [CrossRef]
- Freiberg, M.S.; Chang, C.H.; Skanderson, M.; Patterson, O.V.; DuVall, S.L.; Brandt, C.A.; So-Armah, K.A.; Vasan, R.S.; Oursler, K.A.; Gottdiener, J.; et al. Association between Hiv Infection and the Risk of Heart Failure with Reduced Ejection Fraction and Preserved Ejection Fraction in the Antiretroviral Therapy Era: Results from the Veterans Aging Cohort Study. JAMA Cardiol. 2017, 2, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, A.D.; Gelpi, M.; Afzal, S.; Ronit, A.; Roen, A.; Mocroft, A.; Lundgren, J.; Nordestgaard, B.; Sillesen, H.; Lebech, A.-M.; et al. Brief Report: Prevalence of Peripheral Artery Disease Is Higher in Persons Living With HIV Compared With Uninfected Controls. JAIDS J. Acquir. Immune Defic. Syndr. 2018, 79, 381–385. [Google Scholar] [CrossRef]
- Alonso, A.; Barnes, A.E.; Guest, J.L.; Shah, A.; Shao, I.Y.; Marconi, V. HIV Infection and Incidence of Cardiovascular Diseases: An Analysis of a Large Healthcare Database. J. Am. Hear. Assoc. 2019, 8, e012241. [Google Scholar] [CrossRef]
- Beckman, J.A.; Duncan, M.S.; Alcorn, C.W.; So-Armah, K.; Butt, A.A.; Goetz, M.B.; Tindle, H.A.; Sico, J.J.; Tracy, R.P.; Justice, A.C.; et al. Association of Human Immunodeficiency Virus Infection and Risk of Peripheral Artery Disease. Circ. 2018, 138, 255–265. [Google Scholar] [CrossRef]
- Rao, S.G.; Galaviz, K.I.; Gay, H.C.; Wei, J.; Armstrong, W.S.; del Rio, C.; Narayan, K.V.; Ali, M. Factors Associated With Excess Myocardial Infarction Risk in HIV-Infected Adults: A Systematic Review and Meta-analysis. JAIDS J. Acquir. Immune Defic. Syndr. 2019, 81, 224–230. [Google Scholar] [CrossRef]
- Chattranukulchai, P.; Thimaporn, W.; Siwamogsatham, S.; Satitthunmmanid, S.; Sitticharoenchai, P.; Apornpong, T.; Sangarlangkarn, A.; Kerr, S.J.; Ruxrungtham, K.; Boonyaratavej, S.; et al. Echocardiographic Findings Among Virally Suppressed HIV-Infected Aging Asians Compared with HIV-Negative Individuals. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 85, 379–386. [Google Scholar] [CrossRef]
- Vallilo, N.G.; Durigon, G.S.; Lianza, A.C.; Diniz, M.d.R.; Sawamura, K.S.S.; Brito, C.R.; Marques, H.H.d.; Ferraro, A.A.; Leal, G.N. Echocardiographic Follow-up of Perinatally Hiv-Infected Children and Adolescents: Results from a Single-Center Retrospective Cohort Study in Brazil. Pediatr. Infect Dis. J. 2020, 39, 526–532. [Google Scholar] [CrossRef]
- Shen, F.; Zhu, B.; Ding, Y.; Gao, M.; He, N. Electrocardiographic abnormalities among people with HIV in Shanghai, China. Biosci. Trends 2020, 14, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Kline, E.R.; Sutliff, R.L. The Roles of HIV-1 Proteins and Antiretroviral Drug Therapy in HIV-1-Associated Endothelial Dysfunction. J. Investig. Med. 2008, 56, 752–769. [Google Scholar] [CrossRef]
- Baliga, R.S.; Chaves, A.A.; Jing, L.; Ayers, L.W.; Bauer, J.A. Aids-Related Vasculopathy: Evidence for Oxidative and Inflammatory Pathways in Murine and Human Aids. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1373–H1380. [Google Scholar] [CrossRef] [PubMed]
- Maggi, P.; Bellacosa, C.; Leone, A.; Volpe, A.; Ricci, E.D.; Ladisa, N.; Cicalini, S.; Grilli, E.; Viglietti, R.; Chirianni, A.; et al. Cardiovascular risk in advanced naïve HIV-infected patients starting antiretroviral therapy: Comparison of three different regimens—PREVALEAT II cohort. Atheroscler. 2017, 263, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal Microbiota, Microbial Translocation, and Systemic Inflammation in Chronic HIV Infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef]
