Abstract
In the context of climate change, elevated temperature is a major concern due to the impact on plant–pathogen interactions. Although atmospheric temperature is predicted to increase in the next century, heat waves during summer seasons have already become a current problem. Elevated temperatures strongly influence plant–virus interactions, the most drastic effect being a breakdown of plant viral resistance conferred by some major resistance genes. In this work, we focused on the R-BPMV gene, a major resistance gene against Bean pod mottle virus in Phaseolus vulgaris. We inoculated different BPMV constructs in order to study the behavior of the R-BPMV-mediated resistance at normal (20 °C) and elevated temperatures (constant 25, 30, and 35 °C). Our results show that R-BPMV mediates a temperature-dependent phenotype of resistance from hypersensitive reaction at 20 °C to chlorotic lesions at 35 °C in the resistant genotype BAT93. BPMV is detected in inoculated leaves but not in systemic ones, suggesting that the resistance remains heat-stable up to 35 °C. R-BPMV segregates as an incompletely dominant gene in an F2 population. We also investigated the impact of elevated temperature on BPMV infection in susceptible genotypes, and our results reveal that elevated temperatures boost BPMV infection both locally and systemically in susceptible genotypes.
1. Introduction
Among all plant diseases, viruses account for about half of all known pathogens [1] and constitute particular entities, non-living organisms that are obligate and intra-cellular parasites that need a live host for replication. Plant viruses are biotic pathogens that cause serious epidemics in major crops with annual yield losses of more than $30 billion [2]. No effective pesticide-based control measures are effective against viruses, so the most reliable method of plant protection is increasing plant genetic resistance [2]. Plant resistance relies on different immune responses. First, recognition of viral double-stranded RNAs is a major mechanism in antiviral plant defense [3] that induces both RNA silencing [4] and pattern-triggered immunity [5,6,7]. Viruses have evolved viral suppressors of these responses in order to promote their own replication [8,9,10,11]. Second, plant effector-triggered immunity (ETI) is induced by the recognition of virus effectors by resistance (R) proteins mostly represented by nucleotide-binding domain leucin-rich repeat containing receptors (NLRs) [12]. NLR receptors are intracellular and encoded by dominant genes [13]. Many R genes against viruses have been identified so far, as well as the corresponding viral-encoded effectors that are subsequently referred to as avirulence (Avr) factors [14,15]. Other types of resistances are also effective against viruses such as those controlled by quantitative trait loci [16] or ‘recessive genes’, the latter ones encoding mutated or truncated host factors that are hijacked by viruses in their wild type forms for their life cycle [17].
Plant resistance is strongly affected by environmental conditions, and temperature is undoubtedly one of the key parameters that have a major impact on worldwide plant production. It is well known that elevated temperatures influence plant–virus interactions, as well as the timing and severity of disease epidemics [18,19,20], but little is known about the associated molecular mechanisms in plant–pathogen interactions [21]. The more drastic impact of elevated temperatures is the breakdown of plant viral resistance conferred by major R genes [18]. Indeed, some R genes have been shown to be overcome at high temperatures, usually around 28–30 °C, allowing viral infection and spreading at the whole-plant level. This is the case for the N-mediated resistance that occurs only at temperatures below 28 °C in Nicotiana tabacum [22] and for the Tsw-gene-mediated resistance that is overcome between 25 and 30 °C in Capsicum species [23]. Conversely, for other R genes, resistance is still efficient at temperatures above 28–30 °C, such as, for example, the Rx-mediated resistance that is not compromised against Potato virus X (PVX, Potexvirus) at temperatures up to 32 °C [24]. Alternatively, elevated temperatures may also induce more mild effects on plant resistance conferred by R genes. Indeed, the resistance phenotype can be modified, meaning that local necrotic lesions such as hypersensitive reaction (HR, a form of programmed cell death [25]) can be transformed into systemic necrosis. Additionally, extreme resistance, which inhibits virus replication without apparent HR, can be shifted to HR or even to systemic necrosis at temperatures above 28–30 °C. In the absence of R genes, elevated temperature was shown to promote symptom severity, systemic spreading, and replication potential of viruses [26,27,28]. Other studies reported contradictory results in the sense that elevated temperatures reduced viral symptoms and viral accumulation [29,30,31], which was hypothesized to be attributable to increased efficiency of the RNA silencing pathway at elevated temperature [32]. Therefore, predicting a general outcome for all the pathosystems may be difficult.
Common bean (Phaseolus vulgaris L.) is a major pulse crop of agronomic importance cultivated as a dry grain or fresh vegetable. Indeed, it is the most important grain legume for human consumption worldwide, especially in developing countries such as Central and South America and Southeastern Africa [33]. Common bean varieties are grown over a wide range of latitudes but optimal growth conditions need temperatures comprised between 17.5 and 23 °C [34,35]. Indeed, daytime temperatures above 30 °C and nighttime temperatures above 22 °C lead to yield losses [36,37].
In the main production areas of common bean, several viruses including Potyviruses Bean common mosaic virus (BCMV), Bean common mosaic necrosis virus (BCMNV), Bean yellow mosaic virus (BYMV), and Clover yellow vein virus (ClYVV) affect the quality and quantity of bean productions (reviewed in [38]). The well-known I locus, located at the extremity of chromosome 2, confers resistance to a large part of them. In addition to resistance against Potyviruses, resistance to Comovirus has also been positioned in the region of the I locus [39,40]. This is the case of the R-BPMV gene conferring resistance to Bean pod mottle virus (BPMV) in P. vulgaris genotype BAT93 [41].
The aim of the current study was to address several questions concerning the R-BPMV-mediated resistance in P. vulgaris. Does the R-BPMV-mediated resistance to BPMV depend on temperature? If so, at which temperature does the phenotype switch occur? What is the inheritance of the R-BPMV gene? Here, we report that R-BPMV induces HR lesions at 20 °C. Further analysis revealed that at 25 and 30 °C, R-BPMV-mediated resistance still induces HR lesions, whereas at 35 °C, local HR lesions are replaced by chlorotic lesions but resistance is not overcome at the whole-plant level. Finally, we also investigated the impact of elevated temperature on BPMV infection in a compatible context (i.e., in susceptible genotypes), and our results highlighted that rising temperature boosts BPMV infection.
2. Materials and Methods
2.1. Common Bean Material and Growing Conditions
The following genotypes of Phaseolus vulgaris were used in this study: BAT93 (Mesoamerican breeding line), JaloEEP558, Black Valentine (both Andean landraces), and two Near-Isogenic Lines for the I locus [42], Black Turtle 1 (BT-1; I/I, resistant to BCMV and BCMNV) and Black Turtle 2 (BT-2; i/i susceptible to BCMV and BCMNV). The inheritance of R-BPMV was studied using 60 F2 individuals derived from a cross between BAT93 (R-BPMV/R-BPMV, resistant to BPMV) and the Andean landrace JaloEEP558 (r-bpmv/r-bpmv, susceptible to BPMV).
Growing conditions from sowing to virus inoculation were followed as described in Pflieger et al. [41] with some modifications. Briefly, seeds were sown in soil instead of vermiculite and grown in a growth chamber at 23 °C under a 16 h light/8 h dark cycle and 75% relative humidity until the BPMV-inoculation stage (fully expanded primary leaf stage, 10 days post-sowing in our growth conditions). From sowing to inoculation, seedlings were watered with tap water.
2.2. Viral Material
An infectious BPMV cDNA clone derived from isolate IA-Di1 was provided by C. Zhang and S. Whitham (Iowa State University, Ames, IA, USA) and described previously [43]. Briefly, the infectious clone BPMV-WT contains the WT-RNA1 (on infectious plasmid ‘pBPMV-IA-R1M’) and the WT-RNA2 (infectious plasmid ‘pBPMV-IA-V1’) of BPMV [43]. We also used the GFP-tagged BPMV (BPMV-GFP, infectious plasmids ‘pBPMV-IA-R1M’ + ‘pBPMV-GFP2’) [43] in which a GFP cassette was inserted in the frame between the movement protein (MP) and the large-coat protein (L-CP) coding regions and was flanked with protease recognition sites, allowing excision from the RNA2 polyprotein.
2.3. Viral Rub-Inoculation of P. vulgaris Plants and High-Temperature Assays
Viral rub-inoculation of P. vulgaris plants was performed as described previously [41,44]. Briefly, a viral inoculum was prepared by grinding frozen or fresh infected leaves from P. vulgaris cv. Black Valentine with a mortar and pestle in the presence of a mock buffer (potassium phosphate buffer 0.1 M, pH 7) [44]. Mechanical inoculation was then performed on one primary leaf of a healthy plant, using carborundum as an abrasive [44]. Inoculated plants were then placed in a growth chamber either at constant 20 °C (control temperature) or at constant 25, 30, or 35 °C (high temperature assays) in a growth chamber Aralab (Fitoclima 1.200, Rio de Mouro, Portugal) under 75% constant humidity at 16/8 h light/dark condition. After inoculation, plants were watered with a nutritive solution. Each experiment was reproduced at least twice.
2.4. Cell Death Assays
For HR assays, the leaves of 7 days post-inoculation (dpi) plants were stained with trypan blue in lactophenol solution (lactic acid:glycerol:liquid phenol:distilled water (1:1:1:1), 0.067% w/v trypan blue) in universal tubes and heated in a boiling water bath for 2 min. After cooling, the solution was replaced with chloral hydrate (2.5 g/mL), and samples were shaken until leaves were fully destained. For observations, the chloral hydrate was replaced with 60% glycerol.
