African Swine Fever Virus as a Difficult Opponent in the Fight for a Vaccine—Current Data
Abstract
:1. Introduction
2. Worldwide Occurrence and Spread of ASFV
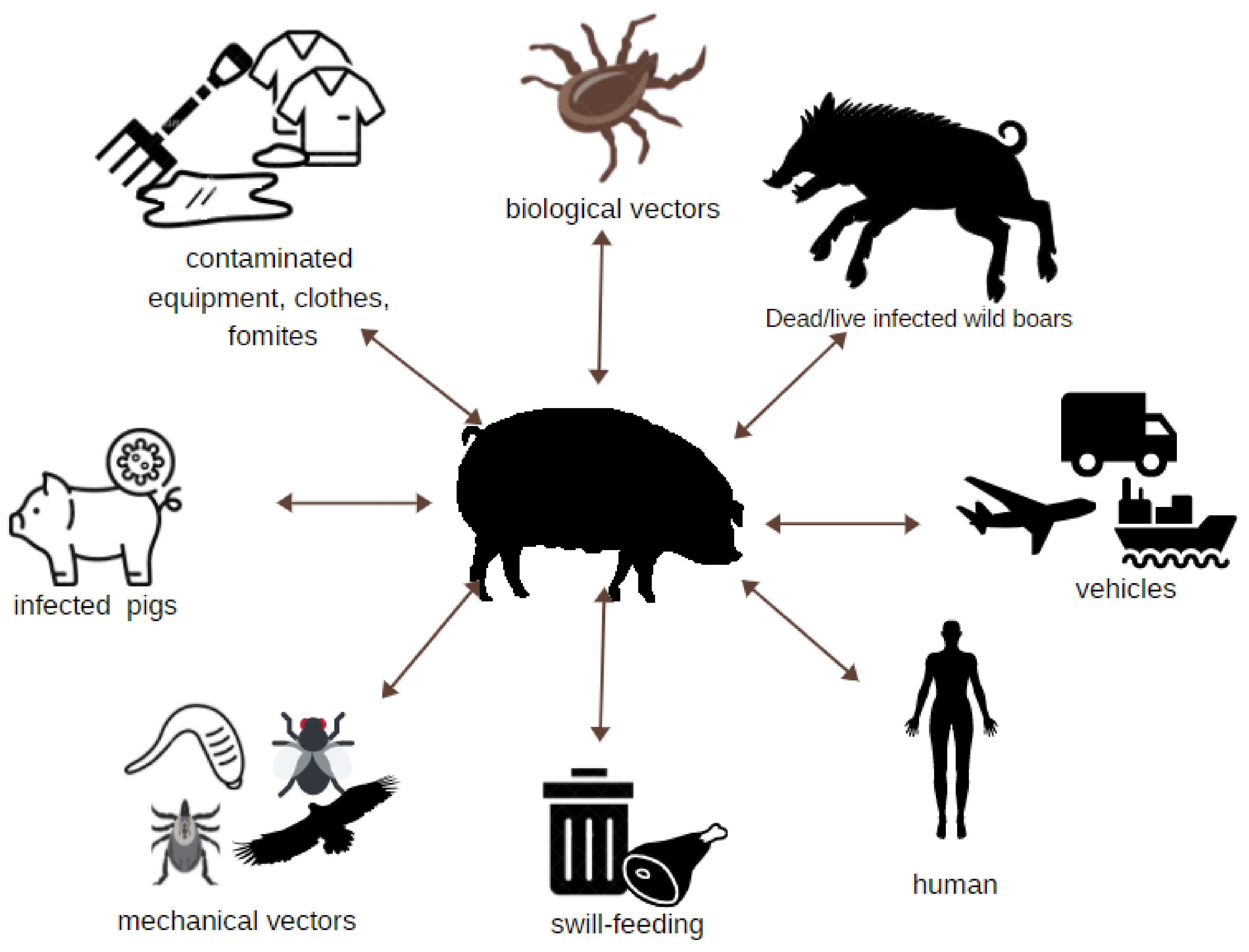
3. Easy and Multi-Directional Transmission of ASFV
4. Vaccines against ASF—A Short Review and the Latest Achievements
4.1. Live Attenuated Vaccines
4.2. Vectored Vaccines and Subunit Vaccines
5. Concerns about Vaccines
5.1. African Swine Fever in Wild Boars
5.2. Complex Nature of the Virus
5.3. Lack of an Established Macrophage Cell Line
5.4. Safety
5.5. DIVA Strategy
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV virus taxonomy profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Śmietanka, K.; Woźniakowski, G.; Kozak, E.; Niemczuk, K.; Frączyk, M.; Bocian, Ł.; Kowalczyk, A.; Pejsak, Z. African Swine Fever Epidemic, Poland, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oie.int. Available online: https://www.oie.int/app/uploads/2021/03/report-47-global-situation-asf.pdf (accessed on 10 June 2021).
