Evidence of West Nile Virus Circulation in Lebanon
Abstract
:1. Introduction
2. Materials and Methods
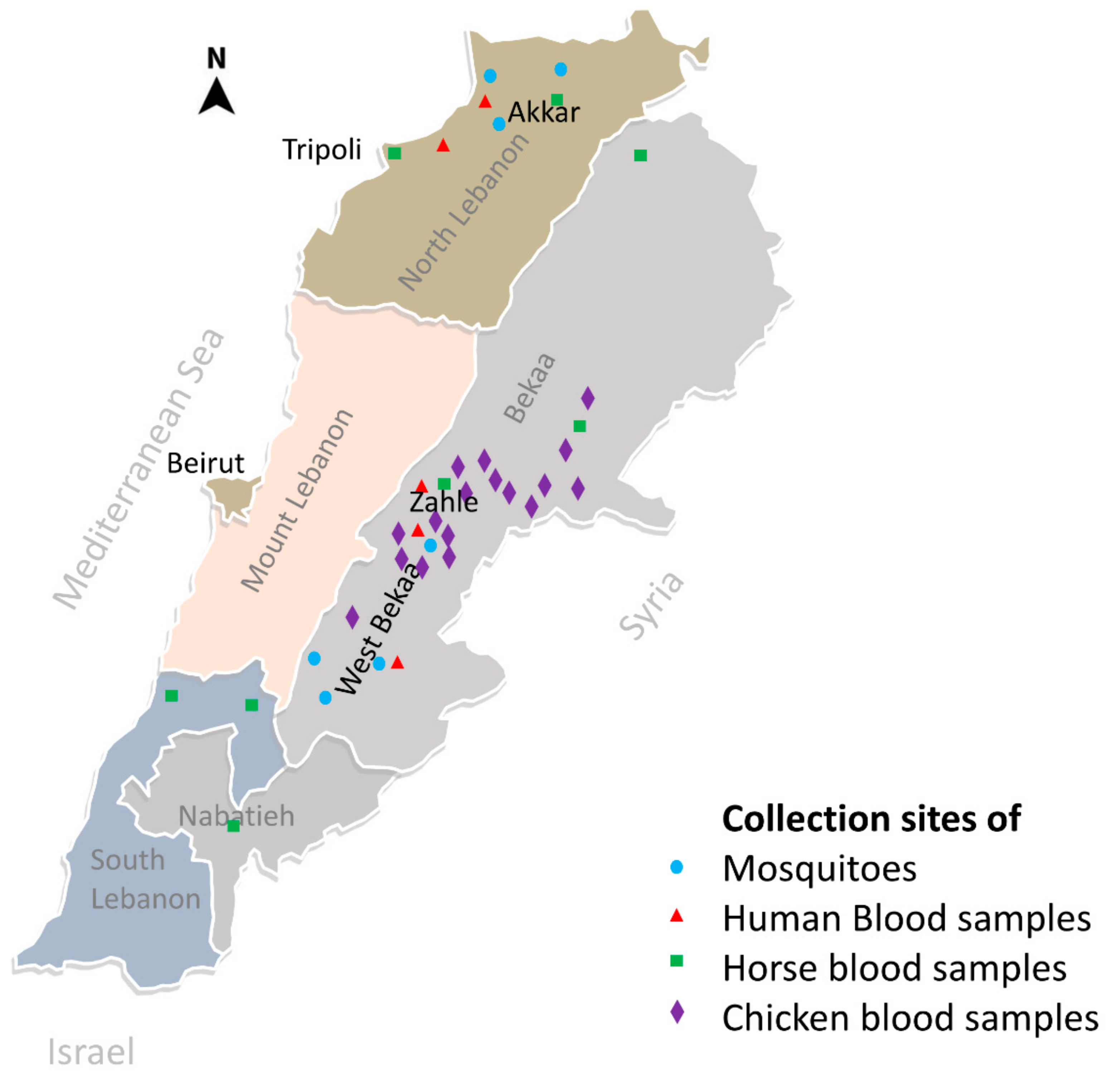
2.1. Blood Collection
2.2. Screening of Sera by Enzyme-Linked Immuno-Assay
2.3. Confirmation by Plaque Reduction Neutralization Test
2.4. Screening for WNV in Mosquitoes
2.4.1. Mosquito Collection and Identification
2.4.2. Screening for WNV
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kramer, L.D.; Bernard, K.A. West Nile virus in the western hemisphere. Curr. Opin. Infect. Dis. 2001, 14, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Roehrig, J.T. West Nile Virus in the United States—A Historical Perspective. Viruses 2013, 5, 3088–3108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- eCDC West Nile Virus Infection—Annual Epidemiological Report for 2018. Available online: https://www.ecdc.europa.eu/en/publications-data/west-nile-virus-infection-annual-epidemiological-report-2018 (accessed on 9 July 2020).