2.5. Detection of GFP Fluorescence in Planta
GFP fluorescence in whole plants was detected by using a Black Ray long-wave UV lamp (high intensity 100-Watt long-wave UV lamp; UVP, Upland, CA, USA). Higher-magnification fluorescence detection was performed with an epifluorescence microscope for temperature assays at 20 and 25 °C (Leica MZ16F, Leica Microsystems GmBH, Wetzlar, Germany) equipped with a fluorescein isothiocyanate–tetramethyl rhodamine isothiacyanate multiband filter. The GFP fluorescence specter was checked using a confocal microscope (Zeiss LMS880, Carl Zeiss Microscopy GmBH, Iena, Germany). For temperature assays at 30 °C, we used an Axio Zoom V16 fluorescence stereomicroscope (Carl Zeiss Microscopy GmBH, Iena, Germany). Specific GFP fluorescence was detected using a short-pass filter (excitation filter, 470/40 nm; barrier filter, 525/50 nm). To detect all UV-fluorescent cell components including chlorophyll and GFP, a long-pass filter (excitation filter, 572/25 nm; barrier filter, 629/62 nm) was used. Images were obtained using a digital video camera (Axiocam 506 mono) coupled to the Axio Zoom microscope.
2.6. RNA Isolation and RT-PCR Analyses
For BPMV RNA detection, inoculated and systemic leaves from BPMV-inoculated plants were sampled at 7 and 14 or 21 dpi, respectively. Total RNA was extracted using the NucleoSpin RNA kit (Macherey-Nagel, Hœrdt, France). RNA concentrations were determined by measuring the absorbance at 260 nm on a NanoDrop 8000 (Thermo Fisher Scientific, Waltham, MA, USA) and integrity was checked by electrophoresis on a 1% agarose BET gel. cDNA was synthesized from 1 µg of total RNA using Reverse Transcription (RT) ImProm-IITM enzyme (Promega Corp., Madison, USA) and Oligo-dT (Promega Corp., Madison, WI, USA) according to the manufacturer’s protocol and finally diluted 2.5 times in Milli-Q H2O.
Semi-quantitative RT-PCR was performed on 1 µL of diluted cDNA using GoTaq G2 Flexi (Promega Corp., Madison, USA) and using primers specific to BPMV RNA1, RNA2, PvUBIQUITIN (PvUBI, reference gene), and PvINSULIN-DEGRADING ENZYME (PvIDE) (reference gene; primers IDE-F 5′-GCAACCAACCTTTCATCAGC-3′ and IDE-R 5′-AGAAATGCCTCAACCCTTTG-3′), as described previously [41]. After either 20 cycles for primers RNA1 and RNA2 or 25 cycles for primers PvUBI and PvIDE, PCR products were analyzed by electrophoresis on a 2% agarose BET gel.
BPMV virus titer was estimated using a method based on a quantitative RT-PCR (RT-qPCR) analysis by determining the quantity of BPMV RNA1 and plant PvIDE mRNA, in four biological and three technical replicates (unless otherwise stated), in order to obtain a ratio of virus RNA to plant RNA. RT-qPCR protocol and analyses were performed as described in Richard et al. [45]. Briefly, RT-qPCR analysis was performed with a LightCycler® 96 instrument in a volume of 15 µL reaction containing 2 µL of diluted cDNA, each specific primer with a final concentration of 0.1 µM, 7.5 µL of SYBR Green (LightCycler® 480 SYBR Green I Master, Roche), and distilled water. The program used consisted of initial denaturation at 95 °C for 5 min and 50 cycles of 15 s of denaturation, 15 s of hybridization, and 15 s of elongation at 95, 60, and 72 °C, respectively. The results were analyzed using LightCycler® 96 software version 1.1.
3. Results
3.1. R-BPMV-Mediated Resistance Is Associated with Local HR Lesions at 20 °C Where BPMV Is Able to Multiply in a First Step
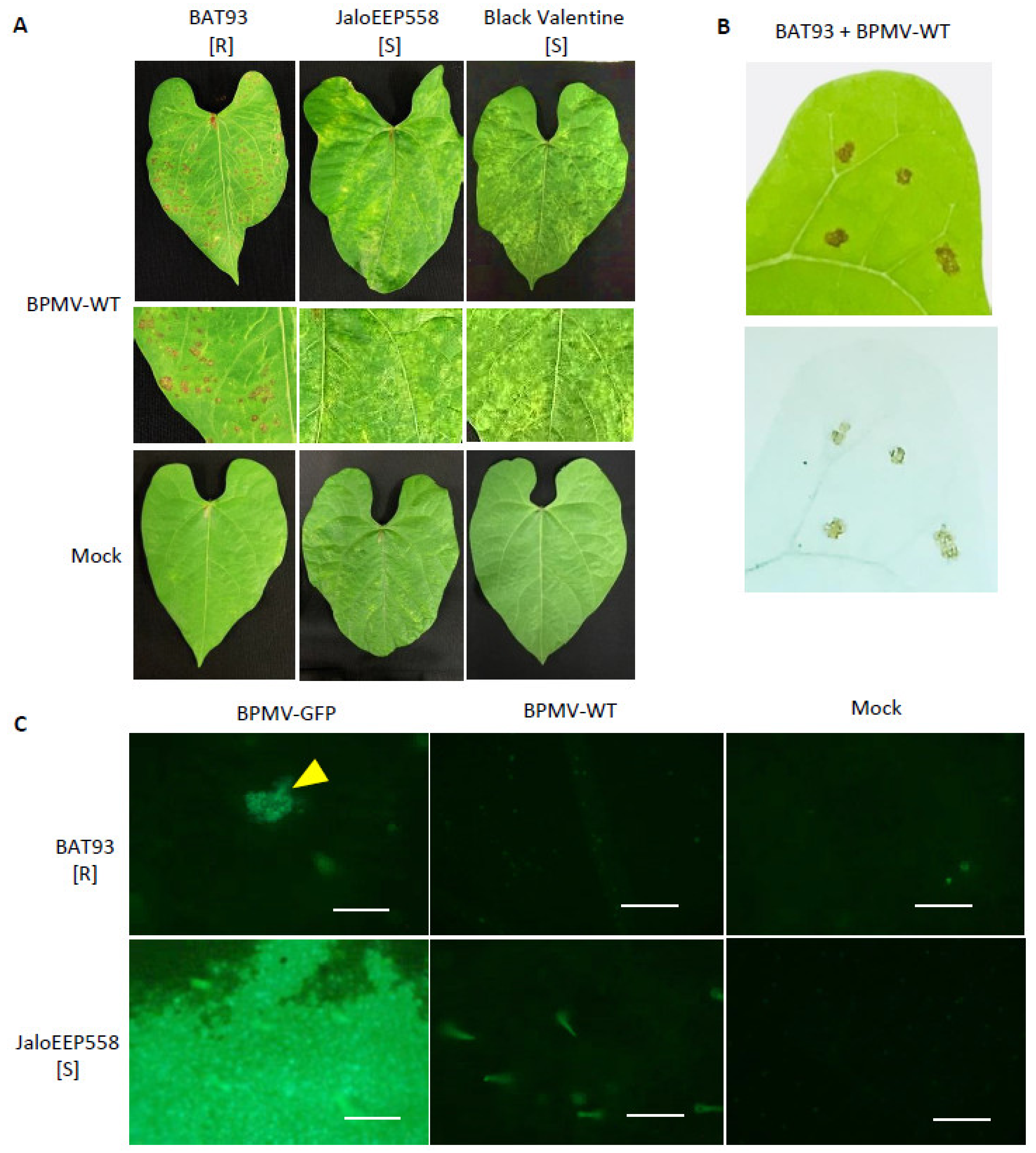
In previous work, we have shown that the Mesoamerican genotype BAT93 is resistant to BPMV and that resistance is controlled by a major gene, named R-BPMV [41]. In order to further characterize the BAT93 resistance phenotype, we performed inoculation assays using BPMV-WT and BPMV-GFP, a BPMV construct expressing GFP as a mature protein processed from the viral RNA2 polyprotein. We observed that at 7 dpi with BPMV-WT, a large number of macroscopic HR lesions developed in the inoculated leaves of BAT93 at 20 °C, whereas mosaic symptoms were visible in JaloEEP558 and Black Valentine, two susceptible genotypes lacking the R-BPMV gene (Figure 1A). Trypan blue staining assays at 7 dpi confirmed that HR lesions contained death cells (Figure 1B), thus attesting that these cells have undergone a cell death process induced by the R-BPMV gene. In order to study if BPMV can multiply in some individual cells before the establishment of HR lesions, we scanned the upper surface of BPMV-GFP-inoculated leaves of BAT93 at 4 dpi using an epifluorescence microscope. JaloEEP558 was used as a susceptible genotype. Interestingly, GFP fluorescence was detected in both BAT93 and JaloEEP558 at 4 dpi in inoculated leaves (Figure 1C). Widespread fluorescence was observed in JaloEEP558 leaves, whereas in BAT93, GFP fluorescence was found concentrated in a number of discrete areas that we will further call ‘GFP foci’ (Figure 1C). This suggests that resistance conferred by R-BPMV in BAT93 allows BPMV multiplication in small cell clusters but finally restricts virus spread, putatively by blocking virus cell-to-cell movement and/or inducing programmed cell death.
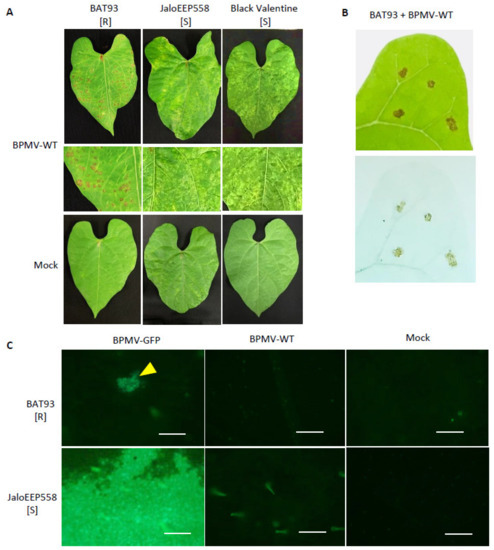
Figure 1.