- Wallet, M.A.; Rodriguez, C.A.; Yin, L.; Saporta, S.; Chinratanapisit, S.; Hou, W.; Sleasman, J.W.; Goodenow, M.M. Microbial translocation induces persistent macrophage activation unrelated to HIV-1 levels or T-cell activation following therapy. AIDS 2010, 24, 1281–1290. [Google Scholar] [CrossRef]
- Peterson, T.E.; Hullsiek, K.H.; Engen, N.W.; Kumarasamy, N.; Lebech, A.-M.; Liappis, A.; Papadopoulos, A.; Polizzotto, M.N.; Schreiner, P.J.; Duprez, D.; et al. Inflammation Associates With Impaired Small Arterial Elasticity Early in HIV Disease. Open Forum Infect. Dis. 2018, 5, 117. [Google Scholar] [CrossRef]
- Rönsholt, F.F.; Ullum, H.; Katzenstein, T.L.; Gerstoft, J.; Ostrowski, S.R. Persistent Inflammation and Endothelial Activation in HIV-1 Infected Patients after 12 Years of Antiretroviral Therapy. PLoS ONE 2013, 8, e65182. [Google Scholar] [CrossRef]
- Lo, J. Dyslipidemia and lipid management in HIV-infected patients. Curr. Opin. Endocrinol. Diabetes Obes. 2011, 18, 144–147. [Google Scholar] [CrossRef]
- Asztalos, B.F.; Mujawar, Z.; Morrow, M.P.; Grant, A.; Pushkarsky, T.; Wanke, C.; Shannon, R.; Geyer, M.; Kirchhoff, F.; Sviridov, D.; et al. Circulating Nef Induces Dyslipidemia in Simian Immunodeficiency Virus–Infected Macaques by Suppressing Cholesterol Efflux. J. Infect. Dis. 2010, 202, 614–623. [Google Scholar] [CrossRef]
- Cui, H.L.; Ditiatkovski, M.; Kesani, R.; Bobryshev, Y.V.; Liu, Y.; Geyer, M.; Mukhamedova, N.; Bukrinsky, M.; Sviridov, D. HIV protein Nef causes dyslipidemia and formation of foam cells in mouse models of atherosclerosis. FASEB J. 2014, 28, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Mujawar, Z.; Tamehiro, N.; Grant, A.; Sviridov, D.; Bukrinsky, M.; Fitzgerald, M.L. Mutation of the ATP Cassette Binding Transporter A1 (ABCA1) C-Terminus Disrupts HIV-1 Nef Binding but Does Not Block the Nef Enhancement of ABCA1 Protein Degradation. Biochemistry 2010, 49, 8338–8349. [Google Scholar] [CrossRef]
- Sharma, H.; Chinnappan, M.; Agarwal, S.; Dalvi, P.; Gunewardena, S.; O’Brien-Ladner, A.; Dhillon, N.K. Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: Role of altered miRNA cargo in response to HIV infection and substance abuse. FASEB J. 2018, 32, 5174–5185. [Google Scholar] [CrossRef]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Wolinsky, S.M.; Gabuzda, D. Small RNA sequencing of extracellular vesicles identifies circulating miRNAs related to inflammation and oxidative stress in HIV patients. BMC Immunol. 2020, 21, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachary, B.; Mahajan, A.; Kumar, A.; Agarwal, S.; Mohan, A.; Chen, L.; Hsue, P.; Chalise, P.; Morris, A.; Dhillon, N.K. Extracellular Vesicle Tgf-Beta1 Is Linked to Cardiopulmonary Dysfunction in Hiv. Am. J. Respir. Cell Mol. Biol. 2021. [Google Scholar] [CrossRef]
- Chelvanambi, S.; Bogatcheva, N.V.; Bednorz, M.; Agarwal, S.; Maier, B.; Alves, N.J.; Li, W.; Syed, F.; Saber, M.M.; Dahl, N.; et al. HIV-Nef Protein Persists in the Lungs of Aviremic Patients with HIV and Induces Endothelial Cell Death. Am. J. Respir. Cell Mol. Biol. 2019, 60, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Chelvanambi, S.; Gupta, S.K.; Chen, X.; Ellis, B.W.; Maier, B.F.; Colbert, T.M.; Kuriakose, J.; Zorlutuna, P.; Jolicoeur, P.; Obukhov, A.G.; et al. HIV-Nef Protein Transfer to Endothelial Cells Requires Rac1 Activation and Leads to Endothelial Dysfunction Implications for Statin Treatment in HIV Patients. Circ. Res. 2019, 125, 805–820. [Google Scholar] [CrossRef]