- Lustig, Y.; Kaufman, Z.; Mannasse, B.; Koren, R.; Katz-Likvornik, S.; Orshan, L.; Glatman-Freedman, A.; Mendelson, E. West Nile virus outbreak in Israel in 2015: Phylogenetic and geographic characterization in humans and mosquitoes. Clin. Microbiol. Infect. 2017, 23, 986–993. [Google Scholar] [CrossRef] [Green Version]
- Weinberger, M.; Pitlik, S.D.; Gandacu, D.; Lang, R.; Nassar, F.; Ben David, D.; Rubinstein, E.; Izthaki, A.; Mishal, J.; Kitzes, R.; et al. West Nile fever outbreak, Israel, 2000: Epidemiologic aspects. Emerg. Infect. Dis. 2001, 7, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Kalaycioglu, H.; Korukluoglu, G.; Ozkul, A.; Oncul, O.; Tosun, S.; Karabay, O.; Gozalan, A.; Uyar, Y.; Caglayık, D.Y.; Atasoylu, G.; et al. Emergence of West Nile virus infections in humans in Turkey, 2010 to 2011. Eurosurveillance 2012, 17, 20182. [Google Scholar] [CrossRef]
- Batieha, A.; Saliba, E.K.; Graham, R.; Mohareb, E.; Hijazi, Y.; Wijeyaratne, P. Seroprevalence of West Nile, Rift Valley, and sandfly arboviruses in Hashimiah, Jordan. Emerg. Infect. Dis. 2000, 6, 358–362. [Google Scholar] [CrossRef]
- Soliman, A.; Mohareb, E.; Salman, D.; Saad, M.; Salama, S.; Fayez, C.; Hanafi, H.; Medhat, I.; Labib, E.; Rakha, M.; et al. Studies on West Nile virus infection in Egypt. J. Infect. Public Health 2010, 3, 54–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebel, G.D.; Dupuis, A.P.; Nicholas, D.; Young, D.; Maffei, J.; Kramer, L.D. Detection by Enzyme-Linked Immunosorbent Assay of Antibodies to West Nile virus in Birds. Emerg. Infect. Dis. 2002, 8, 979–982. [Google Scholar] [CrossRef]
- Frazier, C.L.; Shope, R.E. Detection of antibodies to alphaviruses by enzyme-linked immunosorbent assay. J. Clin. Microbiol. 1979, 10, 583–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubose, W.P.; Curtin, T.J. Identification keys to the adult and larval mosquitoes of the mediterranean area. J. Med. Entomol. 1965, 1, 349–355. [Google Scholar]
- Schaffner, F.; Angel, G.; Geoffroy, B.; Hervy, J.-P.; Rhaim, A.; Brunhes, J. Les Moustiques d’Europe: Logiciel D’identification et D’enseignement = The Mosquitoes of Europe: An Identification and Training Programme; IRD: Paris, France; EID: Montpellier, France, 2001; ISBN 2-7099-1485-9. [Google Scholar]
- Brunhes, J.; Rhaim, A.; Geoffroy, B.; Angel, G.; Hervy, J.-P. Les Moustiques de l’Afrique Méditerranéenne: Logiciel D’identification et D’enseignement; IRD: Paris, France; IPT: Tunis, Tunisia, 2000; ISBN 2-7099-1446-8. [Google Scholar]