BPMV is able to multiply and move from cell-to-cell in the inoculated leaves of genotype BAT93 at 20 °C but infection is finally restricted by the induction of local Hypersensitive-Response (HR) lesions: (A) Representative pictures of local HR lesions on six BAT93-inoculated leaves of six different plants grown at 20 °C, 7 days post-inoculation (dpi) with BPMV-WT. JaloEEP558 and Black Valentine were used as susceptible control genotypes on which mosaic/mottling symptoms are visible on the inoculated leaves at 7 dpi with BPMV-WT. Mock was used as control. This experiment was performed at least 3 times with similar results. (B) Local HR lesions (top) and lesions visualized using trypan blue staining (bottom) on BAT93 leaves inoculated with BPMV-WT at 20 °C. Pictures were taken at 7 dpi. (C) Microscopic observations of GFP fluorescence in six inoculated leaves of six different plants of either BAT93 or JaloEEP558, both grown at 20 °C. Observations were made at 4 dpi. The BPMV-GFP construct expressing GFP as an individual protein processed from the RNA2 polyprotein was used as a reporter of BPMV infection. BPMV-WT and Mock were used as control. Yellow triangle indicates GFP foci. Autofluorescence is visible with BPMV-WT and Mock assays. All infection assays were made at 20 °C. Scale bars = 250 μm.
3.2. The R-BPMV Gene Segregates as an Incompletely Dominant Gene
To determine the inheritance of R-BPMV, we investigated the segregation of resistance versus susceptibility to BPMV in an F2 population of 60 plants derived from the cross BAT93 (resistant to BPMV) xJaloEEP558 (susceptible to BPMV). Each F2 plant was inoculated with the construct BPMV-GFP, and resistant/susceptible phenotypes were scored at 7 to 10 dpi. The observed segregation ratio was 56 resistant: 4 susceptible. The goodness-of-fit test indicated a significant deviation (χ21df = 10.74, p = 0.001) from the expected Mendelian ratio (3:1) with a deficit of susceptible phenotypes. Thus, the R-BPMV gene segregates as an incompletely dominant gene.
3.3. At 25 and 30 °C, R-BPMV Induces More Expanded Local HR Lesions
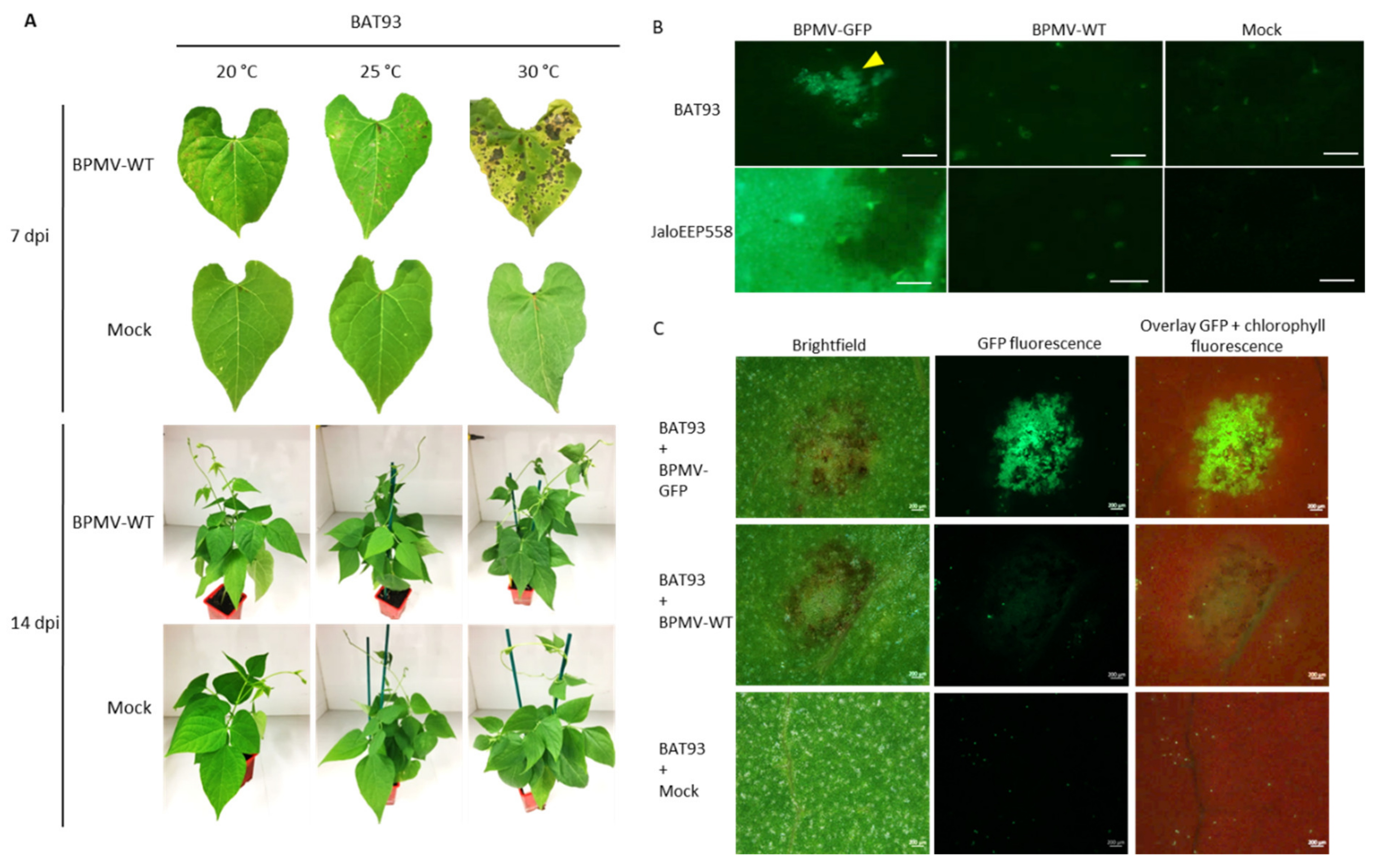
In previous work, we showed that R-BPMV is closely linked to the I gene at one end of chromosome 2 of P. vulgaris [41]. It is known that I-mediated resistance is associated with a systemic necrosis that may kill the host when infected by either BCMV above 28 °C or BCMNV regardless of the temperature [46,47,48]. Thus, the resistance phenotype conferred by the I gene in response to BCMV infection is temperature-dependent and switches at 28 °C from extreme resistance to systemic necrosis. In order to check if the resistance mediated by R-BPMV is dependent on temperature, we studied the resistance phenotype at 25 and 30 °C. We first performed inoculation assays using BPMV-WT on the resistant genotype BAT93. We observed that the resistance phenotype is expressed as local HR lesions in the inoculated leaves at all tested temperatures but that HR lesions become more expanded as the temperature increases (Figure 2A). Nevertheless, no vascular systemic necrosis appears at 14 dpi in apical parts of the inoculated plants (Figure 2A). Moreover, no viral symptoms were visible in these apical parts (Figure 2A) and no viral RNAs were detected in systemic leaves of BAT93-inoculated plants at all temperatures (Figure S1). Thus, in our pathosystem, BPMV was blocked in the inoculated leaves and could not spread systemically at all tested temperatures (20, 25, and 30 °C). In conclusion, no switch of phenotype was observed in BAT93 when inoculated with BPMV at either 25 or 30 °C.
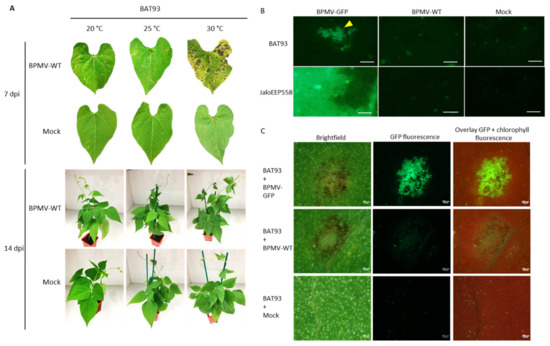
Figure 2.
Elevated temperatures promote BPMV multiplication and cell-to-cell movement in the inoculated leaves of cv. BAT93 and larger local Hypersensitive-Response lesions are induced: (A) Representative pictures of local HR lesions on six BAT93-inoculated leaves from six different plants at 20 °C, 25 °C and 30 °C, at 7 days post-inoculation (dpi) with BPMV-WT (upper panel) and six whole plants of BAT93 at 20 °C, 25 °C and 30 °C, 14 dpi with BPMV-WT (lower panel). Mock was used as control. This experiment was performed at least 3 times with similar results. (B) Microscopic observations of BPMV-GFP accumulation in inoculated leaves of BAT93 and JaloEEP558 grown at 25 °C. The BPMV-GFP construct expressing GFP as an individual protein processed from the RNA2 polyprotein was used as a reporter of BPMV accumulation. BPMV-WT and Mock were used as control. All infection assays were made at 25 °C. Observations of GFP fluorescence (indicated by a yellow triangle) were made at 4 dpi and are representative of six BAT93-inoculated leaves sampled on six different plants. Scale bars = 250 μm. (C) Microscopic observations of BPMV-GFP accumulation in inoculated leaves of BAT93 grown at 30 °C. The BPMV-GFP construct expressing GFP as an individual protein processed from the RNA2 polyprotein was used as a reporter of BPMV accumulation. From left to right: brightfield, BPMV-GFP accumulation (green), overlay of chlorophyll fluorescence (red) and BPMV-GFP accumulation (green). BPMV-WT and Mock were used as control. All infection assays were made at 30 °C. Observations of GFP fluorescence were made at 4 dpi and are representative of six BAT93-inoculated leaves sampled on six different plants. Scale bars = 200 μm.