- Wu, K.; Liu, J.; Wang, L.; Fan, S.; Li, Z.; Li, Y.; Yi, L.; Ding, H.; Zhao, M.; Chen, J. Current State of Global African Swine Fever Vaccine Development under the Prevalence and Transmission of ASF in China. Vaccines 2020, 8, 531. [Google Scholar] [CrossRef] [PubMed]
- Busch, F.; Haumont, C.; Penrith, M.-L.; Laddomada, A.; Dietze, K.; Globig, A.; Guberti, V.; Zani, L.; Depner, K. Evidence-Based African Swine Fever Policies: Do We Address Virus and Host Adequately? Front. Vet. Sci. 2021, 8, 637487. [Google Scholar] [CrossRef]
- Woźniakowski, G.; Frączyk, M.; Kowalczyk, A.; Pomorska-Mól, M.; Niemczuk, K.; Pejsak, Z. Polymerase cross-linking spiral reaction (PCLSR) for detection of African swine fever virus (ASFV) in pigs and wild boars. Sci. Rep. 2017, 7, 42903. [Google Scholar] [CrossRef] [Green Version]
- Bosch-Camós, L.; López, E.; Rodriguez, F. African swine fever vaccines: A promising work still in progress. Porc. Health Manag. 2020, 6, 17. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); Nielsen, S.S.; Alvarez, J.; Bicout, D.J.; Calistri, P.; Depner, K.; Drewe, J.A.; Garin-Bastuji, B.; Gonzales Rojas, J.L.; Gortázar Schmidt, C.; et al. Research priorities to fill knowledge gaps on ASF seasonality that could improve the control of ASF. EFSA J. 2021, 19, e06550. [Google Scholar] [CrossRef]
- Mauroy, A.; Depoorter, P.; Saegerman, C.; Cay, B.; De Regge, N.; Filippitzi, M.E.; Fischer, C.; Laitat, M.; Maes, D.; Morelle, K.; et al. Semi-quantitative risk assessment by expert elicitation of potential introduction routes of African swine fever from wild reservoir to domestic pig industry and subsequent spread during the Belgian outbreak (2018–2019). Transbound. Emerg. Dis. 2021. [Google Scholar] [CrossRef]
- Montgomery, R.E. On a form of swine fever occuring in British East Africa. J. Comp. Pathol. Ther. 1921, 34, 159–191. [Google Scholar] [CrossRef] [Green Version]
- Kramera, M.; Staubacha, C.; Koenenb, F.; Haegemanb, A.; Polc, F.; Le Potierc, M.F.; Greiser-Wilked, I. Scientific reviews on Classical Swine Fever (CSF), African Swine Fever (ASF) and African Horse Sickness (AHS), and evaluation of the distribution of anthropod vectors and their potential for transmitting exotic or emerging vector-borne animal diseases and zoonoses. In Scientific Review on Classical Swine Fever; EFSA Supporting Publications: New York, NY, USA, 2009; pp. 1–141. [Google Scholar] [CrossRef]
- Arias, M.; Sánchez-Vizcaíno, J.M. African swine fever eradication: The Spanish model. In Trends in Emerging Viral Infections of Swine, 1st ed.; Morilla, A., Yoon, K.J., Zimmerman, J.J., Eds.; Iowa State Press: Ames, IA, USA, 2002; pp. 133–139. [Google Scholar]
- Vepkhadze, N.G.; Menteshashvili, I.; Kokhreidze, M.; Goginashvili, K.; Tigilauri, T.; Mamisashvili, E.; Gelashvili, L.; Abramishvili, T.; Donduashvili, M.; Ghvinjilia, G.; et al. Active surveillance of African swine fever in domestic swine herds in Georgia, 2014. Rev. Sci. Tech. 2017, 36, 879–887. [Google Scholar] [CrossRef]
- Cwynar, P.; Stojkov, J.; Wlazlak, K. African Swine Fever Status in Europe. Viruses 2019, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Gogin, A.; Gerasimov, V.; Malogolovkin, A.; Kolbasov, D. African swine fever in the North Caucasus region and the Russian Federation in years 2007–2012. Virus Res. 2013, 173, 198–203. [Google Scholar] [CrossRef]
- Oie.int. Available online: https://wahis.oie.int/ (accessed on 30 April 2021).
- Galindo, C.; Alonso, C. African swine fever virus: A review. Viruses 2017, 9, E103. [Google Scholar] [CrossRef] [Green Version]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L.K. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Vizcaíno, J.M.; Mur, L.; Bastos, A.D.; Penrith, M.L. New insights into the role of ticks in African swine fever epidemiology. Rev. Sci. Tech. 2015, 34, 503–511. [Google Scholar] [CrossRef] [Green Version]
- Frant, M.; Woźniakowski, G.; Pejsak, Z. African swine fever (ASF) and ticks. No risk of tick-mediated ASF spread in Poland and Baltic states. J. Vet. Res. 2017, 61, 375–380. [Google Scholar] [CrossRef] [Green Version]
- De Carvalho Ferreira, H.C.; Tudela Zúquete, S.; Wijnveld, M.; Weendorp, E.; Jongejan, F.; Stegeman, A.; Loeffen, W.L. No evidence of African swine fever virus replication in hard ticks. Ticks Tick-Borne Dis. 2014, 5, 582–589. [Google Scholar] [CrossRef] [Green Version]
- Probst, C.; Globig, A.; Knoll, B.; Conraths, F.J.; Depner, K. Behaviour of free ranging wild boar towards their dead fellows: Potential implications for the transmission of African swine fever. R. Soc. Open Sci. 2017, 4, 170–174. [Google Scholar] [CrossRef] [Green Version]
- Blome, S.; Gabriel, C.; Dietze, K.; Breithaupt, A.; Beer, M. High virulence of African swine fever virus caucasus isolate in European wild boars of all ages. Emerg. Infect. Dis. 2012, 18, 708. [Google Scholar] [CrossRef]
- Pejsak, Z.; Truszczyński, M. Vaccine against African swine fever. Życie Weter 2020, 96, 358–361. [Google Scholar]