- Wang, T.; Green, L.A.; Gupta, S.K.; Kim, C.; Wang, L.; Almodovar, S.; Flores, S.C.; Prudovsky, I.A.; Jolicoeur, P.; Liu, Z.; et al. Transfer of Intracellular HIV Nef to Endothelium Causes Endothelial Dysfunction. PLoS ONE 2014, 9, e91063. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.A.; Huang, M.-B.; Campbell, P.E.; Roth, W.W.; Campbell, T.; Khan, M.; Newman, G.; Villinger, F.; Powell, M.D.; Bond, V.C. Genetic Characterization of HIV Type 1 Nef-Induced Vesicle Secretion. AIDS Res. Hum. Retrovir. 2010, 26, 173–192. [Google Scholar] [CrossRef] [PubMed]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from Human Immunodeficiency Virus Type 1 (HIV-1)-Infected Cells License Quiescent CD4 + T Lymphocytes To Replicate HIV-1 through a Nef- and ADAM17-Dependent Mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef] [PubMed]
- Campbell, T.D.; Khan, M.; Huang, M.-B.; Bond, V.C.; Powell, M.D. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn. Dis. 2008, 18, S2-14-9. [Google Scholar]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotič, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitaš, A.; Peterlin, B. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef]
- Mohan, A.; Agarwal, S.; Clauss, M.; Britt, N.S.; Dhillon, N.K. Extracellular vesicles: Novel communicators in lung diseases. Respir. Res. 2020, 21, 175. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Feng, Z.; Yue, H.; Bazdar, D.; Mbonye, U.; Zender, C.; Harding, C.V.; Bruggeman, L.; Karn, J.; Sieg, S.F.; et al. Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA. Nat. Commun. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ellwanger, J.H.; Veit, T.D.; Chies, J.A.B. Exosomes in HIV infection: A review and critical look. Infect. Genet. Evol. 2017, 53, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Gangoda, L.; Boukouris, S.; Liem, M.; Kalra, H.; Mathivanan, S. Extracellular vesicles including exosomes are mediators of signal transduction: Are they protective or pathogenic? Proteom. 2015, 15, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Hildreth, J.E.K. HIV As Trojan Exosome: Immunological Paradox Explained? Front. Immunol. 2017, 8, 1715. [Google Scholar] [CrossRef]
- Kodidela, S.; Gerth, K.; Haque, S.; Gong, Y.; Ismael, S.; Singh, A.; Ishrat, T.; Kumar, S. Extracellular Vesicles: A Possible Link between HIV and Alzheimer’s Disease-Like Pathology in HIV Subjects? Cells 2019, 8, 968. [Google Scholar] [CrossRef]
- Konadu, K.A.; Chu, J.; Huang, M.B.; Amancha, P.K.; Armstrong, W.S.; Powell, M.D.; Villinger, F.; Bond, V.C. Association of Cytokines With Exosomes in the Plasma of HIV-1–Seropositive Individuals. J. Infect. Dis. 2015, 211, 1712–1716. [Google Scholar] [CrossRef]
- Li, H.; Chi, X.; Li, R.; Ouyang, J.; Chen, Y. HIV-1-infected cell-derived exosomes promote the growth and progression of cervical cancer. Int. J. Biol. Sci. 2019, 15, 2438–2447. [Google Scholar] [CrossRef]
- Madison, M.N.; Okeoma, C.M. Exosomes: Implications in HIV-1 Pathogenesis. Viruses 2015, 7, 4093–4118. [Google Scholar] [CrossRef]
- Lee, J.-H.; Schierer, S.; Blume, K.; Dindorf, J.; Wittki, S.; Xiang, W.; Ostalecki, C.; Koliha, N.; Wild, S.; Schuler, G.; et al. HIV-Nef and ADAM17-containing plasma extracellular vesicles induce and correlate with immune pathogenesis in chronic HIV infection. EBioMedicine 2016, 6, 103–113. [Google Scholar] [CrossRef]
