Functional Features of the Respiratory Syncytial Virus G Protein
Abstract
:1. Background
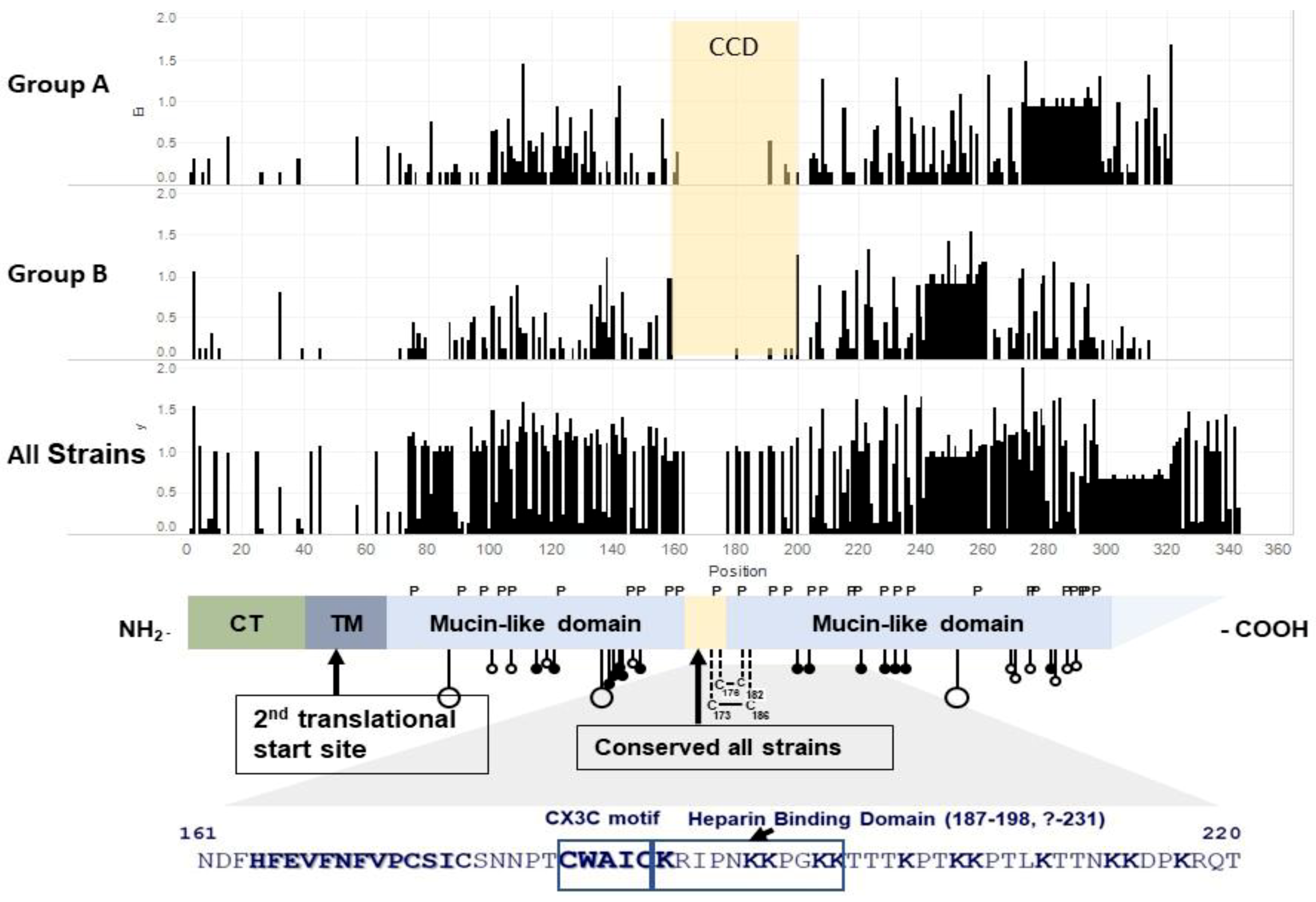
2. Structure of G
3. Secreted G
4. Binding to and Infection of Cells
5. Animal Studies and Disease Pathogenesis
6. In Vitro Studies of the Immune Response
6.1. Innate Response
6.2. Adaptive Response
6.3. Airway Epithelial Cells
7. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [Green Version]
- Stockman, L.J.; Curns, A.T.; Anderson, L.J.; Fischer-Langley, G. Respiratory Syncytial Virus-associated Hospitalizations among Infants and Young Children in the United States, 1997–2006. Pediatr. Infect. Dis. J. 2012, 31, 5–9. [Google Scholar] [CrossRef]
- Falsey, A.R.; Hennessey, P.A.; Formica, M.A.; Cox, C.; Walsh, E.E. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005, 352, 1749–1759. [Google Scholar] [CrossRef] [PubMed]
- Hall, C.B.; Weinberg, G.A.; Iwane, M.K.; Blumkin, A.K.; Edwards, K.M.; Staat, M.A.; Auinger, P.; Griffin, M.R.; Poehling, K.A.; Erdman, D.; et al. The Burden of Respiratory Syncytial Virus Infection in Young Children. N. Engl. J. Med. 2009, 360, 588–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Group TI-RS. Palivizumab, a Humanized Respiratory Syncytial Virus Monoclonal Antibody, Reduces Hospitalization from Respiratory Syncytial Virus Infection in High-risk Infants. Pediatrics 1998, 102, 531–537. [Google Scholar] [CrossRef]
- Groothuis, J.R.; Simoes, E.A.F.; Levin, M.J.; Hall, C.B.; Long, C.E.; Rodriguez, W.J.; Arrobio, J.; Meissner, H.C.; Fulton, D.R.; Welliver, R.C.; et al. Prophylactic administration of respiratory syncytial virus immune globulin in high-risk infants and young children. N. Engl. J. Med. 1993, 329, 1524–1530. [Google Scholar] [CrossRef]
- Mazur, N.I.; Higgins, D.; Nunes, M.C.; Melero, J.A.; Langedijk, A.C.; Horsley, N.; Buchholz, U.J.; Openshaw, P.J.; McLellan, J.; Englund, J.A.; et al. The respiratory syncytial virus vaccine landscape: Lessons from the graveyard and promising candidates. Lancet Infect. Dis. 2018, 18, e295–e311. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.; Dormitzer, P.; Nokes, D.; Rappuoli, R.; Roca, A.; Graham, B. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine 2013, 31, B209–B215. [Google Scholar] [CrossRef] [Green Version]
- Chin, J.; Magoffin, R.L.; Shearer, L.A.; Schieble, J.H.; Lennette, E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric Population. Am. J. Epidemiol. 1969, 89, 449–463. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated Vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Fulginiti, V.A.; Eller, J.J.; Sieber, O.F.; Joyner, J.W.; Minamitani, M.; Meiklejohn, G. Respiratory virus immunization: I. A field of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 1969, 89, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Kapikian, A.Z.; Mitchell, R.H.; Chanock, R.M.; Shvedoff, R.A.; Stewart, C.E. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated rs virus vaccine. Am. J. Epidemiol. 1969, 89, 405–421. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Melero, J.A. Progress in understanding and controlling respiratory syncytial virus: Still crazy after all these years. Virus Res. 2011, 162, 80–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connors, M.; Collins, P.L.; Firestone, C.Y.; Murphy, B.R. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 1991, 65, 1634–1637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, L.J.; Hierholzer, J.C.; Tsou, C.; Hendry, R.M.; Fernie, B.F.; Stone, Y.; McIntosh, K. Antigenic characterization of respiratory syncytial virus strains with monoclonal antibodies. J. Infect. Dis. 1985, 151, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Mufson, M.A.; Örvell, C.; Rafnar, B.; Norrby, E. Two Distinct Subtypes of Human Respiratory Syncytial Virus. J. Gen. Virol. 1985, 66, 2111–2124. [Google Scholar] [CrossRef] [PubMed]
- Peret, T.C.T.; Hall, C.B.; Hammond, G.W.; Piedra, P.A.; Storch, G.A.; Sullender, W.M. Circulation patterns of group A and B human respiratory synyctial virus genotypes in five communities in North America. J. Infect. Dis. 2000, 181, 1891–1896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peret, T.C.; Golub, J.A.; Anderson, L.J.; Hall, C.B.; Schnabel, K.C. Circulation patterns of genetically distinct group A and B strains of human respiratory syncytial virus in a community. J. Gen. Virol. 1998, 79, 2221–2229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, P.R., Jr.; Olmsted, R.A.; Prince, G.A.; Murphy, B.R.; Alling, D.W.; Walsh, E.E. Antigenic relatedness between glycoproteins of human respiratory syncytial virus subgroups A and B: Evaluation of the contributions of F and G glycoproteins to immunity. J. Virol. 1987, 61, 3163–3166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamprecht, C.L.; Krause, H.E.; Mufson, M.A. Role of maternal antibody in pneumonia and bronchiolitis due to respiratory syncytial virus. J. Infect. Dis. 1981, 134, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Ogilvie, M.M.; Vathenen, A.S.; Radford, M.; Codd, J.; Key, S. Maternal antibody and respiratory syncytial virus infection in infancy. J. Med. Virol. 1981, 7, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Agerskov, K.; Meakins, T.; Aaby, P.; Simões, E.A. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 2009, 123, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Walsh, E.E.; Wang, L.; Falsey, A.R.; Qiu, X.; Corbett, A.; Holden-Wiltse, J.; Mariani, T.J.; Topham, D.J.; Caserta, M.T. Virus-Specific Antibody, Viral Load, and Disease Severity in Respiratory Syncytial Virus Infection. J. Infect. Dis. 2018, 218, 208–217. [Google Scholar] [CrossRef]