- Costard, S.; Mur, L.; Lubroth, J.; Sánchez-Vizcaíno, J.M.; Pfeiffer, D.U. Epidemiology of African swine fever virus. Virus Res. 2013, 173, 191–197. [Google Scholar] [CrossRef]
- Pejsak, Z.; Truszczyński, M. Ocena ryzyka szerzenia się ASF w Europie i Azji. In Afrykański Pomór Świń. Monografia; Pejsak, Z., Truszczyński, M., Eds.; Wydawnictwo PIWet-PIB Puławy: Puławy, Poland, 2016; pp. 127–130. [Google Scholar]
- Gaudreault, N.N.; Madden, D.W.; Wilson, W.C.; Trujillo, J.D.; Richt, J.A. African swine fever virus: An emerging DNA arbovirus. Front. Vet. Sci. 2020, 7, 215. [Google Scholar] [CrossRef]
- Olesen, A.S.; Lohse, L.; Hansen, M.F.; Boklund, A.; Halasa, T.; Belsham, G.J.; Rasmussen, T.B.; Bøtner, A.; Bødker, R. Infection of pigs with African swine fever virus via ingestion of stable flies (Stomoxys calcitrans). Transbound. Emerg. Dis. 2018, 65, 1152–1157. [Google Scholar] [CrossRef] [Green Version]
- Karalyan, Z.; Avetisyan, A.; Avagyan, H.; Ghazaryan, H.; Vardanyan, T.; Manukyan, A.; Semerjyan, A.; Voskanyan, H. Presence and survival of African swine fever virus in leeches. Vet. Microbiol. 2019, 237, 108421. [Google Scholar] [CrossRef]
- Dee, S.A.; Bauermann, F.V.; Niederwerder, M.C.; Singrey, A.; Clement, T.; De Lima, M.; Long, C.; Patterson, G.; Sheahan, M.A.; Stoian, A.M.M.; et al. Correction: Survival of viral pathogens in animal feed ingredients under transboundary shipping models. PLoS ONE 2018, 13, e0208130. [Google Scholar] [CrossRef]
- Stoian, A.M.M.; Zimmerman, J.; Ji, J.; Hefley, T.J.; Dee, S.; Diel, D.G.; Rowland, R.R.R.; Niederwerder, M.C. Halflife of African swine fever virus in shipped feed. Emerg. Infect. Dis. 2019, 25, 2261–2263. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African swine fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.; Goatley, L.C.; Guinat, C.; Netherton, C.L.; Gubbins, S.; Dixon, L.K.; Reis, A.L. Survival of African Swine Fever Virus in Excretions from Pigs Experimentally Infected with the Georgia 2007/1 Isolate. Transbound. Emerg. Dis. 2017, 64, 425–431. [Google Scholar] [CrossRef]
- Mazur-Panasiuk, N.; Żmudzki, J.; Woźniakowski, G. African Swine Fever Virus—Persistence in Different Environmental Conditions and the Possibility of its Indirect Transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef] [Green Version]
- McKercher, P.D.; Yedloutschnig, R.J.; Callis, J.J.; Murpfy, R.; Panina, G.F.; Civardi, A.; Bugnetti, M.; Fonn, E.H.; Laddomada, A.; Scarana, C.; et al. Survival of viruses in “Prosciutto di Parma” (Parma ham). Can. Inst. Food Sci. Tech. J. 1987, 20, 267–272. [Google Scholar] [CrossRef]
- Mebus, C.A.; House, C.; Ruiz-Gonzalvo, F.; Pineda, J.M.; Tapiador, J.; Pire, J.J.; Bergada, J.; Yedlontshning, R.J.; Sahu, S.; Becerra, V.; et al. Survival of foot and mouth disease, African swine fever and hog cholera virus in Spanish serrano cured hams and Iberian cured hams, shoulder and loin. Food Microbiol. 1993, 10, 133–143. [Google Scholar] [CrossRef]
- Petrini, S.; Feliziani, F.; Casciari, C.; Giammarioli, M.; Torresi, C.; De Mia, G.M. Survival of African swine fever virus (ASFV) in various traditional Italian dry-cured meat products. Prev. Vet. Med. 2019, 162, 126–130. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Staubach, C.; Blome, S. African and classical swine fever: Similarities, differences and epidemiological consequences. Vet. Res. 2017, 48, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, S.; Rutili, D.; Guberti, V. Preventive measures aimed at minimizing the risk of African swine fever virus spread in pig farming systems. Acta Vet. Scand. 2016, 58, 82. [Google Scholar] [CrossRef] [Green Version]
- Blome, S.; Gabriel, C.; Beer, M. Modern adjuvants do not enhance the efficacy of an inactivated African swine fever virus vaccine preparation. Vaccine 2014, 32, 3879–3882. [Google Scholar] [CrossRef]
- Korenkov, D.; Isakova-Sivak, I.; Rudenko, L. Basics of CD8 T-cell immune responses after influenza infection and vaccination with inactivated or live attenuated influenza vaccine. Expert. Rev. Vaccines 2018, 17, 977–987. [Google Scholar] [CrossRef]
- Hamdy, F.M.; Dardiri, A.H. Clinical and immunologic responses of pigs to African swine fever virus isolated from the Western Hemisphere. Am. J. Vet. Res. 1984, 45, 711–714. [Google Scholar]
- Ruiz-Gonzalvo, F.C.M.; Bruyel, V. Immunological responses of pigs to partially attenuated ASF and their resistance to virulent homologous and heterologous viruses. In Proceedings of the FAO/CEC Expert Consultation in ASF Research, Sassari, Italy, 23 September 1981; Wilkinson, P.J., Ed.; FAO: Rome, Italy, 1981; pp. 206–216. [Google Scholar]
- Onisk, D.V.; Borca, M.V.; Kutish, G.; Kramer, E.; Irusta, P.; Rock, D.L. Passively transferred African swine fever virus antibodies protect swine against lethal infection. Virology 1994, 198, 350–354. [Google Scholar] [CrossRef]
- Wardley, R.C.; Norley, S.G.; Wilkinson, P.J.; Williams, S. The role of antibody in protection against African swine fever virus. Vet. Immunol. Immunopathol. 1985, 9, 201–212. [Google Scholar] [CrossRef]