- Chettimada, S.; Lorenz, D.R.; Misra, V.; Dillon, S.T.; Reeves, R.K.; Manickam, C.; Morgello, S.; Kirk, G.D.; Mehta, S.H.; Gabuzda, D. Exosome markers associated with immune activation and oxidative stress in HIV patients on antiretroviral therapy. Sci. Rep. 2018, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Joos, B.; Niederost, B.; Kaiser, P.; Hafner, R.; Von Wyl, V.; Ackermann, M.; Weber, R.; Gunthard, H.F. Biphasic decay kinetics suggest progressive slowing in turnover of latently HIV-1 infected cells during antiretroviral therapy. Retrovirology 2008, 5, 107. [Google Scholar] [CrossRef]
- Fischer, M.; Joos, B.; Hirschel, B.; Bleiber, G.; Weber, R.; Gunthard, H.F. Cellular Viral Rebound after Cessation of Potent Antiretroviral Therapy Predicted by Levels of Multiply Spliced Hiv-1 Rna Encoding Nef. J. Infect. Dis. 2004, 190, 1979–1988. [Google Scholar] [CrossRef]
- Raymond, A.; Campbell-Sims, T.; Khan, M.A.K.G.; Lang, M.; Huang, M.B.; Bond, V.C.; Powell, M.D. HIV Type 1 Nef Is Released from Infected Cells in CD45+Microvesicles and Is Present in the Plasma of HIV-Infected Individuals. AIDS Res. Hum. Retrovir. 2011, 27, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Green, L.A.; Gupta, S.K.; Amet, T.; Byrd, D.J.; Yu, Q.; Twigg, H.L., 3rd; Clauss, M. Intracellular Nef Detected in Peripheral Blood Mononuclear Cells from Hiv Patients. AIDS Res. Hum. Retrovir. 2015, 31, 217–220. [Google Scholar] [CrossRef] [PubMed]
- Ferdin, J.; Goričar, K.; Dolžan, V.; Plemenitaš, A.; Martin, J.N.; Peterlin, B.M.; Deeks, S.; Lenassi, M. Viral protein Nef is detected in plasma of half of HIV-infected adults with undetectable plasma HIV RNA. PLoS ONE 2018, 13, e0191613. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Wittki, S.; Brau, T.; Dreyer, F.S.; Kratzel, K.; Dindorf, J.; Johnston, I.C.; Gross, S.; Kremmer, E.; Zeidler, R.; et al. Hiv Nef, Paxillin, and Pak1/2 Regulate Activation and Secretion of Tace/Adam10 Proteases. Mol. Cell 2013, 49, 668–679. [Google Scholar] [CrossRef]
- Ostalecki, C.; Wittki, S.; Lee, J.-H.; Geist, M.M.; Tibroni, N.; Harrer, T.; Schuler, G.; Fackler, O.; Baur, A.S. HIV Nef- and Notch1-dependent Endocytosis of ADAM17 Induces Vesicular TNF Secretion in Chronic HIV Infection. EBioMedicine 2016, 13, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Gooz, M. ADAM-17: The enzyme that does it all. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 146–169. [Google Scholar] [CrossRef] [PubMed]
- Arenaccio, C.; Anticoli, S.; Manfredi, F.; Chiozzini, C.; Olivetta, E.; Federico, M. Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1. Retrovirology 2015, 12, 1–17. [Google Scholar] [CrossRef]
- Tang, X.; Lu, H.; Dooner, M.; Chapman, S.; Quesenberry, P.J.; Ramratnam, B. Exosomal Tat protein activates latent HIV-1 in primary, resting CD4+ T lymphocytes. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- McNamara, R.P.; Costantini, L.M.; Myers, T.A.; Schouest, B.; Maness, N.J.; Griffith, J.D.; Damania, B.A.; MacLean, A.G.; Dittmer, D.P. Nef Secretion into Extracellular Vesicles or Exosomes Is Conserved across Human and Simian Immunodeficiency Viruses. mBio 2018, 9. [Google Scholar] [CrossRef]
- Saribas, A.S.; Cicalese, S.; Ahooyi, T.M.; Khalili, K.; Amini, S.; Sariyer, I.K. HIV-1 Nef is released in extracellular vesicles derived from astrocytes: Evidence for Nef-mediated neurotoxicity. Cell Death Dis. 2018, 8, e2542. [Google Scholar] [CrossRef]