- Walsh, E.E.; Falsey, A.R. Humoral and Mucosal Immunity in Protection from Natural Respiratory Syncytial Virus Infection in Adults. J. Infect. Dis. 2004, 190, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Piedra, P.A.; Jewell, A.M.; Cron, S.G.; Atmar, R.L.; Glezen, W.P. Correlates of immunity to respiratory syncytial virus (RSV) associated-hospitalization: Establishment of minimum protective threshold levels of serum neutralizing antibodies. Vaccine 2003, 21, 3479–3482. [Google Scholar] [CrossRef]
- Tripp, R.A.; Power, U.F.; Openshaw, P.J.M.; Kauvar, L.M. Respiratory Syncytial Virus: Targeting the G Protein Provides a New Approach for an Old Problem. J. Virol. 2018, 92, e01302-17. [Google Scholar] [CrossRef] [Green Version]
- Miao, C.; Radu, G.U.; Caidi, H.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab′)2 components mediates reduced pulmonary inflammation in mice. J. Gen. Virol. 2009, 90, 1119–1123. [Google Scholar] [CrossRef]
- Haynes, L.M.; Caidi, H.; Radu, G.U.; Miao, C.; Harcourt, J.L.; Tripp, R.A.; Anderson, L.J. Therapeutic Monoclonal Antibody Treatment Targeting Respiratory Syncytial Virus (RSV) G Protein Mediates Viral Clearance and Reduces the Pathogenesis of RSV Infection in BALB/c Mice. J. Infect. Dis. 2009, 200, 439–447. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Gaston, K.A.; Todd, S.O.; Boyoglu, C.; Chirkova, T.; Barnum, T.R.; Jorquera, P.; Haynes, L.M.; Tripp, R.A.; Moore, M.L.; et al. A Respiratory Syncytial Virus (RSV) Anti-G Protein F(ab′) 2 Monoclonal Antibody Suppresses Mucous Production and Breathing Effort in RSV rA2-line19F-Infected BALB/c Mice. J. Virol. 2013, 87, 10955–10967. [Google Scholar] [CrossRef] [Green Version]
- Boyoglu-Barnum, S.; Todd, S.O.; Chirkova, T.; Barnum, T.R.; Gaston, K.A.; Haynes, L.M.; Tripp, R.A.; Moore, M.L.; Anderson, L.J. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 2015, 483, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Chirkova, T.; Lin, S.; Oomens, A.G.P.; Gaston, K.A.; Boyoglu-Barnum, S.; Meng, J.; Stobart, C.C.; Cotton, C.U.; Hartert, T.V.; Moore, M.L.; et al. CX3CR1 is an important surface molecule for respiratory syncytial virus infection in human airway epithelial cells. J. Gen. Virol. 2015, 96, 2543–2556. [Google Scholar] [CrossRef]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Teng, M.N.; Oomens, A.G.; Walsh, E.E.; Peeples, M.E. Respiratory Syncytial Virus Uses CX3CR1 as a Receptor on Primary Human Airway Epithelial Cultures. PLoS Pathog. 2015, 11, e1005318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLellan, J.; Ray, W.C.; Peeples, M.E. Structure and Function of Respiratory Syncytial Virus Surface Glycoproteins. Curr. Top. Microbiol. Immunol. 2013, 372, 83–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, P.L. O glycosylation of glycoprotein G of human respiratory syncytial virus is specified within the divergent ectodomain. J. Virol. 1990, 64, 4007–4012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, T.; Mejias, A.; Ramilo, O.; Peeples, M.E. The larger attachment glycoprotein of respiratory syncytial virus produced in primary human bronchial epithelial cultures reduces infectivity for cell lines. PLoS Pathog. 2021, 17, e1009469. [Google Scholar] [CrossRef] [PubMed]
- Wootton, J.C.; Federhen, S. Statistics of local complexity in amino acid sequences and sequence databases. Comput. Chem. 1993, 17, 149–163. [Google Scholar] [CrossRef]
- Teng, M.N.; Collins, P.L. The Central Conserved Cystine Noose of the Attachment G Protein of Human Respiratory Syncytial Virus Is Not Required for Efficient Viral Infection In Vitro or In Vivo. J. Virol. 2002, 76, 6164–6171. [Google Scholar] [CrossRef] [Green Version]
- Tripp, R.; Jones, L.; Haynes, L.; Zheng, H.; Murphy, P.; Anderson, L. CX3C chemokine mimicry by respiratory syncytial virus G protein. Nat. Immunol. 2001, 2, 732–738. [Google Scholar] [CrossRef]
- Feldman, S.A.; Hendry, R.M.; Beeler, J.A. Identification of a linear heparin binding domain for human respiratory syncytial virus attachment glycoprotein G. J. Virol. 1999, 73, 6610–6617. [Google Scholar] [CrossRef] [Green Version]
- Fedechkin, S.O.; George, N.L.; Wolff, J.T.; Kauvar, L.M.; Dubois, R.M. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci. Immunol. 2018, 3, eaar3534. [Google Scholar] [CrossRef] [Green Version]
- Jones, H.G.; Ritschel, T.; Pascual, G.; Brakenhoff, J.P.J.; Keogh, E.; Furmanova-Hollenstein, P.; Lanckacker, E.; Wadia, J.S.; Gilman, M.S.A.; Williamson, R.A.; et al. Structural basis for recognition of the central conserved region of RSV G by neutralizing human antibodies. PLoS Pathog. 2018, 14, e1006935. [Google Scholar] [CrossRef] [Green Version]