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef]
- Fernandez, A.; Perez, J.; Martin de las Mulas, L.; Carrasco, L.; Dominguez, J.; Sierra, M.A. Localization of African swine fever viral antigen, swine IgM, IgG and C1q in lung and liver tissues of experimentally infected pigs. J. Comp. Pathol. 1992, 107, 81–90. [Google Scholar] [CrossRef]
- Pan, I.C.; Moulton, J.E.; Hess, W.R. Immunofluorescent studies on chronic pneumonia in swine with experimentally induced African swine fever. Am. J. Vet. Res. 1975, 36, 379–386. [Google Scholar]
- Slauson, D.O.; Sanchez-Vizcaino, J.M. Leukocyte-dependent platelet vasoactive amine release and immune complex deposition in African swine fever. Vet. Pathol. 1981, 18, 813–826. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Pérez-Martín, E.; Gallardo, C.; Salguero, F.J.; Borrego, B.; Lacasta, A.; Accensi, F.; Díaz, I.; Nofrarías, M.; Pujols, J.; et al. Enhancing DNA immunization by targeting ASFV antigens to SLA-II bearing cells. Vaccine 2011, 29, 5379–5385. [Google Scholar] [CrossRef]
- Netherton, C.L.; Goatley, L.C.; Reis, A.L.; Portugal, R.; Nash, R.H.; Morgan, S.B.; Gault, L.; Nieto, R.; Norlin, V.; Gallardo, C.; et al. Identification and immunogenicity of African swine fever virus antigens. Front. Immunol. 2019, 19, 1318. [Google Scholar] [CrossRef] [Green Version]
- Sunwoo, S.Y.; Pérez-Núñez, D.; Morozov, I.; Sánchez, E.; Gaudreault, N.; Trujillo, J.; Mur, L.; Nogal, M.; Madden, D.; Urbaniak, K.; et al. DNA-protein vaccination strategy does not protect from challenge with African swine fever virus Armenia 2007 strain. Vaccines 2019, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Oura, C.A.; Denyer, M.S.; Takamatsu, H.; Parkhouse, R.M. In vivo depletion of CD8+ T lymphocytes abrogates protective immunity to African swine fever virus. J. Gen. Virol. 2005, 86, 2445–2450. [Google Scholar] [CrossRef]
- Netherton, C.L. African swine fever vaccines. In Understanding and Combatting African Swine Fever. A European Perspective; Iacolina, L., Penrith, M.L., Bellini, S., Chenais, E., Jori, F., Montoya, M., Ståhl, K., Gavier-Widén, D., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2021; pp. 161–182. [Google Scholar] [CrossRef]
- Sang, H.; Miller, G.; Lokhandwala, S.; Sangewar, N.; Waghela, S.D.; Bishop, R.; Mwangi, W. Progress Toward Development of Effective and Safe African Swine Fever Virus Vaccines. Front. Vet. Sci. 2020, 7, 84. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Huang, S.; Qiu, H.J. Multifaceted Immune Responses to African Swine Fever Virus: Implications for Vaccine Development. Vet. Microbiol. 2020, 249, 108832. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Holinka, L.G.; Velazquez-Salinas, L.; Zhu, J.; Gladue, D.P. Development of a highly effective African swine fever virus vaccine by deletion of the I177L gene resultsin sterile immunity against the current epidemic Eurasia strain. J. Virol. 2020, 94, e02017-19. [Google Scholar] [CrossRef]
- Borca, M.V.; Ramirez-Medina, E.; Silva, E.; Vuono, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Velazquez-Salinas, L.; Gay, C.G.; Gladue, D.P. ASFV-G-∆I177L as an Effective Oral Nasal Vaccine against the Eurasia Strain of Africa Swine Fever. Viruses 2021, 13, 765. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, D.; He, X.; Liu, R.; Wang, Z.; Zhang, X.; Li, F.; Shan, D.; Chen, H.; Zhang, J.; et al. A seven-gene-deleted African swine fever virus is safe and effective as a live attenuated vaccine in pigs. Sci. China Life Sci. 2020, 63, 623–634. [Google Scholar] [CrossRef]
- Reis, A.L.; Goatley, L.C.; Jabbar, T.; Lopez, E.; Rathakrishnan, A.; Dixon, L.K. Deletion of the Gene for the Type I Interferon Inhibitor I329L from the Attenuated African Swine Fever Virus OURT88/3 Strain Reduces Protection Induced in Pigs. Vaccines 2020, 8, 262. [Google Scholar] [CrossRef]
- Barasona, J.A.; Gallardo, C.; Cadenas-Fernández, E.; Jurado, C.; Rivera, B.; Rodríguez-Bertos, A.; Arias, M.; Sánchez-Vizcaíno, J.M. First Oral Vaccination of Eurasian Wild Boar Against African Swine Fever Virus Genotype II. Front. Vet. Sci. 2019, 6, 137. [Google Scholar] [CrossRef]
- O’Donnell, V.; Holinka, L.G.; Krug, P.W.; Gladue, D.P.; Carlson, J.; Sanford, B.; Alfano, M.; Kramer, E.; Lu, Z.; Arzt, J.; et al. African Swine Fever Virus Georgia 2007 with a Deletion of Virulence-Associated Gene 9GL (B119L), when Administered at Low Doses, Leads to Virus Attenuation in Swine and Induces an Effective Protection against Homologous Challenge. J. Virol. 2015, 89, 8556–8566. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, V.; Risatti, G.R.; Holinka, L.G.; Krug, P.W.; Carlson, J.; Velazquez-Salinas, L.; Azzinaro, P.A.; Gladue, D.P.; Borca, M.V. Simultaneous Deletion of the 9GL and UK Genes from the African Swine Fever Virus Georgia 2007 Isolate Offers Increased Safety and Protection against Homologous Challenge. J. Virol. 2017, 91, e01760-16. [Google Scholar] [CrossRef] [Green Version]