- Khan, M.B.; Lang, M.J.; Huang, M.B.; Raymond, A.; Bond, V.C.; Shiramizu, B.; Powell, M.D. Nef Exosomes Isolated from the Plasma of Individuals with Hiv-Associated Dementia (Had) Can Induce Abeta(1-42) Secretion in Sh-Sy5y Neural Cells. J. Neurovirol. 2016, 22, 179–190. [Google Scholar] [CrossRef]
- Raymond, A.D.; Diaz, P.; Chevelon, S.; Agudelo, M.; Yndart-Arias, A.; Ding, H.; Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Roy, U.; et al. Microglia-derived HIV Nef+ exosome impairment of the blood–brain barrier is treatable by nanomedicine-based delivery of Nef peptides. J. NeuroVirology 2016, 22, 129–139. [Google Scholar] [CrossRef]
- Eckard, A.R.; Mccomsey, G.A. The Role of Statins in the Setting of HIV Infection. Curr. HIV/AIDS Rep. 2015, 12, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Mukhamedova, N.; Hoang, A.; Dragoljevic, D.; Dubrovsky, L.; Pushkarsky, T.; Low, H.; Ditiatkovski, M.; Fu, Y.; Ohkawa, R.; Meikle, P.J.; et al. Exosomes containing HIV protein Nef reorganize lipid rafts potentiating inflammatory response in bystander cells. PLoS Pathog. 2019, 15, e1007907. [Google Scholar] [CrossRef]
- Lowenstein, C.J.; Solomon, S.D. Severe COVID-19 is a Microvascular Disease. Circulation 2020, 142, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Potus, F.; Mai, V.; Lebret, M.; Malenfant, S.; Breton-Gagnon, E.; Lajoie, A.C.; Boucherat, O.; Bonnet, S.; Provencher, S. Novel insights on the pulmonary vascular consequences of COVID-19. Am. J. Physiol. Cell. Mol. Physiol. 2020, 319, L277–L288. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.J.; Nikolaienko, S.I.; Shults, N.V.; Gychka, S.G. COVID-19 patients may become predisposed to pulmonary arterial hypertension. Med. Hypotheses 2021, 147, 110483. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.; Nukala, S.B.; Srivastava, S.; Miyamoto, H.; Ismail, N.I.; Jousma, J.; Rehman, J.; Ong, S.B.; Lee, W.H.; Ong, S.G. Detection of Viral Rna Fragments in Human Ipsc Cardiomyocytes Following Treatment with Extracellular Vesicles from Sars-Cov-2 Coding Sequence Overexpressing Lung Epithelial Cells. Stem. Cell Res. Ther. 2020, 11, 514. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical Course and Outcomes of Critically Ill Patients with Sars-Cov-2 Pneumonia in Wuhan, China: A Single-Centered, Retrospective, Observational Study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef]
- Cocozza, F.; Nevo, N.; Piovesana, E.; Lahaye, X.; Buchrieser, J.; Schwartz, O.; Manel, N.; Tkach, M.; Thery, C.; Martin-Jaular, L. Extracellular Vesicles Containing Ace2 Efficiently Prevent Infection by Sars-Cov-2 Spike Protein-Containing Virus. J. Extracell. Vesicles 2020, 10, e12050. [Google Scholar] [CrossRef] [PubMed]
- Krishnamachary, B.; Cook, C.; Spikes, L.; Chalise, P.; Dhillon, N.K. The Potential Role of Extracellular Vesicles in Covid-19 Associated Endothelial Injury and Pro-Inflammation. medRxiv 2020. [Google Scholar] [CrossRef]
- Abbasifard, M.; Khorramdelazad, H. The bio-mission of interleukin-6 in the pathogenesis of COVID-19: A brief look at potential therapeutic tactics. Life Sci. 2020, 257, 118097. [Google Scholar] [CrossRef]
- Arunachalam, P.S.; Wimmers, F.; Mok, C.K.P.; Perera, R.A.P.M.; Scott, M.; Hagan, T.; Sigal, N.; Feng, Y.; Bristow, L.; Tsang, O.T.-Y.; et al. Systems biological assessment of immunity to mild versus severe COVID-19 infection in humans. Science 2020, 369, 1210–1220. [Google Scholar] [CrossRef]