- Fedechkin, S.O.; George, N.L.; Castrejon, A.M.N.; Dillen, J.R.; Kauvar, L.M.; DuBois, R.M. Conformational Flexibility in Respiratory Syncytial Virus G Neutralizing Epitopes. J. Virol. 2020, 94, e01879-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, B.; Mills, J.; Ghildyal, R.; Gooley, P.; Meanger, J. Multiple heparin binding domains of respiratory syncytial virus G mediate binding to mammalian cells. Arch. Virol. 2003, 148, 1987–2003. [Google Scholar] [CrossRef]
- Teng, M.N.; Whitehead, S.S.; Collins, P.L. Contribution of the Respiratory Syncytial Virus G Glycoprotein and Its Secreted and Membrane-Bound Forms to Virus Replication in Vitro and in Vivo. Virology 2001, 289, 283–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldman, S.A.; Audet, S.; Beeler, J.A. The Fusion Glycoprotein of Human Respiratory Syncytial Virus Facilitates Virus Attachment and Infectivity via an Interaction with Cellular Heparan Sulfate. J. Virol. 2000, 74, 6442–6447. [Google Scholar] [CrossRef] [Green Version]
- Crim, R.L.; Audet, S.A.; Feldman, S.A.; Mostowski, H.S.; Beeler, J.A. Identification of Linear Heparin-Binding Peptides Derived from Human Respiratory Syncytial Virus Fusion Glycoprotein That Inhibit Infectivity. J. Virol. 2007, 81, 261–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tayyari, F.; Marchant, D.; Moraes, T.J.; Duan, W.; Mastrangelo, P.; Hegele, R.G. Identification of nucleolin as a cellular receptor for human respiratory syncytial virus. Nat. Med. 2011, 17, 1132–1135. [Google Scholar] [CrossRef] [PubMed]
- Melero, J.A.; Mas, V.; McLellan, J.S. Structural, antigenic and immunogenic features of respiratory syncytial virus glycoproteins relevant for vaccine development. Vaccine 2017, 35, 461–468. [Google Scholar] [CrossRef] [Green Version]
- Englund, J.A.; Anderson, L.J.; Rhame, F.S. Nosocomial transmission of respiratory syncytial virus in immunocompromised adults. J. Clin. Microbiol. 1991, 29, 115–119. [Google Scholar] [CrossRef] [Green Version]
- Finger, R.; Anderson, L.J.; Dicker, R.C.; Harrison, B.; Doan, R.; Downing, A.; Corey, L. Epidemic Infections Caused by Respiratory Syncytial Virus in Institutionalized Young Adults. J. Infect. Dis. 1987, 155, 1335–1339. [Google Scholar] [CrossRef]
- Mazzulli, T.; Peret, T.C.T.; McGeer, A.; Cann, D.; Macdonald, K.S.; Chua, R.; Erdman, D.D.; Anderson, L.J. Molecular Characterization of a Nosocomial Outbreak of Human Respiratory Syncytial Virus on an Adult Leukemia/Lymphoma Ward. J. Infect. Dis. 1999, 180, 1686–1689. [Google Scholar] [CrossRef] [Green Version]
- Machado, A.F.; Sallum, M.A.M.; Boas, L.S.V.; Tateno, A.F.; Machado, C.M. Molecular Characterization of Strains of Respiratory Syncytial Virus Identified in a Hematopoietic Stem Cell Transplant Outpatient Unit Over 2 Years: Community or Nosocomial Infection? Biol. Blood Marrow Transplant. 2008, 14, 1348–1355. [Google Scholar] [CrossRef] [PubMed]
- Kamau, E.; Otieno, J.R.; Lewa, C.S.; Mwema, A.; Murunga, N.; Nokes, D.J. Evolution of respiratory syncytial virus genotype BA in Kilifi, Kenya, 15 years on. Sci. Rep. 2020, 10, 21176. [Google Scholar] [CrossRef] [PubMed]
- Tabor, D.E.; Fernandes, F.; Langedijk, A.C.; Wilkins, D.; Lebbink, R.J.; Tovchigrechko, A.; Ruzin, A.; Kragten-Tabatabaie, L.; Jin, H.; Esser, M.T.; et al. Global Molecular Epidemiology of Respiratory Syncytial Virus from the 2017−2018 INFORM-RSV Study. J. Clin. Microbiol. 2020, 59, e01828-20. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, R.; Tapia, L.I.; Yang, C.-F.; Torres, J.P.; Chavez-Bueno, S.; Garcia, C.; Jaramillo, L.M.; Moore-Clingenpeel, M.; Jafri, H.; Peeples, M.E.; et al. Respiratory Syncytial Virus Genotypes, Host Immune Profiles, and Disease Severity in Young Children Hospitalized with Bronchiolitis. J. Infect. Dis. 2018, 217, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Agoti, C.N.; Munywoki, P.K.; Phan, M.V.T.; Otieno, J.R.; Kamau, E.; Bett, A.; Kombe, I.; Githinji, G.; Medley, G.F.; Cane, P.A.; et al. Transmission patterns and evolution of respiratory syncytial virus in a community outbreak identified by genomic analysis. Virus Evol. 2017, 3, vex006. [Google Scholar] [CrossRef] [Green Version]
- Grad, Y.H.; Newman, R.; Zody, M.; Yang, X.; Murphy, R.; Qu, J.; Malboeuf, C.M.; Levin, J.Z.; Lipsitch, M.; De Vincenzo, J. Within-host whole-genome deep sequencing and diversity analysis of human respiratory syncytial virus infection reveals dynamics of genomic diversity in the absence and presence of immune pressure. J. Virol. 2014, 88, 7286–7293. [Google Scholar] [CrossRef] [Green Version]
- Vandini, S.; Biagi, C.; Lanari, M. Respiratory Syncytial Virus: The Influence of Serotype and Genotype Variability on Clinical Course of Infection. Int. J. Mol. Sci. 2017, 18, 1717. [Google Scholar] [CrossRef]
- Anderson, L.J.; Peret, T.C.; Piedra, P.A. RSV Strains and Disease Severity. J. Infect. Dis. 2019, 219, 514–516. [Google Scholar] [CrossRef] [PubMed]