In order to assess if larger HR lesions at elevated temperature were correlated with a more expanded multiplication area of BPMV, we scanned the upper surface of the BPMV-GFP-inoculated leaves of BAT93 at 4 dpi using an epifluorescence microscope (25 °C) or Axio Zoom microscope (30 °C). JaloEEP558 was used as a susceptible genotype [41]. As expected, at 4 dpi, many GFP foci were detected on the upper surface of BPMV-GFP inoculated leaves of BAT93 at 25 and 30 °C (Figure 2B,C). Overall, the GFP foci approximately doubled when shifting from 20 to 25 °C and from 25 to 30 °C. Thus, BPMV is still able to multiply and to move from cell-to-cell in BAT93 at 25 and 30 °C. Consequently, the plant defense response mediated by R-BPMV seems to become less efficient when temperature increases from 20 to 30 °C as larger GFP foci are observed.
3.4. The Resistance Mediated by R-BPMV Is Heat-Stable up to 35 °C in BAT93, But Local HR Lesions Are Replaced by Chlorotic Lesions
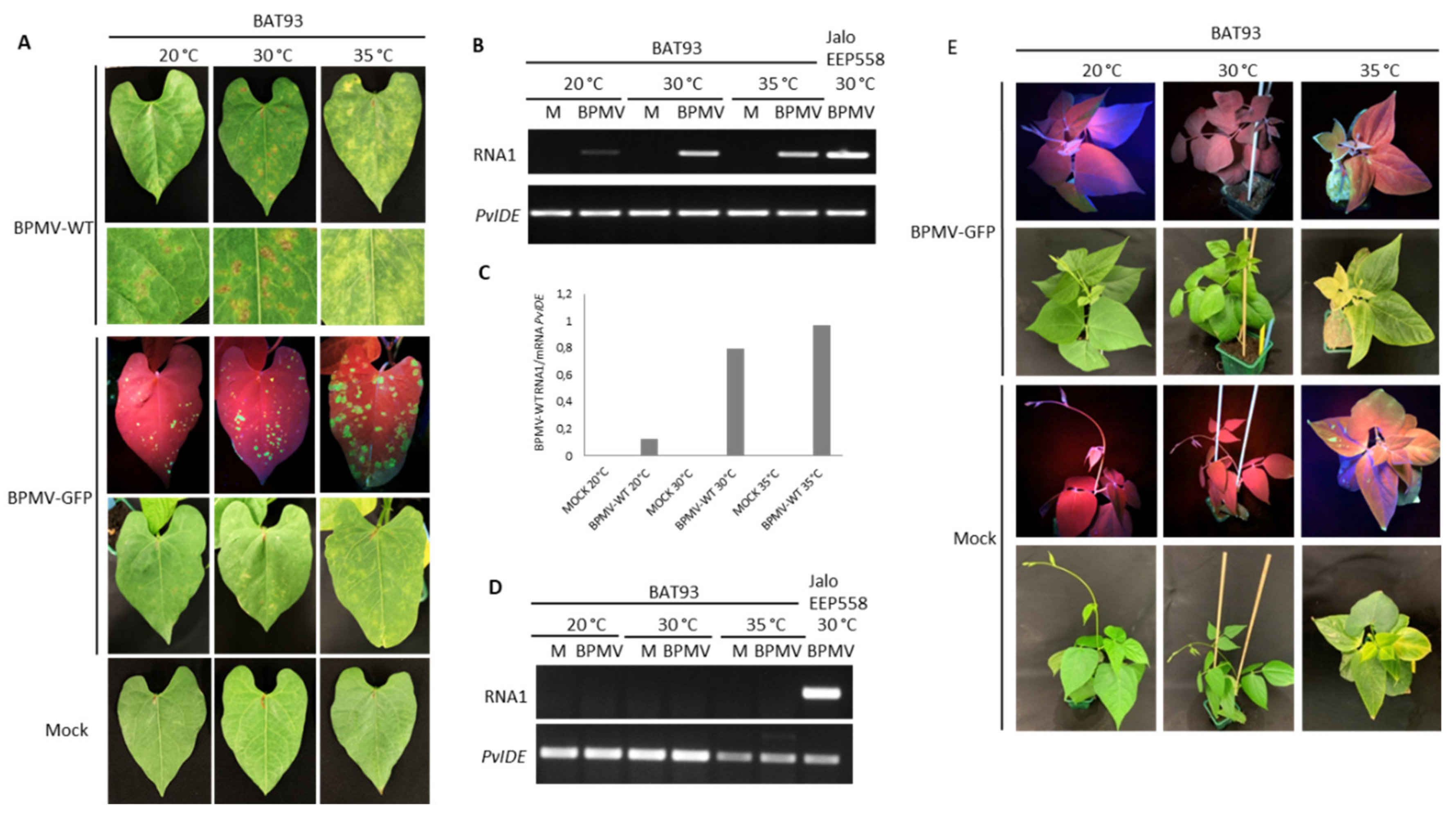
It is known that some dominant R genes against viruses are overcome at elevated temperatures (e.g., at 28 °C for N or at 30 °C for Tsw) [22,23,49], meaning that the resistance status of the genotype is broken and systemic infection of upper leaves occurs, leading to a susceptible state. We found that R-BPMV-mediated resistance was still efficient at temperatures up to 30 °C, but we wanted to test whether higher temperatures (35 °C) could break the resistance conferred by R-BPMV. For this purpose, we inoculated BAT93 plants with BPMV-WT and BPMV-GFP and placed the plants at constant 35 °C. As expected, at 7 dpi, HR lesions developed on inoculated leaves of BAT93 + BPMV-WT at 30 °C, and GFP fluorescence was macroscopically detected on inoculated leaves of BAT93 + BPMV-GFP (Figure 3A). By contrast, at 35 °C, only chlorotic lesions (i.e., light-green or yellow local lesions with no visible cell death/necrosis) were visible at 7 dpi on inoculated leaves of BAT93 + BPMV-WT. This appears to correspond to large infection areas, as confirmed by expanded GFP foci visible in inoculated leaves of BAT93 + BPMV-GFP (Figure 3A). Quantification of virus titer by RT-PCR in the inoculated leaves of BAT93 confirmed a higher accumulation of viral RNAs at 35 °C compared to 30 and 20 °C (Figure 3B,C).
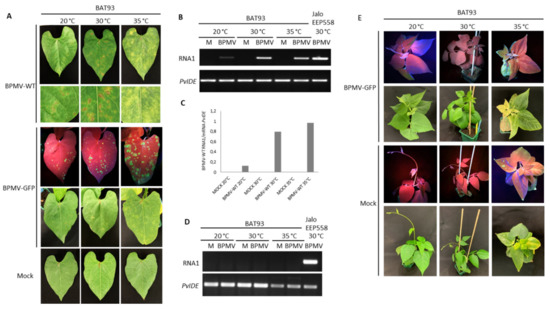
Figure 3.
The R-BPMV gene of BAT93 is still efficient to confer BPMV resistance at 35 °C: (A) Representative pictures of BAT93-inoculated leaves at 20 °C, 30 °C and 35 °C, 7 days post-inoculation (dpi) with BPMV-WT (upper panel; six plants), BPMV-GFP (middle panel; six plants) and Mock (lower panel; six plants). BPMV-GFP was detected under UV light. This experiment was performed at least three times with similar results. (B) Semi-quantitative RT-PCR of BPMV RNA1 (upper panel) in BAT93-inoculated leaves. Plants were inoculated with either mock (M) buffer or BPMV-WT (BPMV) at 20 °C, 30 °C and 35 °C. PvIde was used as an internal control. Total RNA was extracted at 7 dpi from a pool of three BAT93-inoculated leaves sampled on three different plants. A sample of inoculated leaves of JaloEEP558 at 30 °C was taken as positive control for PCR amplification. (C) Quantification of BPMV titer at 7 dpi in BPMV-WT-inoculated leaves of BAT93 by calculation of the relative ratio of BPMV RNA1 to plant mRNA of PvIde using a quantitative RT-PCR procedure. Data are mean ratios of pools of three BAT93-inoculated leaves sampled on three different plants. (D) Semi-quantitative RT-PCR of BPMV RNA1 (upper panel) in systemic leaves of BAT93 plants inoculated with either mock (M) buffer or BPMV-WT (BPMV) at 20 °C, 30 °C and 35 °C. PvIde was used as an internal control. Total RNA was extracted at 14 dpi from a pool of two systemic leaves (third and fourth trifoliate) sampled on two-three different plants of BAT93. A sample of inoculated leaves of JaloEEP558+BPMV-WT at 30 °C was taken as positive control for PCR amplification. This experiment was performed twice with similar results. (E) Representative pictures of six whole plants of BAT93 at 20 °C, 30 °C and 35 °C, 11 dpi with either BPMV-GFP (upper panel) or mock buffer (lower panel). BPMV-GFP was detected under UV light.
To ensure that no virus moved systemically, we studied the systemic leaves of BAT93 plants grown at 35 °C and inoculated with either BPMV-WT or BPMV-GFP. No viral RNAs were detected in systemic leaves at 7 dpi, and no GFP fluorescence was visible (Figure 3D,E), attesting to the fact that although local infection is of higher intensity, the systemic infection is prevented. Furthermore, no systemic necrosis occurred (Figure 3E). In parallel, we used the susceptible genotype Black Valentine as a control to assess if at 35 °C, BPMV-GFP conserved infectiousness and capacity for systemic movement. We observed that GFP fluorescence was widespread in all systemic leaves of Black Valentine plants at 35 °C 7 dpi, including stems, while no GFP was detected in the systemic leaves of plants grown at 20 °C (Figure S2). Overall, our results demonstrate that R-BPMV is heat-stable up to 35 °C. The R-BPMV-mediated phenotype is expressed as chlorotic lesions in the inoculated leaves, and no symptoms are detected in the systemic parts.