- Favalli, E.G. Understanding the Role of Interleukin-6 (IL-6) in the Joint and Beyond: A Comprehensive Review of IL-6 Inhibition for the Management of Rheumatoid Arthritis. Rheumatol. Ther. 2020, 7, 473–516. [Google Scholar] [CrossRef]
- Blake, G.J.; Ridker, P.M. C-reactive protein and other inflammatory risk markers in acute coronary syndromes. J. Am. Coll. Cardiol. 2003, 41, S37–S42. [Google Scholar] [CrossRef]
- Lee, K.W.; Blann, A.D.; Lip, G.Y. Plasma markers of endothelial damage/dysfunction, inflammation and thrombogenesis in relation to TIMI risk stratification in acute coronary syndromes. Thromb. Haemost. 2005, 94, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Borges, Á.H.; O’Connor, J.L.; Phillips, A.N.; Neaton, J.D.; Grund, B.; Neuhaus, J.; Vjecha, M.J.; Calmy, A.; Koelsch, K.K.; Lundgren, J.D. Interleukin 6 Is a Stronger Predictor of Clinical Events Than High-Sensitivity C-Reactive Protein or D-Dimer During HIV Infection. J. Infect. Dis. 2016, 214, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Mair, J.; Lindahl, B.; Hammarsten, O.; Müller, C.; Giannitsis, E.; Huber, K.; Möckel, M.; Plebani, M.; Thygesen, K.; Jaffe, A.S. How is cardiac troponin released from injured myocardium? Eur. Hear. J. Acute Cardiovasc. Care 2018, 7, 553–560. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, P.; Piper, H.M.; Spahr, R.; Spieckermann, P.G. Ultrastructure of cultured adult myocardial cells during anoxia and reoxygenation. Am. J. Pathol. 1984, 115, 349–361. [Google Scholar]
- Hickman, P.E.; Potter, J.M.; Aroney, C.; Koerbin, G.; Southcott, E.; Wu, A.H.; Roberts, M. Cardiac troponin may be released by ischemia alone, without necrosis. Clin. Chim. Acta 2010, 411, 318–323. [Google Scholar] [CrossRef]
- Lala, A.; Johnson, K.; Januzzi, J.L.; Russak, A.J.; Paranjpe, I.; Richter, F.; Zhao, S.; Somani, S.; Van Vleck, T.; Vaid, A.; et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J. Am. Coll. Cardiol. 2020, 76, 533–546. [Google Scholar] [CrossRef]
- Rosell, A.; Havervall, S.; Von Meijenfeldt, F.; Hisada, Y.; Aguilera, K.; Grover, S.P.; Lisman, T.; Mackman, N.; Thålin, C. Patients With COVID-19 Have Elevated Levels of Circulating Extracellular Vesicle Tissue Factor Activity That Is Associated With Severity and Mortality—Brief Report. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 878–882. [Google Scholar] [CrossRef]
- Lacroix, R.; Vallier, L.; Bonifay, A.; Simoncini, S.; Mege, D.; Aubert, M.; Panicot-Dubois, L.; Dubois, C.; Dignat-George, F. Microvesicles and Cancer Associated Thrombosis. Semin. Thromb. Hemost. 2019, 45, 593–603. [Google Scholar] [CrossRef]
- Chen, W.; Huang, Y.; Han, J.; Yukai, H.; Li, Y.; Lu, Z.; Li, H.; Liu, Z.; Shi, C.; Duan, F.; et al. Immunomodulatory effects of mesenchymal stromal cells-derived exosome. Immunol. Res. 2016, 64, 831–840. [Google Scholar] [CrossRef]
- Iyer, S.S.; Rojas, M. Anti-inflammatory effects of mesenchymal stem cells: Novel concept for future therapies. Expert Opin. Biol. Ther. 2008, 8, 569–581. [Google Scholar] [CrossRef]
- Abreu, S.C.; Weiss, D.J.; Rocco, P.R.M. Extracellular vesicles derived from mesenchymal stromal cells: A therapeutic option in respiratory diseases? Stem Cell Res. Ther. 2016, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cao, X.; Qin, L. Mesenchymal Stem Cell-Derived Extracellular Vesicles: A Novel Cell-Free Therapy for Sepsis. Front. Immunol. 2020, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Gardin, C.; Ferroni, L.; Chachques, J.C.; Zavan, B. Could Mesenchymal Stem Cell-Derived Exosomes Be a Therapeutic Option for Critically Ill COVID-19 Patients? J. Clin. Med. 2020, 9, 2762. [Google Scholar] [CrossRef] [PubMed]