- Kauffman, E.B.; Franke, M.A.; Kramer, L.D. Detection Protocols for West Nile Virus in Mosquitoes, Birds, and Nonhuman Mammals. Methods Mol. Biol. 2016, 1435, 175–206. [Google Scholar] [PubMed]
- Lanciotti, R.S.; Kerst, A.J.; Nasci, R.S.; Godsey, M.S.; Mitchell, C.J.; Savage, H.M.; Komar, N.; Panella, N.A.; Allen, B.C.; Volpe, K.E.; et al. Rapid detection of west nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J. Clin. Microbiol. 2000, 38, 4066–4071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bassal, R.; Shohat, T.; Kaufman, Z.; Mannasse, B.; Shinar, E.; Amichay, D.; Barak, M.; Ben-Dor, A.; Bar Haim, A.; Cohen, D.; et al. The seroprevalence of West Nile Virus in Israel: A nationwide cross sectional study. PLoS ONE 2017, 12, e0179774. [Google Scholar] [CrossRef] [Green Version]
- Mostashari, F.; Bunning, M.L.; Kitsutani, P.T.; Singer, D.A.; Nash, D.; Cooper, M.J.; Katz, N.; Liljebjelke, K.A.; Biggerstaff, B.J.; Fine, A.D.; et al. Epidemic West Nile encephalitis, New York, 1999: Results of a household-based seroepidemiological survey. Lancet 2001, 358, 261–264. [Google Scholar] [CrossRef]
- Pierro, A.; Gaibani, P.; Spadafora, C.; Ruggeri, D.; Randi, V.; Parenti, S.; Finarelli, A.C.; Rossini, G.; Landini, M.P.; Sambri, V. Detection of specific antibodies against West Nile and Usutu viruses in healthy blood donors in northern Italy, 2010–2011. Clin. Microbiol. Infect. 2013, 19, E451–E453. [Google Scholar]
- Hadjichristodoulou, C.; Pournaras, S.; Mavrouli, M.; Marka, A.; Tserkezou, P.; Baka, A.; Billinis, C.; Katsioulis, A.; Psaroulaki, A.; Papa, A.; et al. West Nile Virus Seroprevalence in the Greek Population in 2013: A Nationwide Cross-Sectional Survey. PLoS ONE 2015, 10, e0143803. [Google Scholar] [CrossRef] [Green Version]
- Tezcan, S.; Kızıldamar, S.; Ulger, M.; Aslan, G.; Tiftik, N.; Ozkul, A.; Emekdaş, G.; Niedrig, M.; Ergünay, K. [Flavivirus seroepidemiology in blood donors in Mersin province, Turkey]. Mikrobiyol. Bul. 2014, 48, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Meshkat, Z.; Chinikar, S.; Shakeri, M.; Manavifar, L.; Moradi, M.; Mirshahabi, H.; Jalali, T.; Khakifirouz, S.; Shahhosseini, N. Prevalence of West Nile virus in Mashhad, Iran: A population–based study. Asian Pac. J. Trop. Med. 2015, 8, 203–205. [Google Scholar] [PubMed] [Green Version]
- Gallian, P.; Micco, P.; Ghorra, P. Seroprevalence of West Nile virus in blood donors at Hôtel Dieu de France, Beirut, Lebanon. Transfusion 2010, 50, 1156–1158. [Google Scholar]
- OIE. OIE World Animal Health Information System. Available online: https://www.oie.int/wahis_2/public/wahid.php/Diseaseinformation/Diseasetimelines (accessed on 9 July 2020).