3.5. Elevated Temperatures Boost BPMV Infection in Susceptible Genotypes
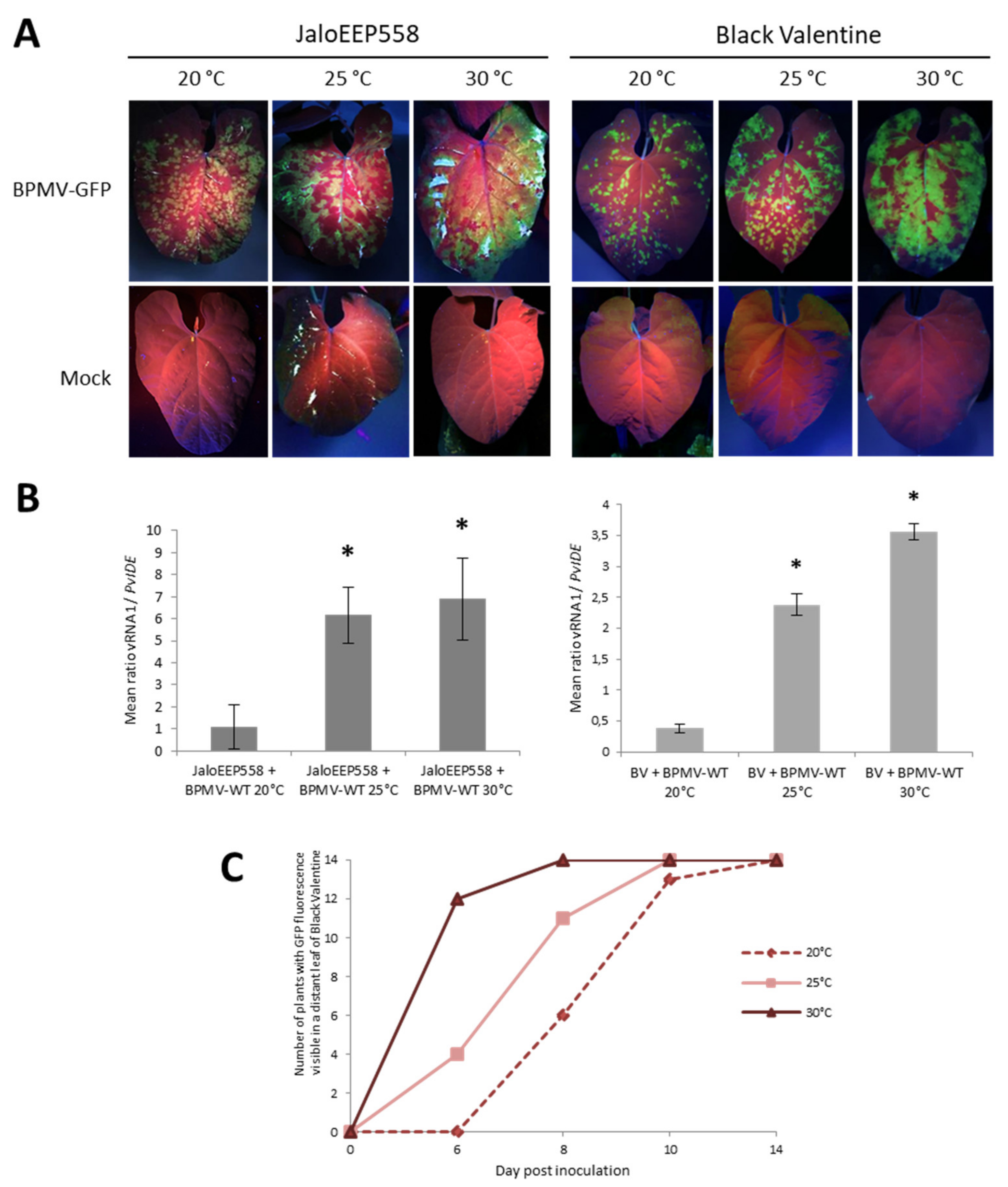
In the resistant genotype BAT93, elevated temperatures (especially 35 °C) promoted viral replication and cell-to-cell movement in the inoculated leaves (Figure 3A). Therefore, we investigated the effect of elevated temperatures on BPMV infection in the two susceptible genotypes, JaloEEP558 and Black Valentine. We followed the BPMV-GFP accumulation by visual inspection of plants inoculated with BPMV-GFP grown either at 25 or 30 °C. Compared to control plants at 20 °C, GFP foci at 25 and 30 °C on inoculated leaves of JaloEEP558 and Black Valentine at 7 dpi were more expanded and confluent on the upper side of the leaves (Figure 4A). Quantification of BPMV RNA1 in leaves inoculated with BPMV-WT confirmed the higher accumulation of BPMV at 25 and 30 °C compared to 20 °C (Figure 4B). More precisely, significantly more RNAs were detected at 25 and 30 °C compared to 20 °C (~6 fold- and ~7–9-fold increase, respectively) (Figure 4B). Consequently, BPMV replication and cell-to-cell movement are more efficient from 0 to 7 dpi in the susceptible genotypes JaloEEP558 and Black Valentine at elevated temperature (25 and 30 °C) compared to 20 °C.
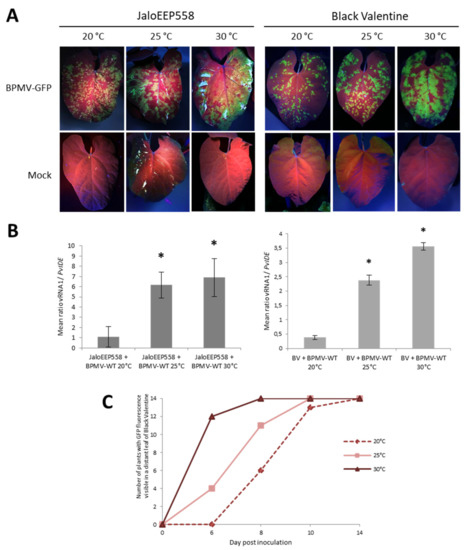
Figure 4.
Elevated temperatures promote BPMV infection in the inoculated leaf and BPMV systemic spreading in whole plants of two susceptible genotypes of P. vulgaris: (A) Representative pictures of four inoculated leaves from four different plants of either JaloEEP558 or Black Valentine (left and right panel, respectively) at 20 °C, 25 °C and 30 °C, 7 days post-inoculation (dpi) with BPMV-GFP (upper panel) and Mock (lower panel). BPMV-GFP was detected under UV light. This experiment was performed at least three times with similar results. (B) Quantification of BPMV titer at 7 dpi in four BPMV-WT-inoculated leaves of four different plants of JaloEEP558 or Black Valentine (BV) by calculation of the relative ratio of BPMV RNA1 to plant mRNA of PvIde using a quantitative RT-PCR procedure. Asteriks indicate significant differences between the tested temperature and 20 °C, the control temperature (t-test, p-value < 0,05). No significant difference of viral titer was found between the two elevated temperatures 25 °C and 30 °C. Data are mean ratios ± SD of four biological replicates. (C) BPMV-GFP systemic spreading in whole plants of Black Valentine at 20 °C, 25 °C, and 30 °C. The graph represents the number of plants with GFP fluorescence visible in a distant leaf scored at four dates after inoculation with BPMV-GFP: 0, 6, 8, 10, and 14 dpi on a total of fourteen plants per temperature assay. BPMV-GFP was detected under UV light. This experiment was performed twice with similar results.
Furthermore, the effect of temperature on the rate of systemic spreading of BPMV-GFP was investigated by scoring the date of appearance of GFP foci on systemic leaves of Black Valentine plants at 20, 25, and 30 °C between 6 and 14 dpi. From 6 to 10 dpi, the systemic spreading is faster at 30 °C compared to 25 °C, and at 25 °C compared to 20 °C (Figure 4C). At 10 dpi, GFP foci in systemic leaves of plants at 20 °C were tiny, whereas GFP fluorescence was widespread at 25 and 30 °C (Figure S3A). At 14 dpi, all plants at 20, 25, and 30 °C were systemically infected by BPMV-GFP (Figure 4C). High levels of fluorescence were visible in all inoculated parts of plants grown at 25 and 30 °C (Figure S3B). These results confirm that BPMV systemic movement becomes faster when temperature increases from 20 to 30 °C, resulting in a higher disease incidence in susceptible genotypes.
3.6. BT-1 and BT-2, Two Near Isogenic Lines for the I. locus, Are Both Resistant to BPMV Systemic Movement
The R-BPMV gene is closely linked to I, a famous locus conferring a broad-spectrum resistance to at least ten different Potyviruses (reviewed in [38]) and several Comoviruses. In this work, we showed that R-BPMV segregates as an incompletely dominant gene. BT-1 (resistant to BCMV, I/I) and BT-2 (susceptible to BCMV, i/i) are two near-isogenic lines created to study the I locus [42]. We tested them to decipher the resistance mechanism mediated by the R-BPMV gene at the tissue and cell levels, as done for the I locus [50,51,52]. We challenged BT-1 and BT-2 for their resistance/susceptibility to BPMV at three temperatures: 20, 25, and 30 °C.