| Study | Country | No. of HIV + Patients | Outcome |
|---|---|---|---|
| Lang et al. [11] (2010) | France | 74,958 | Myocardial Infarction (Standardized Morbidity Ratio 1.5) |
| Hsue et al. [12] (2010) | USA | 196 | Left Ventricular Mass Index (77.2 g/m (2) vs. 66.5 g/m (2) p < 0.0001) |
| Diastolic Dysfunction (50% vs. 29% p = 0.008) | |||
| Durand et al. [13] (2010) | Canada | 7053 | Acute Myocardial Infarction (Adjusted Incidence Ratio 2.11) |
| Butt et al. [14] (2011) | USA | 2391 | Heart Failure (Hazard Ratio 1.81) |
| Sliwa et al. [15] (2012) | South Africa | 518 | Pericarditis (13% vs. 1.2%, p < 0.001) |
| Lorgis et al. [16] (2012) | USA | 608 | Ischemic Cardiomyopathy (7.6% vs. 4.2% p = 0.02) |
| Freiberg et al. [17] (2013) | USA | 27,350 | Acute Myocardial Infarction (Hazard Ratio 1.48) |
| Womack et al. [18] (2014) | USA | 710 | Cardiovascular Disease Outcome (Hazard Ratio = 2.8) |
| Luo et al. [19] (2014) | China | 325 | Diastolic Dysfunction (Prevalence 16.5% vs. 7.2%, p = 0.027) |
| Fontes-Carvalho et al. [20] (2015) | Portugal | 206 | Diastolic Dysfunction (Prevalence 19% vs. 3.3%, p < 0.05) |
| Rasmussen et al. [21] (2015) | Denmark | 3251 | Myocardial Infarction (Incidence Rate Ratio 1.78) |
| Chow et al. [22] (2015) | USA | 100 | Subclinical Atherosclerosis (mean Coronary Artery Calcium Score, Relative Risk = 1.20, p < 0.05) |
| Al-Kindi et al. [23] (2016) | USA | 36,400 | Heart Failure (Relative Risk 1.66) |
| Freiberg et al. [24] (2017) | USA | 31,523 | HFpEF (Hazard Ratio 1.21) |
| HFrEF (Hazard Ratio 1.61) | |||
| Knudsen et al. [25] (2018) | Denmark | 908 | Peripheral Artery Disease (Adjusted Odds Ratio 1.9) |
| Alonso et al. [26] (2019) | USA | 19,798 | Stroke (Hazard Ratio 2.3) |
| Myocardial Infarction (Hazard Ratio 1.2) | |||
| Heart Failure (Hazard Ratio 2.8) | |||
| Atrial Fibrillation (Hazard Ratio1.3) | |||
| Beckman et al. [27] (2019) | USA | 28,714 | Peripheral Artery Disease (Hazard Ratio 1.19) |
| Rao et al. [28] (2019) | Various | 248,145 * | Acute Myocardial Infarction (Relative Risk 1.96) |
| Chattranukulchai et al. [29] | Thailand | 298 | Diastolic Dysfunction (Adjusted Odds Ratio 3.5) |
| LV Hypertrophy (Adjusted Odds Ratio 1.91) | |||
| Gaspar Vallilo et al. [30] | Brazil | 148 | LV Dilation and ART (Adjusted Odds Ratio 0.98) |
| Septal Hypertrophy and ART (Adjusted Odds Ratio 0.96) | |||
| Shen et al. [31] | China | 587 | ST-T segment elevation and ART (Adjusted Odds Ratio 0.96) |
| All ECG abnormality and ART (Adjusted Odds Ratio 0.95) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clauss, M.; Chelvanambi, S.; Cook, C.; ElMergawy, R.; Dhillon, N. Viral Bad News Sent by EVAIL. Viruses 2021, 13, 1168. https://doi.org/10.3390/v13061168
Clauss M, Chelvanambi S, Cook C, ElMergawy R, Dhillon N. Viral Bad News Sent by EVAIL. Viruses. 2021; 13(6):1168. https://doi.org/10.3390/v13061168
Chicago/Turabian StyleClauss, Matthias, Sarvesh Chelvanambi, Christine Cook, Rabab ElMergawy, and Navneet Dhillon. 2021. "Viral Bad News Sent by EVAIL" Viruses 13, no. 6: 1168. https://doi.org/10.3390/v13061168
APA StyleClauss, M., Chelvanambi, S., Cook, C., ElMergawy, R., & Dhillon, N. (2021). Viral Bad News Sent by EVAIL. Viruses, 13(6), 1168. https://doi.org/10.3390/v13061168