- Teklue, T.; Sun, Y.; Abid, M.; Luo, Y.; Qiu, H.J. Current status and evolving approaches to African swine fever vaccine development. Transbound. Emerg. Dis. 2020, 67, 529–542. [Google Scholar] [CrossRef]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African swine fever epidemiology and control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [Green Version]
- Portugal, R.; Coelho, J.; Höper, D.; Little, N.S.; Smithson, C.; Upton, C.; Martins, C.; Leitão, A.; Keil, G.M. Related strains of African swine fever virus with different virulence: Genome comparison and analysis. J. Gen. Virol. 2015, 96, 408–419. [Google Scholar] [CrossRef]
- Gallardo, C.; Sanchez, E.G.; Perez-Nunez, D.; Nogal, M.; De Leon, P.; Carrascosa, A.L.; Nieto, R.; Soler, A.; Arias, M.L.; Revilla, Y. African swine fever virus (ASFV) protection mediated by NH/P68 and NH/P68 recombinant live-attenuated viruses. Vaccine 2018, 36, 2694–2704. [Google Scholar] [CrossRef]
- Gladue, D.P.; O’Donnell, V.; Ramirez-Medina, E.; Rai, A.; Pruitt, S.; Vuono, E.A.; Silva, E.; Velazquez-Salinas, L.; Borca, M.V. Deletion of CD2-Like (CD2v) and C-Type Lectin-Like (EP153R) Genes from African Swine Fever Virus Georgia-∆9GL Abrogates Its Effectiveness as an Experimental Vaccine. Viruses 2020, 12, 1185. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, V.; Holinka, L.G.; Gladue, D.P.; Sanford, B.; Krug, P.W.; Lu, X.; Arzt, J.; Reese, B.; Carrillo, C.; Risatti, G.R.; et al. African Swine Fever Virus Georgia Isolate Harboring Deletions of MGF360 and MGF505 Genes Is Attenuated in Swine and Confers Protection against Challenge with Virulent Parental Virus. J. Virol. 2015, 89, 6048–6056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudreault, N.N.; Richt, J.A. Subunit Vaccine Approaches for African Swine Fever Virus. Vaccines 2019, 7, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogan, D.; Babiuk, L.A. Novel vaccines from biotechnology. Rev. Sci. Tech. 2005, 24, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Feng, Z.; Chen, J.; Liang, W.; Chen, W.; Li, Z.; Chen, Q.; Cai, S. The recombinant pseudorabies virus expressing African swine fever virus CD2v protein is safe and effective in mice. Virol. J. 2020, 17, 180. [Google Scholar] [CrossRef]
- Goatley, L.C.; Reis, A.L.; Portugal, R.; Goldswain, H.; Shimmon, G.L.; Hargreaves, Z.; Ho, C.-S.; Montoya, M.; Sánchez-Cordón, P.J.; Taylor, G.; et al. A pool of eight virally vectored african swine fever antigens protect pigs against fatal disease. Vaccines 2020, 8, 234. [Google Scholar] [CrossRef]
- Lokhandwala, S.; Waghela, S.D.; Bray, J.; Martin, C.L.; Sangewar, N.; Charendoff, C.; Shetti, R.; Ashley, C.; Chen, C.H.; Berghman, L.R.; et al. Induction of Robust Immune Responses in Swine by Using a Cocktail of Adenovirus-Vectored African Swine Fever Virus Antigens. Clin. Vaccine Immunol. 2016, 23, 888–900. [Google Scholar] [CrossRef] [Green Version]
- Lokhandwala, S.; Waghela, S.D.; Bray, J.; Sangewar, N.; Charendoff, C.; Martin, C.L.; Hassan, W.S.; Koynarski, T.; Gabbert, L.; Burrage, T.G.; et al. Adenovirus-vectored novel African Swine Fever Virus antigens elicit robust immune responses in swine. PLoS ONE 2017, 12, e0177007. [Google Scholar] [CrossRef]
- Lopera-Madrid, J.; Osorio, J.E.; He, Y.; Xiang, Z.; Adams, L.G.; Laughlin, R.C.; Mwangi, W.; Subramanya, S.; Neilan, J.; Brake, D.; et al. Safety and immunogenicity of mammalian cell derived and Modified Vaccinia Ankara vectored African swine fever subunit antigens in swine. Vet. Immunol. Immunopathol. 2017, 185, 20–33. [Google Scholar] [CrossRef]
- Argilaguet, J.M.; Perez-Martin, E.; Lopez, S.; Goethe, M.; Escribano, J.M.; Giesow, K.; Keil, G.M.; Rodriguez, F. BacMam immunization partially protects pigs against sublethal challenge with African swine fever virus. Antivir. Res. 2013, 98, 61–65. [Google Scholar] [CrossRef]
- Chen, X.; Yang, J.; Ji, Y.; Okoth, E.; Liu, B.; Li, X.; Yin, H.; Zhu, Q. Recombinant Newcastle disease virus expressing African swine fever virus protein 72 is safe and immunogenic in mice. Virol. Sin. 2016, 31, 150–159. [Google Scholar] [CrossRef]
- Murgia, M.V.; Mogler, M.; Certoma, A.; Green, D.; Monaghan, P.; Williams, D.T.; Rowland, R.R.R.; Gaudreault, N.N. Evaluation of an African swine fever (ASF) vaccine strategy incorporating priming with an alphavirus-expressed antigen followed by boosting with attenuated ASF virus. Arch. Virol. 2019, 164, 359–370. [Google Scholar] [CrossRef]
- Jarząb, A.; Skowicki, M.; Witkowska, D. Subunit vaccines—antigens, carriers, conjugation methods and the role of adjuvants. Postepy Hig. Med. Dosw. 2013, 67, 1128–1143. [Google Scholar] [CrossRef]
- Neilan, J.G.; Zsak, L.; Lu, Z.; Burrage, T.G.; Kutish, G.F.; Rock, D.L. Neutralizing antibodies to African swine fever virus proteins p30, p54, and p72 are not sufficient for antibody-mediated protection. Virology 2004, 319, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, V.; Efremov, E.E.; Novikov, B.V.; Balyshev, V.M.; Tsibanov, S.Z.; Kalinovsky, T.; Kolbasov, D.V.; Niedzwiecki, A.; Rath, M. Vaccination with viral protein-mimicking peptides postpones mortality in domestic pigs infected by African swine fever virus. Mol. Med. Rep. 2011, 4, 395–401. [Google Scholar] [CrossRef]