- Moore, M.L.; Chi, M.H.; Luongo, C.; Lukacs, N.W.; Polosukhin, V.V.; Huckabee, M.M.; Newcomb, D.C.; Buchholz, U.J.; Crowe, J.E.; Goleniewska, K.; et al. A Chimeric A2 Strain of Respiratory Syncytial Virus (RSV) with the Fusion Protein of RSV Strain Line 19 Exhibits Enhanced Viral Load, Mucus, and Airway Dysfunction. J. Virol. 2009, 83, 4185–4194. [Google Scholar] [CrossRef] [Green Version]
- Stokes, K.L.; Chi, M.H.; Sakamoto, K.; Newcomb, D.C.; Currier, M.G.; Huckabee, M.M.; Lee, S.; Goleniewska, K.; Pretto, C.; Williams, J.; et al. Differential Pathogenesis of Respiratory Syncytial Virus Clinical Isolates in BALB/c Mice. J. Virol. 2011, 85, 5782–5793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, L.M.; Oosterheert, J.J.; Kuil, S.D.; Viveen, M.; Bont, L.J.; Hoepelman, A.I.M. High epidemic burden of RSV disease coinciding with genetic alterations causing amino acid substitutions in the RSV G-protein during the 2016/2017 season in The Netherlands. J. Clin. Virol. 2019, 112, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Trento, A.; Galiano, M.; Videla, C.; Carballal, G.; García-Barreno, B.; Melero, J.A.; Palomo, C. Major changes in the G protein of human respiratory syncytial virus isolates introduced by a duplication of 60 nucleotides. J. Gen. Virol. 2003, 84, 3115–3120. [Google Scholar] [CrossRef] [PubMed]
- Eshaghi, A.; Duvvuri, V.; Lai, R.; Nadarajah, J.T.; Li, A.; Patel, S.N.; Low, D.E.; Gubbay, J.B. Genetic Variability of Human Respiratory Syncytial Virus A Strains Circulating in Ontario: A Novel Genotype with a 72 Nucleotide G Gene Duplication. PLoS ONE 2012, 7, e32807. [Google Scholar] [CrossRef] [Green Version]
- Trento, A.; Casas, I.; Calderón, A.; Garcia-Garcia, M.L.; Calvo, C.; Perez-Breña, P.; Melero, J.A. Ten Years of Global Evolution of the Human Respiratory Syncytial Virus BA Genotype with a 60-Nucleotide Duplication in the G Protein Gene. J. Virol. 2010, 84, 7500–7512. [Google Scholar] [CrossRef] [Green Version]
- Duvvuri, V.R.; Granados, A.; Rosenfeld, P.; Bahl, J.; Eshaghi, A.; Gubbay, J.B. Genetic diversity and evolutionary insights of respiratory syncytial virus A ON1 genotype: Global and local transmission dynamics. Sci. Rep. 2015, 5, srep14268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hotard, A.L.; Laikhter, E.; Brooks, K.; Hartert, T.V.; Moore, M.L. Functional Analysis of the 60-Nucleotide Duplication in the Respiratory Syncytial Virus Buenos Aires Strain Attachment Glycoprotein. J. Virol. 2015, 89, 8258–8266. [Google Scholar] [CrossRef] [Green Version]
- Cui, G.; Liu, H.; Li, X.; Ming, L. Preliminary functional and phylogeographic analyses of the 72 nucleotide duplication region in the emerging human respiratory syncytial virus ON1 strain attachment glycoprotein gene. Biomed. Pharmacother. 2020, 123, 109800. [Google Scholar] [CrossRef]
- Escribano-Romero, E.; Rawling, J.; García-Barreno, B.; Melero, J.A. The Soluble Form of Human Respiratory Syncytial Virus Attachment Protein Differs from the Membrane-Bound Form in Its Oligomeric State but Is Still Capable of Binding to Cell Surface Proteoglycans. J. Virol. 2004, 78, 3524–3532. [Google Scholar] [CrossRef] [Green Version]
- Schwarze, J.; Schauer, U. Enhanced virulence, airway inflammation and impaired lung function induced by respiratory syncytial virus deficient in secreted G protein. Thorax 2004, 59, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maher, C.F.; Hussell, T.; Blair, E.; Ring, C.J.; Openshaw, P.J. Recombinant respiratory syncytial virus lacking secreted glycoprotein G is attenuated, non-pathogenic but induces protective immunity. Microbes Infect. 2004, 6, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Bukreyev, A.; Serra, M.E.; Laham, F.R.; Melendi, G.A.; Kleeberger, S.R.; Collins, P.L.; Polack, F.P. The Cysteine-Rich Region and Secreted Form of the Attachment G Glycoprotein of Respiratory Syncytial Virus Enhance the Cytotoxic T-Lymphocyte Response despite Lacking Major Histocompatibility Complex Class I-Restricted Epitopes. J. Virol. 2006, 80, 5854–5861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukreyev, A.; Yang, L.; Fricke, J.; Cheng, L.; Ward, J.M.; Murphy, B.R.; Collins, P.L. The Secreted Form of Respiratory Syncytial Virus G Glycoprotein Helps the Virus Evade Antibody-Mediated Restriction of Replication by Acting as an Antigen Decoy and through Effects on Fc Receptor-Bearing Leukocytes. J. Virol. 2008, 82, 12191–12204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukreyev, A.; Yang, L.; Collins, P.L. The Secreted G Protein of Human Respiratory Syncytial Virus Antagonizes Antibody-Mediated Restriction of Replication Involving Macrophages and Complement. J. Virol. 2012, 86, 10880–10884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, R.; König, B.; Werchau, H.; König, W. Respiratory syncytial virus deficient in soluble G protein induced an increased proinflammatory response in human lung epithelial cells. Virology 2004, 330, 384–397. [Google Scholar] [CrossRef]
- Polack, F.P.; Irusta, P.M.; Hoffman, S.J.; Schiatti, M.P.; Melendi, G.A.; Delgado, M.F.; Laham, F.R.; Thumar, B.; Hendry, R.M.; Melero, J.A.; et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc. Natl. Acad. Sci. USA 2005, 102, 8996–9001. [Google Scholar] [CrossRef] [Green Version]