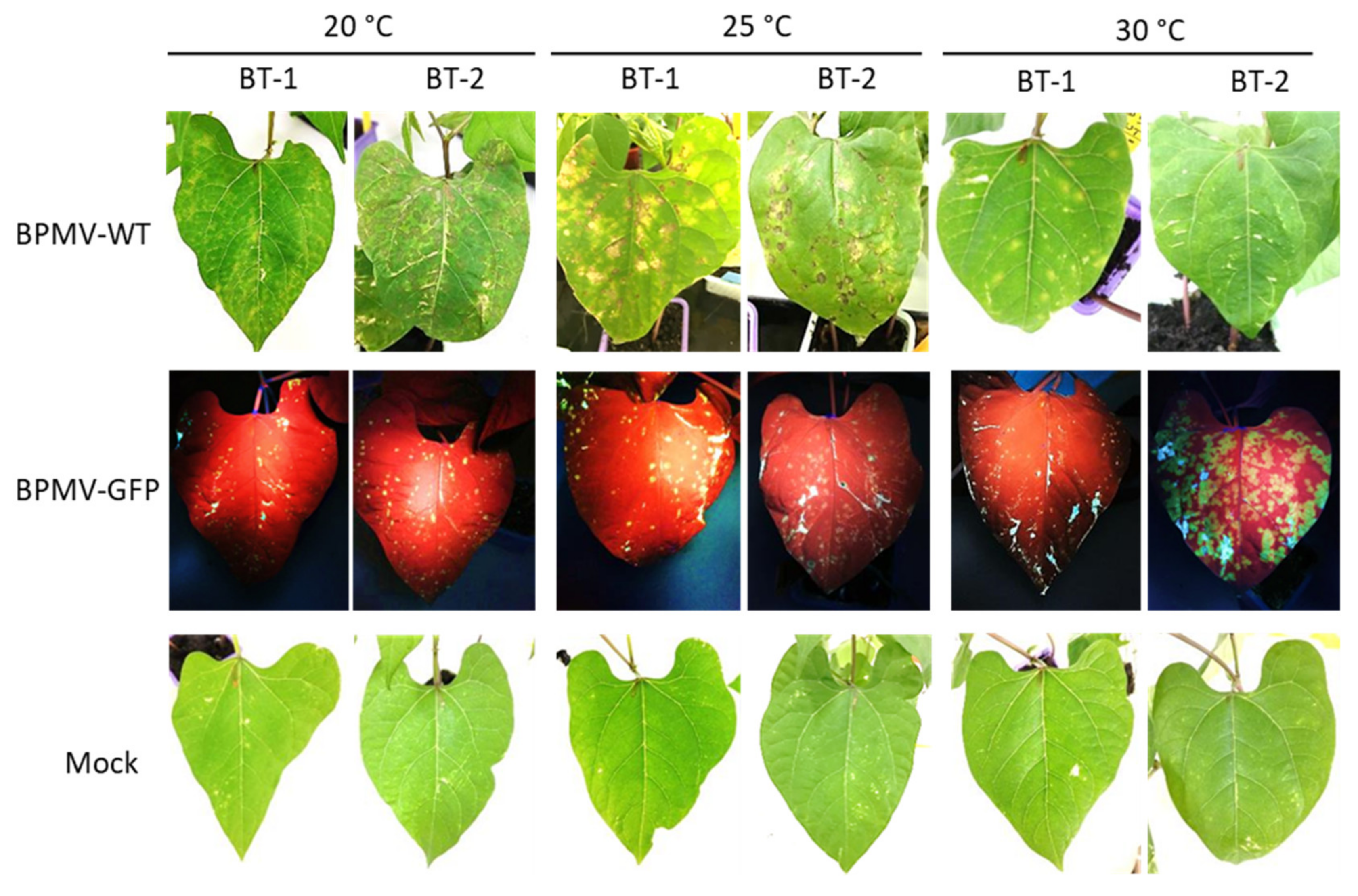
At 7 dpi, local HR lesions developed on inoculated leaves of BT-1 and BT-2 at 20 and 25 °C (Figure 5). As in BAT93, these local HR lesions corresponded to infection areas in which BPMV can first multiply, since small GFP foci were visible after inoculation with BPMV-GFP in BT-1 and BT-2 (Figure 5). Thus, both BT-1 and BT-2 behave as resistant genotypes to BPMV at 20, 25, and 30 °C, and this is strengthened by the observation that no BPMV RNAs were detected in systemic leaves at 21 dpi (Figure S4) as well as no viral symptoms or systemic necrosis (data not shown). In conclusion, BT-1 and BT-2 will not be useful tools to dissect R-BPMV-mediated resistance at the cellular level.
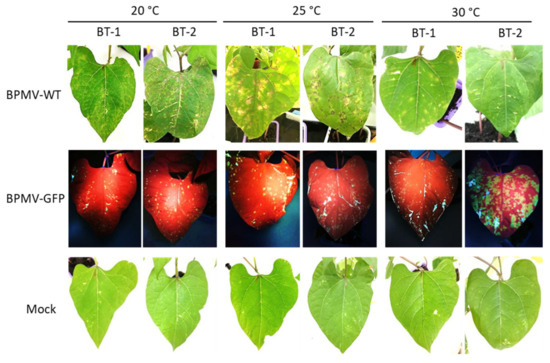
Figure 5.
BT-1 and BT-2, two Near Isogenic Lines for the I locus are both resistant to BPMV systemic movement: Representative pictures of four BT-1- and four BT-2-inoculated leaves (sampled on four different plants) grown respectively at 20 °C, 30 °C and 35 °C, 7 days post-inoculation (dpi) with BPMV-WT (upper panel), BPMV-GFP (middle panel), and Mock (lower panel). BPMV-GFP was detected under UV light. This experiment was performed twice with similar results.
4. Discussion
In this work, we studied the behavior of the R-BPMV-mediated resistance to BPMV in P. vulgaris at normal (20 °C) and elevated temperatures (25, 30, and 35 °C). Strikingly, we found that R-BPMV-mediated resistance is heat-stable up to constant 35 °C at the whole plant level (Figure 3), in the sense that no systemic infection is observed and BPMV remains confined to the inoculated leaf.
This is in agreement with results obtained for other plant–virus pathosystems for which heat-stable R genes have been described. In pepper, different natural allelic variants from the same gene may have different behaviors as reported for the L1c allele, which is heat-stable at 30 °C, whereas other alleles (L1) are not [53]. More recently, the potato NLR Rx1 gene was reported to confer a temperature-insensitive resistance to PVX in Nicotiana benthamiana that is not overcome at temperatures up to 32 °C, a temperature at which the virus was no longer infectious [24]. Additional heat-stable R-gene-mediated resistance has also been reported for pathogens other than viruses. For instance, the tomato Mi-9 gene confers a heat-stable resistance to root-knot nematodes (Meloidogyne sp.) [54,55], despite being homologous to the NLR Mi-1, which is heat-sensitive [56]. In pepper, several heat-stable R genes against Meloidogyne species, named Me, have been reported [57,58]. In Solanum species, Rysto and Rychc R genes present in S. stoloniferum and S. chacoense, respectively, confer extreme resistance to the Tobacco veinal necrosis strain of PVY (PVYN) and are functional at both low (16–20 °C) and elevated temperatures (above 24 °C) [59,60].
On the other hand, temperature sensitivity has more often been reported in the literature. In tobacco, the NLR N is unable to confer resistance to the Tobacco mosaic virus (TMV) above 28 °C [22,49]. Accordingly, the Capsicum sp. NLR Tsw fails to trigger resistance to the Tomato spotted wilt virus (TSWV) at 32 °C and above [23,61]. In potato, some R genes conferring HR to PVY including Ny in Solanum sparsipilum and S. sucrense, or Ny-1 in S. tuberosum cv. Rywal, confer resistance only at low temperatures (16–20 °C), whereas at higher temperatures (24–28 °C), resistance is inhibited and PVY infects plants systemically [60]. Concerning the other kinds of pathogens, temperature sensitivity has been reported for the NLR Mi-1 in tomato (resistance to Meloidogyne incognita, [62]), for the NLR Bs2 in pepper (resistance to Xanthomonas axonopodis pv. vesicatoria, [63]), the non-NLR Cf-4 and Cf-9 genes (resistance to Cladosporium fulvum, [64,65]), as well as for many R genes against bacteria in Arabidopsis (e.g., RPS2 and RPM1 resistance to Pseudomonas syringae, [66]; RPS2 and ZAR1 resistance to P. syringae, [67]; RPS4/RRS1-R resistance to Ralstonia solanacearum, [68]; and SNC1 and RPS4 resistance to P. syringae and Peronospora parasitica, [69,70,71]). All these data converge to assess that temperature sensitivity of resistance is widespread, whereas heat-stable R genes are scarce.
Mechanisms underlying heat sensitivity are poorly understood. Zhu et al. [70] demonstrated that the R proteins, involved in the recognition of pathogen effectors, can be themselves the causal temperature-sensitive component in defense responses. Indeed, autoimmunity-associated mutations (e.g., mutants in which ETI and spontaneous cell death are constitutively activated) in SNC1, an Arabidopsis NLR-homolog, cause increased SNC1 nuclear localization at 22 °C. In contrast, at 28 °C, the reduced nuclear localization is associated with the suppression of autoimmune phenotypes. Similarly, nuclear localization of the R protein N of tobacco was observed after recognition of the TMV coat protein at 22 but not at 28 °C [70]. When the two SNC1 mutations were introduced into the N gene, resistance was effective at elevated temperature, thus suggesting that these mutations may prevent the temperature-sensitive conformational loss of function of the NLR N [70] and maintaining its interaction with the TMV coat protein [22,49]. It is not excluded that such differential point mutations could exist between R-BPMV and I genes, and one way to confirm this hypothesis would be to clone their corresponding gene sequences. Other putative mechanisms of temperature sensitivity have been proposed such as homeostasis of R-interacting chaperons, reduced R protein amounts resulting in a lower R activity, and deregulation of nucleo-cytoplasmic localization of R proteins (reviewed in [18]). More recently, the implication of the methionine cycle [72] and deregulation of the intercellular communication via plasmodesmata [73] have also been supposed to influence virus spread within their hosts.