- Jancovich, J.K.; Chapman, D.; Hansen, D.T.; Robida, M.D.; Loskutov, A.; Craciunescu, F.; Borovkov, A.; Kibler, K.; Goatley, L.; King, K.; et al. Immunization of pigs by DNA prime and recombinant Vaccinia virus boost to identify and rank African swine fever virus immunogenic and protective proteins. J. Virol. 2018, 92, e02219-17. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Núñez, D.; Sunwoo, S.Y.; Sánchez, E.G.; Haley, N.; García-Belmonte, R.; Nogal, M.; Morozov, I.; Madden, D.; Gaudreault, N.N.; Mur, L.; et al. Evaluation of a viral DNA-protein immunization strategy against African swine fever in domestic pigs. Vet. Immunol. Immunopathol. 2019, 208, 34–43. [Google Scholar] [CrossRef]
- Freitas, F.B.; Simões, M.; Frouco, G.; Martins, C.; Ferreira, F. Towards the Generation of an ASFV-pA104R DISC Mutant and a Complementary Cell Line—A Potential Methodology for the Production of a Vaccine Candidate. Vaccines 2019, 7, 68. [Google Scholar] [CrossRef] [Green Version]
- Urbano, A.C.; Ferreira, F. Role of the DNA-Binding Protein pA104R in ASFV Genome Packaging and as a Novel Target for Vaccine and Drug Development. Vaccines 2020, 8, 585. [Google Scholar] [CrossRef]
- Pejsak, Z.; Niemczuk, K.; Frant, M.; Mazur, M.; Pomorska-Mól, M.; Ziętek-Barszcz, A.; Bocian, Ł.; Łyjak, M.; Borowska, D.; Woźniakowski, G. Four years of African swine fever in Poland. New insights into epidemiology and prognosis of future disease spread. Pol. J. Vet. Sci. 2018, 21, 835–841. [Google Scholar] [CrossRef]
- Tarasiuk, K.; Giżejewski, Z. Wild boar behaviour and the epidemiological significance of this animal species as the main reservoir of the African swine fever virus. Med. Weter. 2021, 77, 115–121. [Google Scholar] [CrossRef]
- Bieber, C.; Ruf, T. Population dynamics in wild boar Sus scrofa: Ecology, elasticity, of growth rate and implications for the management of pulsed resource consumers. J. Appl. Ecol. 2005, 42, 1203–1213. [Google Scholar] [CrossRef]
- Sanchez-Vizcaino, J.M.; Laddomada, A.; Arias, M.L. African swine fever virus. In Diseases of Swine; Zimmerman, J.J., Karriker, L.A., Ramirez, A., Schwartz, K.J., Stevenson, G.W., Zhang, J., Eds.; John Willey& Sons Inc.: Hoboken, NJ, USA, 2019; pp. 396–404. [Google Scholar]
- Redrejo-Rodríguez, M.; Salas, M.L. Repair of base damage and genome maintenance in the nucleocytoplasmic large DNA viruses. Virus Res. 2014, 179, 12–25. [Google Scholar] [CrossRef]
- Brun, A.; Rivas, C.; Esteban, M.; Escribano, J.M.; Alonso, C. African swine fever virus gene A179L, a viral homologue of bcl-2, protects cells from programmed cell death. Virology 1996, 225, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Galindo, I.; Hernaez, B.; Díaz-Gil, G.; Escribano, J.M.; Alonso, C. A179L, a viralBcl-2 homologue, targets the core Bcl-2 apoptotic machinery and its upstream BH3 activators with selective binding restrictions for Bid and Noxa. Virology 2008, 375, 561–572. [Google Scholar] [CrossRef] [Green Version]
- Nogal, M.; Buitrago, G.G.; Rodríguez, C.; Cubelos, B.; Carrascosa, A.; Salas, M.; Revilla, Y. African swine fever virus IAP homologue inhibits caspase activation and promotes cell survival in mammalian cells. J. Virol. 2001, 75, 2535–2543. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.; Abrams, C.; Hernáez, B.; Alcázar, A.; Escribano, J.M.; Dixon, L.; Alonso, C. The MyD116 African swine fever virus homologue interacts with the catalytic subunit of protein phosphatase 1 and activates its phosphatase activity. J. Virol. 2007, 81, 2923–2929. [Google Scholar] [CrossRef] [Green Version]
- Revilla, Y.; Cebrián, A.; Baixerás, E.; Martínez, C.; Viñuela, E.; Salas, M.L. Inhibition of apoptosis by the African swine fever virus Bcl-2 homologue: Role of the BH1 domain. Virology 1997, 228, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Golding, J.P.; Goatley, L.; Goodbourn, S.; Dixon, L.K.; Taylor, G.; Netherton, C.L. Sensitivity of African swine fever virus to type I interferon is linked to genes within multigene families 360 and 505. Virology 2016, 493, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Reis, A.L.; Abrams, C.C.; Goatley, L.C.; Netherton, C.; Chapman, D.G.; Sanchez-Cordon, P.; Dixon, L.K. Deletion of African swine fever virus interferon inhibitors from the genome of a virulent isolate reduces virulence in domestic pigs and induces a protective response. Vaccine 2016, 34, 4698–4705. [Google Scholar] [CrossRef] [Green Version]