- Ray, R.; Hoft, D.F.; Meyer, K.; Brown, R.; Lagging, L.; Belshe, R.B. Immunoregulatory role of secreted glycoprotein G from respiratory syncytial virus. Virus Res. 2001, 75, 147–154. [Google Scholar] [CrossRef]
- Barr, F.E.; Pedigo, H.; Johnson, T.R.; Shepherd, V.L. Surfactant Protein-A Enhances Uptake of Respiratory Syncytial Virus by Monocytes and U937 Macrophages. Am. J. Respir. Cell Mol. Biol. 2000, 23, 586–592. [Google Scholar] [CrossRef]
- Malhotra, R.; Ward, M.; Bright, H.; Priest, R.; Foster, M.R.; Hurle, M.; Blair, E.; Bird, M. Isolation and characterisation of potential respiratory syncytial virus receptor(s) on epithelial cells. Microbes Infect. 2003, 5, 123–133. [Google Scholar] [CrossRef]
- Johnson, T.R.; McLellan, J.S.; Graham, B.S. Respiratory syncytial virus glycoprotein G interacts with DC-SIGN and L-SIGN to activate ERK1 and ERK2. J. Virol. 2012, 86, 1339–1347. [Google Scholar] [CrossRef] [Green Version]
- Jeong, K.-I.; Piepenhagen, P.A.; Kishko, M.; Di Napoli, J.M.; Groppo, R.P.; Zhang, L.; Almond, J.; Kleanthous, H.; Delagrave, S.; Parrington, M. CX3CR1 Is Expressed in Differentiated Human Ciliated Airway Cells and Co-Localizes with Respiratory Syncytial Virus on Cilia in a G Protein-Dependent Manner. PLoS ONE 2015, 10, e0130517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Combadiere, C.; Gao, J.; Tiffany, H.; Murphy, P.M. Gene Cloning, RNA Distribution, and Functional Expression of mCX3CR1,a Mouse Chemotactic Receptor for the CX3C Chemokine Fractalkine. Biochem. Biophys. Res. Commun. 1998, 253, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Hieshima, K.; Haskell, C.; Baba, M.; Nagira, M.; Nishimura, M.; Kakizaki, M.; Takagi, S.; Nomiyama, H.; Schall, T.J.; et al. Identification and Molecular Characterization of Fractalkine Receptor CX3CR1, which Mediates Both Leukocyte Migration and Adhesion. Cell 1997, 91, 521–530. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Umehara, H.; Nakayama, T.; Yoneda, O.; Hieshima, K.; Kakizaki, M.; Dohmae, N.; Yoshie, O.; Imai, T. Dual Functions of Fractalkine/CX3C Ligand 1 in Trafficking of Perforin+/Granzyme B+Cytotoxic Effector Lymphocytes That Are Defined by CX3CR1 Expression. J. Immunol. 2002, 168, 6173–6180. [Google Scholar] [CrossRef] [Green Version]
- Bar-On, L.; Birnberg, T.; Lewis, K.L.; Edelson, B.T.; Bruder, D.; Hildner, K.; Buer, J.; Murphy, K.M.; Reizis, B.; Jung, S. CX3CR1 + CD8 + dendritic cells are a steady-state population related to plasmacytoid dendritic cells. Proc. Natl. Acad. Sci. USA 2010, 107, 14745–14750. [Google Scholar] [CrossRef] [Green Version]
- Corcione, A.; Ferretti, E.; Bertolotto, M.; Fais, F.; Raffaghello, L.; Gregorio, A. CX3CR1 is expressed by human B lymphocytes and mediates [corrected] CX3CL1 driven chemotaxis of tonsil centrocytes. PLoS ONE 2009, 4, e8485. [Google Scholar] [CrossRef] [Green Version]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef] [Green Version]
- Perros, F.; Dorfmüller, P.; Souza, R.; Durand-Gasselin, I.; Godot, V.; Capel, F.; Adnot, S.; Eddahibi, S.; Mazmanian, M.; Fadel, E.; et al. Fractalkine-induced smooth muscle cell proliferation in pulmonary hypertension. Eur. Respir. J. 2007, 29, 937–943. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Fuentes, S.; Salgado-Aguayo, A.; Arratia-Quijada, J.; Gorocica-Rosete, P. Regulation and biological functions of the CX3CL1-CX3CR1 axis and its relevance in solid cancer: A mini-review. J. Cancer 2021, 12, 571–583. [Google Scholar] [CrossRef]
- Lee, M.; Lee, Y.; Song, J.; Lee, J.; Chang, S.-Y. Tissue-specific Role of CX3CR1 Expressing Immune Cells and Their Relationships with Human Disease. Immune Netw. 2018, 18, e5. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Q.; Ou, J.; Zhang, S.; Ming, Y. Crosstalk between the CX3CL1/CX3CR1 Axis and Inflammatory Signaling Pathways in Tissue Injury. Curr. Protein Pept. Sci. 2019, 20, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Inokuchi, S.; Brenner, D.A.; Seki, E. CX3CL1-CX3CR1 interaction prevents carbon tetrachloride-induced liver inflammation and fibrosis in mice. Hepatology 2010, 52, 1390–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, Y.; Hoshino-Negishi, K.; Kuboi, Y.; Tago, F.; Yasuda, N.; Imai, T. Emerging Role of Fractalkine in the Treatment of Rheumatic Diseases. Immunotargets Ther. 2020, 9, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, P.; Ziemka-Nalecz, M.; Sypecka, J.; Zalewska, T. The Impact of the CX3CL1/CX3CR1 Axis in Neurological Disorders. Cells 2020, 9, 2277. [Google Scholar] [CrossRef] [PubMed]
- Darbandi-Tehrani, K.; Hermand, P.; Carvalho, S.; Dorgham, K.; Couvineau, A.; Lacapère, J.; Combadière, C.; Deterre, P. Subtle conformational changes between CX3CR1 genetic variants as revealed by resonance energy transfer assays. FASEB J. 2010, 24, 4585–4598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xia, T.; Meng, H.; Xue, M.; George, S.; Ji, Z.; Wang, X.; Liu, R.; Wang, M.; France, B.; et al. Differential Expression of Syndecan-1 Mediates Cationic Nanoparticle Toxicity in Undifferentiated versus Differentiated Normal Human Bronchial Epithelial Cells. ACS Nano 2011, 5, 2756–2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Bukreyev, A.; Thompson, C.I.; Watson, B.; Peeples, M.E.; Collins, P.L.; Pickles, R.J. Infection of Ciliated Cells by Human Parainfluenza Virus Type 3 in an In Vitro Model of Human Airway Epithelium. J. Virol. 2005, 79, 1113–1124. [Google Scholar] [CrossRef] [Green Version]