- Azmi, K.; Tirosh-Levy, S.; Manasrah, M.; Mizrahi, R.; Nasereddin, A.; Al-Jawabreh, A.; Ereqat, S.; Abdeen, Z.; Lustig, Y.; Gelman, B.; et al. West Nile Virus: Seroprevalence in Animals in Palestine and Israel. Vector-Borne Zoonotic Dis. 2017, 17, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Abutarbush, S.M.; Al-Majali, A.M. West Nile virus infection in horses in Jordan: Clinical cases, seroprevalence and risk factors. Transbound. Emerg. Dis. 2014, 61 (Suppl. S1), 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ozkul, A.; Ergunay, K.; Koysuren, A.; Alkan, F.; Arsava, E.M.; Tezcan, S.; Emekdas, G.; Hacioglu, S.; Turan, M.; Us, D. Concurrent occurrence of human and equine West Nile virus infections in Central Anatolia, Turkey: The first evidence for circulation of lineage 1 viruses. Int. J. Infect. Dis. 2013, 17, e546–e551. [Google Scholar] [CrossRef] [Green Version]
- Chinikar, S.; Shah-Hosseini, N.; Mostafavi, E.; Moradi, M.; Khakifirouz, S.; Jalali, T.; Goya, M.M.; Shirzadi, M.R.; Zainali, M.; Fooks, A.R. Seroprevalence of West Nile virus in Iran. Vector Borne Zoonotic Dis. 2013, 13, 586–589. [Google Scholar] [CrossRef] [PubMed]
- Mannasse, B.; Mendelson, E.; Orshan, L.; Mor, O.; Shalom, U.; Yeger, T.; Lustig, Y. Usutu Virus RNA in Mosquitoes, Israel, 2014-2015. Emerg. Infect. Dis. 2017, 23, 1699–1702. [Google Scholar] [CrossRef] [PubMed]
- Schvartz, G.; Tirosh-Levy, S.; Erster, O.; Shenhar, R.; Levy, H.; Bazanow, B.; Gelman, B.; Steinman, A. Exposure of Horses in Israel to West Nile Virus and Usutu Virus. Viruses 2020, 12, 1099. [Google Scholar] [CrossRef]
- Constant, O.; Bollore, K.; Clé, M.; Barthelemy, J.; Foulongne, V.; Chenet, B.; Gomis, D.; Virolle, L.; Gutierrez, S.; Desmetz, C.; et al. Evidence of Exposure to USUV and WNV in Zoo Animals in France. Pathogens 2020, 9, 1005. [Google Scholar] [CrossRef]
- Hitti, J.K.; Khairallah, A.A. A Report on the Recent Epidemic of Dengue in Beirut, Lebanon, and some of its Complications. J. Palest. Arab Med. Assoc. 1946, 1, 150–153. [Google Scholar]
- Haddad, N.; Harbach, R.E.; Chamat, S.; Bouharoun-Tayoun, H. Presence of Aedes albopictus in Lebanon and Syria. J. Am. Mosq. Control Assoc. 2007, 23, 226–228. [Google Scholar] [CrossRef]
- Haddad, N.; Mousson, L.; Vazeille, M.; Chamat, S.; Tayeh, J.; Osta, M.A.; Failloux, A.-B. Aedes albopictus in Lebanon, a potential risk of arboviruses outbreak. BMC Infect. Dis. 2012, 12, 300. [Google Scholar] [CrossRef] [Green Version]
- Youssef, M.; El Zein, S.; Kanj, S. Dengue fever in Lebanon: First confirmed case since 1945 and review from the region. J. Infect. Dev. Ctries. 2018, 12, 286–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakhia, R.; Mousson, L.; Vazeille, M.; Haddad, N.; Failloux, A.-B. Experimental transmission of West Nile Virus and Rift Valley Fever Virus by Culex pipiens from Lebanon. PLoS Negl. Trop. Dis. 2018, 12, e0005983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, J.; Ruiz, S.; Soriguer, R.; Alcaide, M.; Viana, D.S.; Roiz, D.; Vázquez, A.; Figuerola, J. Feeding patterns of potential West Nile virus vectors in south-west Spain. PLoS ONE 2012, 7, e39549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lustig, Y.; Hindiyeh, M.; Orshan, L.; Weiss, L.; Koren, R.; Katz-Likvornik, S.; Zadka, H.; Glatman-Freedman, A.; Mendelson, E.; Shulman, L.M. Mosquito Surveillance for 15 Years Reveals High Genetic Diversity Among West Nile Viruses in Israel. J. Infect. Dis. 2016, 213, 1107–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Districts | Serum | WNV | DENV | Travel History | Age (Years) | Gender | |