- Alejo, A.; Matamoros, T.; Guerra, M.; Andrés, G. A proteomic atlas of the African swine fever virus particle. J. Virol. 2018, 92, e01293-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez, E.G.; Quintas, A.; Pérez-Núñez, D.; Nogal, M.; Barroso, S.; Carrascosa, Á.L.; Revilla, Y. African swine fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 2012, 8, e1002754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Belmonte, R.; Pérez-Núñez, D.; Pittau, M.; Richt, J.A.; Revilla, Y. African Swine Fever Virus Armenia/07 Virulent Strain Controls Interferon Beta Production through the cGAS-STING Pathway. J. Virol. 2019, 93, e02298-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Granja, A.G.; Sabina, P.; Salas, M.L.; Fresno, M.; Revilla, Y. Regulation of inducible nitric oxide synthase expression by viral A238L-mediated inhibition of p65/RelA acetylation and p300 transactivation. J. Virol. 2006, 80, 10487–10496. [Google Scholar] [CrossRef] [Green Version]
- Silk, R.N.; Bowick, G.C.; Abrams, C.C.; Dixon, L.K. African swine fever virus A238L inhibitor of NF-kappaB and of calcineurin phosphatase is imported actively into the nucleus and exported by a CRM1-mediated pathway. J. Gen. Virol. 2007, 88, 411–419. [Google Scholar] [CrossRef]
- Granja, A.G.; Nogal, M.L.; Hurtado, C.; Del Aguila, C.; Carrascosa, A.L.; Salas, M.L.; Fresno, M.; Revilla, Y. The viral protein A238L inhibits TNF-alpha expression through a CBP/p300 transcriptional coactivators pathway. J. Immunol. 2006, 176, 451–462. [Google Scholar] [CrossRef] [Green Version]
- Achenbach, J.E.; Gallardo, C.; Nieto-Pelegrín, E.; Rivera-Arroyo, B.; Degefa-Negi, T.; Arias, M.; Jenberie, S.; Mulisa, D.D.; Gizaw, D.; Gelaye, E.; et al. Identification of a new genotype of African swine fever virus in domestic pigs from Ethiopia. Transbound. Emerg. Dis. 2017, 64, 1393–1404. [Google Scholar] [CrossRef]
- Quembo, C.J.; Jori, F.; Vosloo, W.; Heath, L. Genetic characterization of African swine fever virus isolates from soft ticks at the wildlife/domestic interface in Mozambique and identification of a novel genotype. Transbound. Emerg. Dis. 2017, 65, 420–431. [Google Scholar] [CrossRef] [Green Version]
- Vergne, T.; Chen-Fu, C.; Li, S.; Cappelle, J.; Edwards, J.; Martin, V.; Pfeiffer, D.U.; Fusheng, G.; Roger, F.L. Pig empire under infectious threat: Risk of African swine fever introduction into the People’s Republic of China. Vet. Rec. 2017, 181, 117. [Google Scholar] [CrossRef]
- Lopez, E.; Van Heerden, J.; Bosch-Camós, L.; Accensi, F.; Navas, M.J.; López-Monteagudo, P.; Argilaguet, J.; Gallardo, C.; Pina-Pedrero, S.; Salas, M.L.; et al. Live Attenuated African Swine Fever Viruses as Ideal Tools to Dissect the Mechanisms Involved in Cross-Protection. Viruses 2020, 12, 1474. [Google Scholar] [CrossRef]
- Portugal, R.; Goatley, L.C.; Husmann, R.; Zuckermann, F.A.; Dixon, L.K. A porcine macrophage cell line that supports high levels of replication of OURT88/3, an attenuated strain of African swine fever virus. Emerg. Microbes. Infect. 2020, 9, 1245–1253. [Google Scholar] [CrossRef]
- Dixon, L.K.; Islam, M.; Nash, R.; Reis, A.L. African swine fever virus evasion of host defences. Virus Res. 2019, 266, 25–33. [Google Scholar] [CrossRef]
- Wang, N.; Zhao, D.; Wang, J.; Zhang, Y.; Wang, M.; Gao, Y.; Li, F.; Wang, J.; Bu, Z.; Rao, Z.; et al. Architecture of African swine fever virus and implications for viral assembly. Science 2019, 366, 640–644. [Google Scholar] [CrossRef]
- Alonso, C.; Galindo, I.; Cuesta-Geijo, M.A.; Cabezas, M.; Hernaez, B.; Munoz-Moreno, R. African swine fever virus-cell interactions: From virus entry to cell survival. Virus Res. 2013, 173, 42–57. [Google Scholar] [CrossRef]
- Hurtado, C.; Bustos, M.J.; Carrascosa, A.L. The use of COS-1 cells for studies of field and laboratory African swine fever virus samples. J. Virol. Methods 2009, 164, 131–134. [Google Scholar] [CrossRef] [Green Version]
- Krug, P.W.; Holinka, L.G.; O’Donnell, V.; Reese, B.; Sanford, B.; Fernandez-Sainz, I.; Gladue, D.P.; Arzt, J.; Rodriguez, L.; Risatti, G.R.; et al. The progressive adaptation of a georgian isolate of African swine fever virus to vero cells leads to a gradual attenuation of virulence in swine corresponding to major modifications of the viral genome. J. Virol. 2015, 89, 2324–2332. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.G.; Riera, E.; Nogal, M.; Gallardo, C.; Fernández, P.; Bello-Morales, R.; López-Guerrero, J.A.; Chitko-McKown, C.G.; Richt, J.A.; Revilla, Y. Phenotyping and susceptibility of established porcine cells lines to African Swine Fever Virus infection and viral production. Sci. Rep. 2017, 7, 10369. [Google Scholar] [CrossRef]
- Borca, M.V.; Rai, A.; Ramirez-Medina, E.; Silva, E.; Velazquez-Salinas, L.; Vuono, E.; Pruitt, S.; Espinoza, N.; Gladue, D.P. A cell culture-adapted vaccine virus against the current pandemic African swine fever virus strain. J. Virol. 2021. [Google Scholar] [CrossRef]
- Coggins, L.; Moulton, J.E.; Colgrove, G.S. Studies with HINDE attenuated African swine fever virus. Cornell Vet. 1968, 4, 525–540. [Google Scholar]
- Ribeiro, M.; Nunes Petisca, J.L.; Lopez Frazao, F.; Sobral, M. Vaccination contre la pest porcine africaine. Bul. Off. Internatl. Epizoot. 1963, 60, 921. [Google Scholar]
- Bøtner, A.; Strandbygaard, B.; Sørensen, K.J.; Have, P.; Madsen, K.G.; Madsen, E.S.; Alexandersen, S. Appearance of acute PRRS-like symptoms in sow herds after vaccination with a modified live PRRS vaccine. Vet. Rec. 1997, 141, 497–499. [Google Scholar] [CrossRef]