Our results show that the major gene R-BPMV in P. vulgaris genotype BAT93 confers resistance to BPMV by inducing local HR lesions at 20 °C, where BPMV is able to multiply in a first step (Figure 1). HR lesions have also been observed at 25 and 30 °C 7 dpi (Figure 2). Interestingly, at 7 dpi, we observed larger HR lesions at 25 and 30 °C compared to 20 °C, and this observation was correlated with (i) more expanded multiplication areas of BPMV visualized as green fluorescence areas in BPMV-GFP infected leaves at 4 dpi (Figure 2) and (ii) a rising virus titer in the inoculated leaves at 7 dpi (Figure 3B,C). Consequently, we propose that the higher levels of BPMV accumulation and cell-to-cell spreading in the primary sites of infection could be attributed to a delayed defense response when temperature increases from 20 to 30 °C. As biotrophic pathogens, viruses mainly induce defense responses regulated by salicylic acid (SA) signaling [74], and SA was shown to be a key component that orchestrates the events restricting viral spread in HR [75,76]. Moreover, HR-mediated resistance against Turnip crinkle virus (TCV) was impaired in Arabidopsis eds5 and sid2 SA-deficient mutants without affecting HR cell death [77]. Lukan et al. [78] showed that Potato virus Y (PVY, Potyvirus) spread is even faster in SA-depleted plants (NahG-potato transgenic plants) with rapid lesion expansion. Interestingly, the expression of SA-dependent responses was reduced at elevated temperature in tobacco and potato plants infected with Cucumber mosaic virus (CMV) and PVY, respectively [79,80]. Whether the SA accumulation or other defenses are inhibited or downregulated at elevated temperature needs further work in our pathosystem. Moreover, it has been shown that virus resistance is uncoupled from cell death and that these two events are likely independent [77,78,81,82,83,84,85,86,87]. For example, in potato plants bearing the temperature-sensitive Ny-1 R gene against PVY, temperature shift assays from 22 to 28 °C induced the detection of PVY in infected cells outside the cell death zone [78]. Nevertheless, the molecular mechanisms restricting both virus spread and cell death remain unknown [88].
Interestingly, at 35 °C, HR lesions that developed on BPMV-inoculated leaves of BAT93 were replaced by chlorotic lesions, while the R-BPMV-mediated resistance remains functional at the whole-plant level (Figure 3). We confirmed that BPMV is still infectious and capable of systemic movement at 35 °C (Figure S2). Thus, it seems that cell death is completely abolished at 35 °C, whereas the local defense response is still efficient to block BPMV systemic infection (Figure 3E). The reduction of cell death at elevated temperature has already been reported in soybean (Glycine max) infected by strains G1 and G7 of Soybean mosaic virus (SMV) [89]. Indeed, a stem-tip necrosis (STN) develops in several soybean cultivars carrying the R genes Rsv1 and Rsv1-n. The STN in genotype V262 induced by strain G1 and in genotype V94-3971 by strain G7 developed at 10, 15, 20, 25, and 30 °C, on 44, 41, 14, 8, and 8 days after inoculation, respectively. By contrast, at 35 °C, no STN reaction and, consequently, no cell death was induced by any strain. Interestingly, cell death reduction at elevated temperature has also been reported for plant–bacteria interactions. AvrRpt2-mediated cell death in Dex-AvrRpt2 plants was significantly reduced at 28 °C and was almost completely abolished at 32 °C [66]. Interestingly, the expression of the WORKY46 transcription factor acting as an upstream regulator of SA metabolism was also temperature-sensitive with a peak at 16 °C and a decrease at temperatures above 28 °C. In the same way, AvrRpm1- and AvrB-mediated cell death was significantly attenuated at elevated temperatures (32 °C) [66]. Thus, the data indicate that elevated temperature could suppress ETI signaling in R-gene-mediated response.
We previously showed that R-BPMV is present in genotype BAT93 of P. vulgaris and that this gene is genetically linked to the I locus on chromosome 2 [41,90]. The I locus is a broad-spectrum resistance locus that confers resistance to BCMV, BCMNV [91,92], and nine other potyviral species (Watermelon mosaic virus-2 [93,94], Cowpea aphid-borne mosaic virus [93,95], Soybean mosaic virus [96,97], Peanut mottle virus [98,99], Zucchini yellow mosaic virus, Thaïland passiflora mosaic virus, and Passionfruit woodiness virus-K [100], BYMV, and ClYVV [101,102]). Thus, we hypothesized that R-BPMV and I could correspond to the same gene with pleiotropic effects and if so, one important question would be: do R-BPMV- and I-mediated resistance have the same behavior regarding temperature?
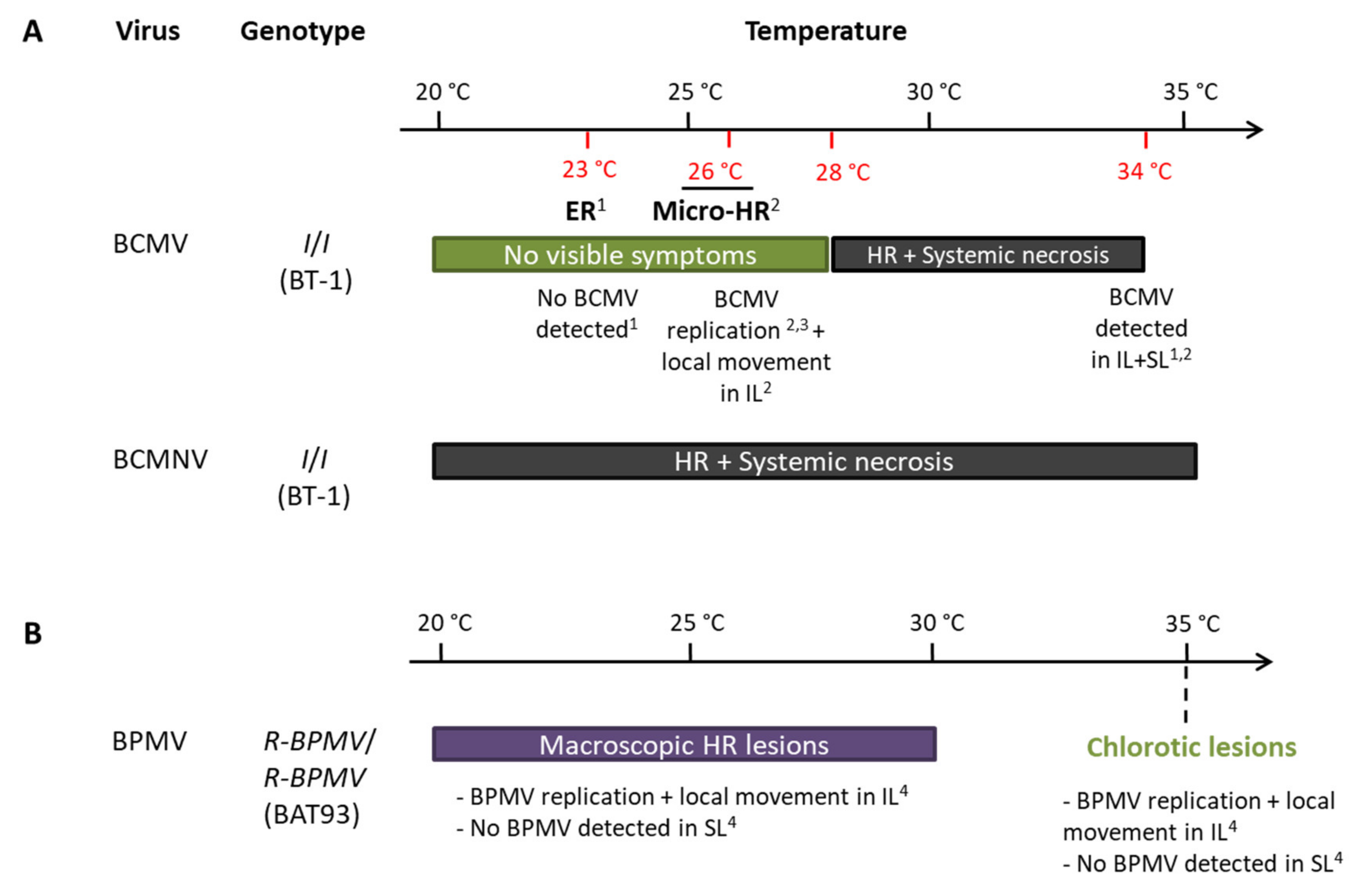
I was reported to be temperature-sensitive for BCMV resistance [47,48]: an extreme resistance or micro-HR phenotypes are observed on inoculated leaves below 28 °C, and BCMV replication was effective [51], but above 28 °C, the ER evolves into a systemic necrosis [48,50,52] (Figure 6). However, for BCMNV, a local HR in inoculated leaves evolves into a systemic necrosis regardless of temperature [46,47,48] (Figure 6). With BPMV, we observed local HR lesions in inoculated leaves of BAT93 at 20, 25, and 30 °C at 7 dpi in which BPMV is able to multiply in a first step (Figure 1 and Figure 2). By contrast, at 35 °C, chlorotic lesions develop in inoculated leaves with no BPMV detected in the systemic leaves (Figure 3A), although BPMV is still infectious at 35 °C in the susceptible genotype BV (Figure S2) with a higher rate of systemic spread at 25, 30, and 35 °C compared to 20 °C (Figures S2 and S3). Thus, our results highlight a switch in the resistance phenotype mediated by R-BPMV that occurs between 30 and 35 °C (Figure 6). Compared to the I gene, this is a significant difference of behavior of the two genetically linked-resistance genes, since I induces local HR and systemic necrosis from 28 °C with BCMV detected in the inoculated leaves and systemic leaves [50,52] (Figure 6).