|---|---|---|---|---|---|---|---|
| OD Ratio (ELISA) | Titre (PRNT90 *) | Titre (PRNT90 *) | |||||
| West Bekaa and Zahle | B9 | 2.64 | 40 | Neg | No | 75 | Female |
| B11 | 5.47 | ≥320 | 40 | No | 70 | Female | |
| B42 | 8.08 | ≥320 ** | 20 | Canada | 83 | Male | |
| B116 | 7.30 | ≥320 | 20 | No | 80 | Male | |
| B118 | 3.24 | 10 | ≥40 ** | No | 43 | Male | |
| B121 | 2.61 | 10 | ≥40 | Venezuela and Syria | 51 | Female | |
| B133 | 5.00 | 160 | Neg | Egypt | 31 | Male | |
| B162 | 5.22 | ≥320 | Neg | No | 65 | Female | |
| 12 | 2.83 | ≥320 | ND | No | 85 | Male | |
| 23 | 2.71 | 10 | ND | Venezuela | 34 | Male | |
| 99 | 3.23 | 10 | ND | No | 58 | Female | |
| 235 | 3.85 | 160 | ND | Brazil and Syria | 66 | Female | |
| 237 | 3.25 | 160 | ND | Syria | 73 | Male | |
| T57 | 5.08 | ≥320 | Neg | USA | 76 | Female | |
| T167 | 5.35 | ≥320 | Neg | USA | 82 | Male | |
| Akkar | U91 | 2.08 | 20 | ND | Germany | 67 | Female |
| U284 | 2.86 | 20 | ND | No | 81 | Male | |
| Serum # | Titre (PRNT90 *) | OD Ratio (ELISA) | Age (Years) | Sex ** | District/Region |
|---|---|---|---|---|---|
| 83 | 160 | 1.371 | 7 | Male | Tripoli/North |
| 128 | 160 | 1.511 | 7 | Female | Akkar/North |
| 183 | 10 | 1.246 | 20 | Female | Nabatieh/South |
| 199 | 40 | 1.238 | 10 | Female | Nabatieh/South |
| 202 | 320 | 1.701 | 7 | Female | Nabatieh/South |
| District | West Bekaa | Akkar | Total | |||||
|---|---|---|---|---|---|---|---|---|
| Nb of Trap-Days | 78 | 88 | ||||||
| Male | Female | Total | Male | Female | Total | Nb | % | |
| Culex pipiens | 440 | 2249 | 2689 | 511 | 2771 | 3282 | 5971 | 96.83 |
| Culex perexiguus | 0 | 10 | 10 | 0 | 82 | 82 | 92 | 1.49 |
| Culex martinii | 2 | 30 | 32 | 0 | 9 | 9 | 41 | 0.67 |
| Culiseta longiareolata | 19 | 17 | 36 | 0 | 12 | 12 | 48 | 0.78 |
| Orthopodomyia pulcripalpis | 12 | 2 | 14 | 0 | 0 | 0 | 14 | 0.23 |
| Total | 473 | 2308 | 2781 | 511 | 2864 | 3385 | 6166 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zakhia, R.; Dupuis, A.P., II; Khodr, F.; Fadel, M.; Kramer, L.D.; Haddad, N. Evidence of West Nile Virus Circulation in Lebanon. Viruses 2021, 13, 994. https://doi.org/10.3390/v13060994
Zakhia R, Dupuis AP II, Khodr F, Fadel M, Kramer LD, Haddad N. Evidence of West Nile Virus Circulation in Lebanon. Viruses. 2021; 13(6):994. https://doi.org/10.3390/v13060994
Chicago/Turabian StyleZakhia, Renée, Alan P. Dupuis, II, Fayçal Khodr, Mahdi Fadel, Laura D. Kramer, and Nabil Haddad. 2021. "Evidence of West Nile Virus Circulation in Lebanon" Viruses 13, no. 6: 994. https://doi.org/10.3390/v13060994
APA StyleZakhia, R., Dupuis, A. P., II, Khodr, F., Fadel, M., Kramer, L. D., & Haddad, N. (2021). Evidence of West Nile Virus Circulation in Lebanon. Viruses, 13(6), 994. https://doi.org/10.3390/v13060994