- Cortjens, B.; Yasuda, E.; Yu, X.; Wagner, K.; Claassen, Y.B.; Bakker, A.Q.; van Woensel, J.B.M.; Beaumont, T. Broadly Reactive Anti-Respiratory Syncytial Virus G Antibodies from Exposed Individuals Effectively Inhibit Infection of Primary Airway Epithelial Cells. J. Virol. 2017, 91, e02357-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, S.M.; McNally, B.A.; Ioannidis, I.; Flano, E.; Oomens, A.G.; Partida-Sanchez, S. Indentification of CX3CR1 as a cellular receptor for respiratory syncytial virus on primary well-differentiated human airway epithelial clutures. PLoS Pathog. 2015, 11, e1005318. [Google Scholar] [CrossRef] [Green Version]
- Anderson, L.J.; Bingham, P.; Hierholzer, J.C. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 1988, 62, 4232–4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Erp, E.A.; Luytjes, W.; Ferwerda, G.; van Kasteren, P.B. Fc-Mediated Antibody Effector Functions During Respiratory Syncytial Virus Infection and Disease. Front. Immunol. 2019, 10, 548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mekseepralard, C.; Toms, G.L.; Routledge, E.G. Protection of mice against Human respiratory syncytial virus by wild-type and aglycosyl mouse–human chimaeric IgG antibodies to subgroup-conserved epitopes on the G glycoprotein. J. Gen. Virol. 2006, 87, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Corbeil, S.; Seguin, C.; Trudel, M. Involvement of the complement system in the protection of mice from challenge with respiratory syncytial virus Long strain following passive immunization with monoclonal antibody 18A2B2. Vaccine 1996, 14, 521–525. [Google Scholar] [CrossRef]
- Hallak, L.K.; Spillmann, D.; Collins, P.L.; Peeples, M.E. Glycosaminoglycan Sulfation Requirements for Respiratory Syncytial Virus Infection. J. Virol. 2000, 74, 10508–10513. [Google Scholar] [CrossRef] [Green Version]
- Tayyari, F.; Hegele, R. Identifying targets in the hunt for effective respiratory syncytial virus interventions. Expert Rev. Respir. Med. 2012, 6, 215–222. [Google Scholar] [CrossRef]
- Clark, H.W. Untapped Therapeutic Potential of Surfactant Proteins: Is There a Case for Recombinant SP-D Supplementation in Neonatal Lung Disease? Neonatology 2010, 97, 380–387. [Google Scholar] [CrossRef]
- Watson, A.; Kronqvist, N.; Spalluto, C.M.; Griffiths, M.; Staples, K.; Wilkinson, T.; Holmskov, U.; Sørensen, G.L.; Rising, A.; Johansson, J.; et al. Novel expression of a functional trimeric fragment of human SP-A with efficacy in neutralisation of RSV. Immunobiology 2017, 222, 111–118. [Google Scholar] [CrossRef] [Green Version]
- Stokes, K.L.; Currier, M.G.; Sakamoto, K.; Lee, S.; Collins, P.L.; Plemper, R.K.; Moore, M.L. The Respiratory Syncytial Virus Fusion Protein and Neutrophils Mediate the Airway Mucin Response to Pathogenic Respiratory Syncytial Virus Infection. J. Virol. 2013, 87, 10070–10082. [Google Scholar] [CrossRef] [Green Version]
- Meng, J.; Hotard, A.L.; Currier, M.G.; Lee, S.; Stobart, C.C.; Moore, M.L. Respiratory Syncytial Virus Attachment Glycoprotein Contribution to Infection Depends on the Specific Fusion Protein. J. Virol. 2016, 90, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Haynes, L.M.; Jones, L.P.; Barskey, A.; Anderson, L.J.; Tripp, R.A. Enhanced Disease and Pulmonary Eosinophilia Associated with Formalin-Inactivated Respiratory Syncytial Virus Vaccination Are Linked to G Glycoprotein CX3C-CX3CR1 Interaction and Expression of Substance P. J. Virol. 2003, 77, 9831–9844. [Google Scholar] [CrossRef] [Green Version]
- Radu, G.U.; Caidi, H.; Miao, C.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J. Virol. 2010, 84, 9632–9636. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-J.; Lee, J.-Y.; Park, M.-H.; Kim, J.; Chang, J. Monoclonal Antibody against G Glycoprotein Increases Respiratory Syncytial Virus Clearance In Vivo and Prevents Vaccine-Enhanced Diseases. PLoS ONE 2017, 12, e0169139. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Klenow, L.; Coyle, E.M.; Golding, H.; Khurana, S. Protective antigenic sites in respiratory syncytial virus G attachment protein outside the central conserved and cysteine noose domains. PLoS Pathog. 2018, 14, e1007262. [Google Scholar] [CrossRef]
- Murawski, M.R.; McGinnes, L.W.; Finberg, R.W.; Kurt-Jones, E.A.; Massare, M.J.; Smith, G.; Heaton, P.M.; Fraire, A.E.; Morrison, T.G. Newcastle disease virus-like particles containing respiratory syncytial virus G protein induced protection in BALB/c mice, with no evidence of immunopathology. J. Virol. 2010, 84, 1110–1123. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Choi, Y.; Haynes, L.M.; Harcourt, J.L.; Anderson, L.J.; Jones, L.P.; Tripp, R.A. Vaccination To Induce Antibodies Blocking the CX3C-CX3CR1 Interaction of Respiratory Syncytial Virus G Protein Reduces Pulmonary Inflammation and Virus Replication in Mice. J. Virol. 2010, 84, 1148–1157. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.; Quan, F.-S.; Kwon, Y.; Sakamoto, K.; Kang, S.-M.; Compans, R.W.; Moore, M.L. Additive protection induced by mixed virus-like particles presenting respiratory syncytial virus fusion or attachment glycoproteins. Antivir. Res. 2014, 111, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Joo, D.-H.; Lee, J.-B.; Shim, B.-S.; Cheon, I.S.; Jang, J.-E.; Song, H.-H.; Kim, K.-H.; Song, M.K.; Chang, J. Dual Role of Respiratory Syncytial Virus Glycoprotein Fragment as a Mucosal Immunogen and Chemotactic Adjuvant. PLoS ONE 2012, 7, e32226. [Google Scholar] [CrossRef] [PubMed]