- Madsen, K.G.; Hansen, C.M.; Madsen, E.S.; Strandbygaard, B.; Bøtner, A.; Sørensen, K.J. Sequence analysis of porcine reproductive and respiratory syndrome virus of the American type collected from Danish swine herds. Arch. Virol. 1998, 143, 1683–1700. [Google Scholar] [CrossRef]
- Storgaard, T.; Oleksiewicz, M.; Bøtner, A. Examination of the selective pressures on a live PRRS vaccine virus. Arch. Virol. 1999, 144, 2389–2401. [Google Scholar] [CrossRef]
- Wang, C.; Wu, B.; Amer, S.; Luo, J.; Zhang, H.; Guo, Y.; Dong, G.; Zhao, B.; He, H. Phylogenetic analysis and molecular characteristics of seven variant Chinese field isolates of PRRSV. BMC Microbiol. 2010, 10, 146. [Google Scholar] [CrossRef] [Green Version]
- Alonso, L. African swine fever virus, NL gene is not required for virus virulence. J. Gen. Virol. 1998, 79, 2543–2547. [Google Scholar]
- Sanford, B.; Holinka, L.; O’Donnell, V.; Krug, P.; Carlson, J.; Alfano, M.; Carrillo, C.; Wu, P.; Lowe, A.; Risatti, G.; et al. Deletion of the thymidine kinase gene induces complete attenuation of the Georgia isolate of African swine fever virus. Virus Res. 2016, 213, 165–171. [Google Scholar] [CrossRef]
- Uttenthal, Å.; Parida, S.; Rasmussen, T.B.; Paton, D.J.; Haas, B.; Dundon, W.G. Strategies for differentiating infection in vaccinated animals (DIVA) for foot-and-mouth disease, classical swine fever and avian influenza. Expert Rev. Vaccines 2010, 9, 73–87. [Google Scholar] [CrossRef]
- Arias, M.; De la Torre, A.; Dixon, L.; Gallardo, C.; Jori, F.; Laddomada, A.; Martins, C.; Parkhouse, R.M.; Revilla, Y.; Rodriguez, F.; et al. Approaches and Perspectives for Development of African Swine Fever Virus Vaccines. Vaccines 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]

| Region | Country | Animal Category |
|---|---|---|
| Africa | Namibia | Domestic |
| Nigeria | Domestic | |
| Sierra Leone | Domestic | |
| South Africa | Domestic | |
| Tanzania | Domestic | |
| Zambia | Domestic | |
| Asia | China (People’s Rep. of) | Domestic and wild |
| Hong Kong | Domestic | |
| India | Domestic | |
| Indonesia | Domestic | |
| Korea (Dem People’s Rep. of) | Domestic | |
| Korea (Rep. of) | Domestic and wild | |
| Laos | Domestic | |
| Malaysia | Domestic and wild | |
| Myanmar | Domestic | |
| Philippines | Domestic | |
| Timor-Leste | Domestic | |
| Vietnam | Domestic | |
| Europe | Bulgaria | Domestic |
| Estonia | Domestic and wild | |
| Germany | Wild | |
| Greece | Domestic | |
| Hungary | Wild | |
| Latvia | Wild | |
| Lithuania | Domestic and wild | |
| Moldova | Domestic and wild | |
| Poland | Domestic and wild | |
| Romania | Domestic and wild | |
| Russia | Domestic and wild | |
| Serbia | Domestic and wild | |
| Slovakia | Domestic and wild | |
| Ukraine | Domestic and wild | |
| Oceania | Papua New Guinea | Domestic |
| ASFV Strain | Virulence | Genotype (P27) | Deleted Genes | Deletion Mutant | Effects | Reference |
|---|---|---|---|---|---|---|
| Georgia 2010 | II | I177L | ASFV-G-ΔI177L | full attenuation in pigs | [57] | |
| Georgia 2010 | II | I177L | ASFV-G-ΔI177L | Oronasal administration is as effective as i.m. administration, animals remained clinically healthy after challenge with ASFV-G, and the specific antibody response was on the same level | [58] | |
| HLJ/18 | High | II | Gene segments MGF505-1R, MGF505-2R, MGF505-3R, MGF360-12L, MGF360-13L, MGF360-14L | HLj/18-7GD | full attenuation in pigs, assurance of complete immunity against lethal ASFV challenge, unable to be converted to the virulent strain | [59] |
| OURT 88/3 | Low | I | I329L | OURT88/3ΔI329L | inhibits the host’s innate immune response, | [60] |
| L v17/WB/Rie1217 | II | 92% protection in wild boars after challenge with the virulent ASF virus isolate Arm07 | [61] | |||
| Georgia 2007 | High | II | 9GL (B119L) UK (DP96R) | full attenuation in pigs | [62,63] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turlewicz-Podbielska, H.; Kuriga, A.; Niemyjski, R.; Tarasiuk, G.; Pomorska-Mól, M. African Swine Fever Virus as a Difficult Opponent in the Fight for a Vaccine—Current Data. Viruses 2021, 13, 1212. https://doi.org/10.3390/v13071212
Turlewicz-Podbielska H, Kuriga A, Niemyjski R, Tarasiuk G, Pomorska-Mól M. African Swine Fever Virus as a Difficult Opponent in the Fight for a Vaccine—Current Data. Viruses. 2021; 13(7):1212. https://doi.org/10.3390/v13071212
Chicago/Turabian StyleTurlewicz-Podbielska, Hanna, Anna Kuriga, Rafał Niemyjski, Grzegorz Tarasiuk, and Małgorzata Pomorska-Mól. 2021. "African Swine Fever Virus as a Difficult Opponent in the Fight for a Vaccine—Current Data" Viruses 13, no. 7: 1212. https://doi.org/10.3390/v13071212
APA StyleTurlewicz-Podbielska, H., Kuriga, A., Niemyjski, R., Tarasiuk, G., & Pomorska-Mól, M. (2021). African Swine Fever Virus as a Difficult Opponent in the Fight for a Vaccine—Current Data. Viruses, 13(7), 1212. https://doi.org/10.3390/v13071212