- Plotnicky-Gilquin, H.; Huss, T.; Aubry, J.-P.; Haeuw, J.-F.; Beck, A.; Bonnefoy, J.-Y.; Nguyen, T.N.; Power, U.F. Absence of Lung Immunopathology Following Respiratory Syncytial Virus (RSV) Challenge in Mice Immunized with a Recombinant RSV G Protein Fragment. Virology 1999, 258, 128–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Openshaw, P.J.M.; Clarke, S.L.; Record, F.M. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. Int. Immunol. 1992, 4, 493–500. [Google Scholar] [CrossRef]
- Yang, J.; Ma, C.; Zhao, Y.; Fan, A.; Zou, X.; Pan, Z. Hepatitis B Virus Core Particles Containing a Conserved Region of the G Protein Combined with Interleukin-35 Protected Mice against Respiratory Syncytial Virus Infection without Vaccine-Enhanced Immunopathology. J. Virol. 2020, 94, e00007-20. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.B.; Pryharski, K.S.; Yu, Q.; Boutilier, L.A.; Campeol, N.; Melville, K.; Laughlin, T.S.; Gupta, C.K.; Lerch, R.A.; Randolph, V.B.; et al. Characterization of Recombinant Respiratory Syncytial Viruses with the Region Responsible for Type 2 T-Cell Responses and Pulmonary Eosinophilia Deleted from the Attachment (G) Protein. J. Virol. 2004, 78, 8446–8454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparer, T.E.; Matthews, S.; Hussell, T.; Rae, A.J.; Garcia-Barreno, B.; Melero, J.A.; Openshaw, P.J.M. Eliminating a region of respiratory synctial virus attachment protein allows induction of protective immunity without vaccine-enhanced lung eosinophilia. J. Exp. Med. 1998, 187, 1921–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, T.R.; Teng, M.N.; Collins, P.L.; Graham, B.S. Respiratory Syncytial Virus (RSV) G Glycoprotein Is Not Necessary for Vaccine-Enhanced Disease Induced by Immunization with Formalin-Inactivated RSV. J. Virol. 2004, 78, 6024–6032. [Google Scholar] [CrossRef] [Green Version]
- Rey, G.U.; Miao, C.; Caidi, H.; Trivedi, S.U.; Harcourt, J.L.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Decrease in Formalin-Inactivated Respiratory Syncytial Virus (FI-RSV) Enhanced Disease with RSV G Glycoprotein Peptide Immunization in BALB/c Mice. PLoS ONE 2013, 8, e83075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, J.; Takeda, K.; Wang, M.; Zeng, W.; Jia, Y.; Shiraishi, Y.; Okamoto, M.; Dakhama, A.; Gelfand, E.W. Effects of Anti-G and Anti-F Antibodies on Airway Function after Respiratory Syncytial Virus Infection. Am. J. Respir. Cell Mol. Biol. 2014, 51, 143–154. [Google Scholar] [CrossRef] [Green Version]
- Caidi, H.; Miao, C.; Thornburg, N.J.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antivir. Res. 2018, 154, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Mavunda, K.; Krilov, L.R. Current State of Respiratory Syncytial Virus Disease and Management. Infect. Dis. Ther. 2021, 10, 1–12. [Google Scholar] [CrossRef]
- Caidi, H.; Harcourt, J.L.; Tripp, R.A.; Anderson, L.J.; Haynes, L.M. Combination Therapy Using Monoclonal Antibodies against Respiratory Syncytial Virus (RSV) G Glycoprotein Protects from RSV Disease in BALB/c Mice. PLoS ONE 2012, 7, e51485. [Google Scholar] [CrossRef]
- Boyoglu-Barnum, S.; Todd, S.O.; Meng, J.; Barnum, T.R.; Chirkova, T.; Haynes, L.M.; Jadhao, S.J.; Tripp, R.A.; Oomens, A.G.; Moore, M.L.; et al. Mutating the CX3C Motif in the G Protein Should Make a Live Respiratory Syncytial Virus Vaccine Safer and More Effective. J. Virol. 2017, 91, e02059-16. [Google Scholar] [CrossRef] [Green Version]
- Das, S.; Raundhal, M.; Chen, J.; Oriss, T.B.; Huff, R.; Williams, J.V.; Ray, A.; Ray, P. Respiratory syncytial virus infection of newborn CX3CR1-deficient mice induces a pathogenic pulmonary innate immune response. JCI Insight 2017, 2, e94605. [Google Scholar] [CrossRef] [Green Version]
- Johnson, C.H.; Miao, C.; Blanchard, E.G.; Caidi, H.; Radu, G.U.; Harcourt, J.L.; Haynes, L.M. Effect of Chemokine Receptor CX3CR1 Deficiency in a Murine Model of Respiratory Syncytial Virus Infection. Comp. Med. 2012, 62, 14–20. [Google Scholar]
- Ralston, S.; Hill, V. Incidence of Apnea in Infants Hospitalized with Respiratory Syncytial Virus Bronchiolitis: A Systematic Review. J. Pediatr. 2009, 155, 728–733. [Google Scholar] [CrossRef]
- Tripp, R.A.; Dakhama, A.; Jones, L.P.; Barskey, A.; Gelfand, E.W.; Anderson, L.J. The G Glycoprotein of Respiratory Syncytial Virus Depresses Respiratory Rates through the CX3C Motif and Substance P. J. Virol. 2003, 77, 6580–6584. [Google Scholar] [CrossRef] [Green Version]
- Tripp, R.A.; Moore, D.; Winter, J.; Anderson, L.J. Respiratory Syncytial Virus Infection and G and/or SH Protein Expression Contribute to Substance P, Which Mediates Inflammation and Enhanced Pulmonary Disease in BALB/c Mice. J. Virol. 2000, 74, 1614–1622. [Google Scholar] [CrossRef] [Green Version]
- Tripp, R.A.; Barskey, A.; Goss, L.; Anderson, L.J. Substance P receptor expression on lymphocytes is associated with the immune response to respiratory syncytial virus infection. J. Neuroimmunol. 2002, 129, 141–153. [Google Scholar] [CrossRef]
- Haynes, L.M.; Tonkin, J.; Anderson, L.J.; Tripp, R.A. Neutralizing Anti-F Glycoprotein and Anti-Substance P Antibody Treatment Effectively Reduces Infection and Inflammation Associated with Respiratory Syncytial Virus Infection. J. Virol. 2002, 76, 6873–6881. [Google Scholar] [CrossRef] [Green Version]
- Tognarelli, E.I.; Bueno, S.M.; González, P.A. Immune-Modulation by the Human Respiratory Syncytial Virus: Focus on Dendritic Cells. Front. Immunol. 2019, 10, 810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirkova, T.; Boyoglu-Barnum, S.; Gaston, K.A.; Malik, F.M.; Trau, S.P.; Oomens, A.G.P.; Anderson, L.J. Respiratory Syncytial Virus G Protein CX3C Motif Impairs Human Airway Epithelial and Immune Cell Responses. J. Virol. 2013, 87, 13466–13479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirkova, T.; Ha, B.; Rimawi, B.H.; Oomens, A.G.P.; Hartert, T.V.; Anderson, L.J. In vitro model for the assessment of human immune responses to subunit RSV vaccines. PLoS ONE 2020, 15, e0229660. [Google Scholar] [CrossRef] [Green Version]
- Shingai, M.; Azuma, M.; Ebihara, T.; Sasai, M.; Funami, K.; Ayata, M.; Ogura, H.; Tsutsumi, H.; Matsumoto, M.; Seya, T. Soluble G protein of respiratory syncytial virus inhibits Toll-like receptor 3/4-mediated IFN-beta induction. Int. Immunol. 2008, 20, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Levitz, R.; Gao, Y.; Dozmorov, I.; Song, R.; Wakeland, E.K.; Kahn, J.S. Distinct patterns of innate immune activation by clinical isolates of respiratory syncytial virus. PLoS ONE 2017, 12, e0184318. [Google Scholar] [CrossRef] [Green Version]
- Harcourt, J.; Alvarez, R.; Jones, L.P.; Henderson, C.; Anderson, L.J.; Tripp, R.A. Respiratory Syncytial Virus G Protein and G Protein CX3C Motif Adversely Affect CX3CR1+T Cell Responses. J. Immunol. 2006, 176, 1600–1608. [Google Scholar] [CrossRef] [Green Version]
- Melendi, G.A.; Bridget, D.; Monsalvo, A.C.; Laham, F.F.; Acosta, P.; Delgado, M.F.; Polack, F.P.; Irusta, P.M. Conserved cysteine residues within the attachment G glycoprotein of respiratory syncytial virus play a critical role in the enhancement of cytotoxic T-lymphocyte responses. Virus Genes 2010, 42, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Zeng, R.-H.; Gong, W.; Fan, C.-F.; Wang, Y.-F.; Mei, X.-G. Induction of balanced immunity in BALB/c mice by vaccination with a recombinant fusion protein containing a respiratory syncytial virus G protein fragment and a CTL epitope. Vaccine 2006, 24, 941–947. [Google Scholar] [CrossRef]
- Zhivaki, D.; Lemoine, S.; Lim, A.; Morva, A.; Vidalain, P.-O.; Schandene, L.; Casartelli, N.; Rameix-Welti, M.-A.; Hervé, P.-L.; Dériaud, E.; et al. Respiratory Syncytial Virus Infects Regulatory B Cells in Human Neonates via Chemokine Receptor CX3CR1 and Promotes Lung Disease Severity. Immunity 2017, 46, 301–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, E.C.; Barber, J.; Tripp, R.A. Respiratory syncytial virus (RSV) attachment and nonstructural proteins modify the type I interferon response associated with suppressor of cytokine signaling (SOCS) proteins and IFN-stimulated gene-15 (ISG15). Virol. J. 2008, 5, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakre, A.A.; Harcourt, J.L.; Haynes, L.M.; Anderson, L.J.; Tripp, R.A. The Central Conserved Region (CCR) of Respiratory Syncytial Virus (RSV) G Protein Modulates Host miRNA Expression and Alters the Cellular Response to Infection. Vaccines 2017, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Oshansky, C.M.; Barber, J.P.; Crabtree, J.; Tripp, R.A. Respiratory Syncytial Virus F and G Proteins Induce Interleukin 1α, CC, and CXC Chemokine Responses by Normal Human Bronchoepithelial Cells. J. Infect. Dis. 2010, 201, 1201–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.S.; Chirkova, T.; Slaunwhite, C.G.; Qiu, X.; Walsh, E.E.; Anderson, L.J.; Mariani, T.J. CX3CR1 Engagement by Respiratory Syncytial Virus Leads to Induction of Nucleolin and Dysregulation of Cilium-Related Genes. J. Virol. 2021, 95. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anderson, L.J.; Jadhao, S.J.; Paden, C.R.; Tong, S. Functional Features of the Respiratory Syncytial Virus G Protein. Viruses 2021, 13, 1214. https://doi.org/10.3390/v13071214
Anderson LJ, Jadhao SJ, Paden CR, Tong S. Functional Features of the Respiratory Syncytial Virus G Protein. Viruses. 2021; 13(7):1214. https://doi.org/10.3390/v13071214
Chicago/Turabian StyleAnderson, Larry J., Samadhan J. Jadhao, Clinton R. Paden, and Suxiang Tong. 2021. "Functional Features of the Respiratory Syncytial Virus G Protein" Viruses 13, no. 7: 1214. https://doi.org/10.3390/v13071214