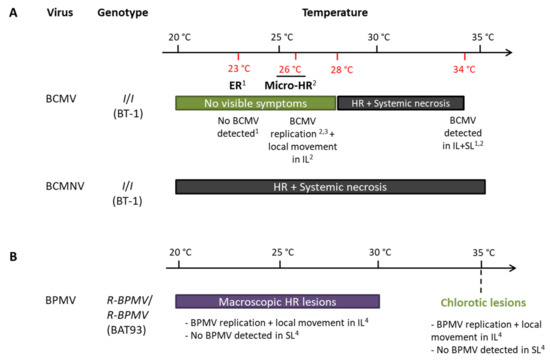
Figure 6.
Resistance phenotypes of R-BPMV- and I-derived resistances do not share the same features regarding temperature: (A)Potyviruses Bean common mosaic virus (BCMV) and Bean common mosaic necrosis virus (BCMNV); (B)Comovirus Bean pod mottle virus (BPMV) Abreviations: ER: Extreme resistance, HR: Hypersensitive Reaction, IL: inoculated leaf, Micro-HR: microscopic Hypersensitive Reaction, SL: systemic leaf. References: 1: Collmer et al. 2000 (whole-plant study); 2: Cadle-Davidson and Jahn 2006 (whole-plant study); 3: Cadle-Davidson and Jahn 2005 (BCMV transfection in protoplasts); 4: this study.
Overall, we show here that R-BPMV- and I-mediated resistance phenotypes do not behave in the same way at an elevated temperature. Thus, although genetically tightly linked, BPMV and I could correspond to two different genes. Another hypothesis is that resistance to BPMV and BCMV is controlled by the same gene but that the downstream signaling cascades differ, explaining the two contrasting kinds of resistance phenotypes. Neither I nor R-BPMV has been cloned but both were hypothesized to encode NLR receptors [90,91,103]. Consistent with this hypothesis, the resistance phenotypes are expressed for I as either extreme resistance or systemic necrosis or for R-BPMV as local HR, which are hallmarks of ETI mediated by NLR receptors. Moreover, Vallejos et al. [91] reported an over-expression of several NLR genes of the I region after BCMV or BCMNV inoculations, suggesting a role of NLR genes in the response to BCMV/BCMNV.
Finally, it is worth noting that both R-BPMV (our study) and I segregate as incompletely dominant genes [50,51,91]. Indeed, when investigating the inheritance of R-BPMV in an F2 population of 60 plants, we found a deficit of susceptible phenotypes (56 resistant: 4 susceptible) and thus, a significant deviation from the expected Mendelian ratio (3:1). A similar segregation deviation was reported for the I gene in an F2 population obtained from the cross Calima (susceptible Andean genotype) × Jamapa (resistant Mesoamerican genotype) [91]. Vallejos et al. [91] suggested that this phenomenon was a consequence of the partial compatibility of the Andean and Mesoamerican genomes interacting in this type of crosses [91].
To study the effect of elevated temperatures in a P. vulgaris-BPMV compatible interaction, we performed experiments on two BPMV-susceptible genotypes P. vulgaris cv. Black Valentine and cv. JaloEEP558. We reported that in both genotypes, the area of primary infection sites at 7 dpi was larger on BPMV-GFP-inoculated leaves at 25 and 30 °C compared to 20 °C, and these results were correlated with a higher virus titer in these leaves (Figure 4A,B). Moreover, in Black Valentine, the virus spreads systemically even more rapidly when the temperature increases (Figure 4C). Overall, our results show a positive effect of temperature elevation on the level of susceptibility in two susceptible genotypes. Our results are in agreement with several other studies on different plant–virus interactions in compatible contexts. For example, Chung et al. [29] investigated the effects of different temperature regimes on the speed of systemic spread after inoculation of Turnip mosaic virus (TuMV) in Chinese cabbage. It took 48 days for systemic infection to occur at 13 °C but only 6 days at 22–33 °C. Likewise, in Solanum tuberosum, plants became 100% systemically infected at 24 and 28 °C, while at 20 °C, only 20% of the plants were systemically infected [26,27]. Nancarrow et al. [27] studied the effects of elevated (10–21 °C, night/ day) or ambient (5–16 °C, night/day) temperature winter growing season regimes on wheat plants infected with Barley yellow dwarf virus (BYDV, Luteovirus). Infected plants grown under an elevated temperature developed virus symptoms earlier and had higher virus titers than plants grown at an ambient temperature. In potato, susceptibility to PVY was dramatically increased in systemic infected leaves at higher temperatures [80]. In cassava, geminiviruses responsible for cassava mosaic disease cause more symptoms and have higher viral titer at 25 °C compared to 30 °C [32]. Nevertheless, other studies have shown opposite results, showing a negative effect on the level of susceptibility in susceptible genotypes. In N. benthamiana, plants infected with the potyviruses PVY and Potato virus A had fewer symptoms and reduced CP protein accumulation at 20 °C compared to 30 °C [104]. In the same way, Del Toro et al. [30] worked in N. benthamiana infected with PVY and PVX and showed attenuated symptoms for PVY and no symptoms for PVX at 30 °C compared to 25 °C. Aguilar et al. [105] described that elevated temperatures decrease both virulence and virus titers in the synergistic infection PVX/Plum pox virus (PPV) in N. benthamiana. Importantly, the RNA silencing machinery was shown to be more active at elevated temperatures in several plants including cassava and N. benthamiana [32,106], suggesting that more efficient RNA silencing is responsible for impaired viral replication (or the viral RNA suppressor is less active). To conclude, all these studies demonstrate that depending on the virus and plant host, elevated temperature may either increase or decrease virus susceptibility. To investigate this differential impact, further studies are needed on other pathosystems in the future. Moreover, molecular mechanisms underlying the differential susceptibility to viruses must be investigated in more depth, especially concerning the implication of the RNA silencing pathway in pathosystems for which viral infection is boosted at elevated temperature such as P. vulgaris-BPMV.
Global warming leads to rising temperatures, which alter plant–virus interactions [73], potentially inducing yield losses and decreasing quality of crop productions [107]. It is therefore timely to study the effect of elevated temperatures on plant responses to virus infections. Importantly, future experiments on the effect of temperature on plant–virus interactions will need to consider the dynamic nature of field conditions, with diurnal fluctuating temperature cycles and heat waves such as those experienced by crops in the field. As performed in Nancarrow et al. [27], growing chambers will need to more closely resemble the dynamic conditions to which plants and pathogens are subjected in nature so that field trials can be mimicked. After studying the effect of a single environmental factor in order to have a specific response, it will be important to test the effect of several combined environmental factors (elevated temperature + high CO2 for example) to analyze whether their effects are additive or antagonistic. In C3 plants like P. vulgaris, high CO2 improves photosynthesis and activates hormonal pathways, whereas high temperatures decrease the efficiency of Rubisco and the solubility of CO2. Consequently, combined high CO2 and elevated temperatures may induce antagonistic effects. At the international level, little work has been published on the combined effects of elevated temperatures and high CO2 on plant–virus interactions (reviewed in [20]). Del Toro et al. [108] focused on combined climate conditions (CCC, elevated temperature (30 versus 25 °C) and high CO2 level (970 versus 405 ppm)) by studying CMV, PVY, and PVX in the model plant N. benthamiana. They showed that viral titers in systemic leaves under CCC showed no significant differences at 7 and 12 dpi for CMV. Conversely, viral titers of PVY and PVX were significantly lower under CCC compared to the standard condition at both 7 and 12 dpi. In the case of PVY, viral titer was lower at 12 dpi compared to 7 dpi under CCC, and the contrary was observed for PVX [108]. In potato (S. tuberosum), Chung et al. [109] demonstrated that Potato leaf roll virus (PLRV) RNA accumulated at higher levels, and larger numbers of potato plants were infected by PLRV under combined high CO2 levels (940 ppm) and elevated temperature (30 °C). More recently, Aguilar et al. [110] studied a multifactorial system combining biotic (virus and bacteria) and abiotic (drought) stresses in A. thaliana (compatible context) and N. benthamiana plants (incompatible context). They demonstrated that infection by the PVX/PPV virus combination induced resistance to the bacteria P. syringae pv. tomato and to drought in both compatible and incompatible host–bacteria interactions. However, combined high CO2 levels (970 ppm) and elevated temperature (30 °C) negatively affected resistance to Pst and to drought induced by a virus infection, and this correlated with diminished H2O2 production, decreased expression of defense genes, and a drop in virus titers.
5. Conclusions
To conclude, our study gives a first insight into the impact of elevated temperature on the level of resistance/susceptibility to viruses in common bean. Indeed, natural genetic resistance is often the most effective and environmentally friendly way of controlling plant diseases, in particular against a virus where no chemical alternative is available. This research needs to be continued in order to decipher the underlying cellular and molecular mechanisms of heat tolerance and to engineer robust thermostable resistances in the context of global warming.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/v13071239/s1, Figure S1, Semi-quantitative RT-PCR showing that P. vulgaris cv. BAT93 is resistant to BPMV systemic infection; Figure S2, BPMV is still infectious at 35 °C and spreads systemically in the susceptible genotype P. vulgaris cv. Black Valentine; Figure S3, Elevated temperatures promote BPMV spreading in the systemic leaves and in whole plants in the susceptible genotype P. vulgaris cv. Black Valentine; Figure S4, Semi-quantitative RT-PCR showing that P. vulgaris cv. BT-1 and BT-2, two Near Isogenic Lines for the I locus, are both resistant to BPMV systemic infection.
Author Contributions
Conceptualization, C.M. and S.P.; methodology, C.M. and S.P.; formal analysis, C.M., J.L., A.G.-W. and S.P.; investigation, C.M., J.L., C.G., M.N., S.B., A.N. and S.P.; writing, C.M., J.L., S.B., M.N. and S.P.; manuscript editing, C.M., J.L., M.N., S.B., A.G.-W., V.G. and S.P.; supervision and project administration, S.P.; funding acquisition, V.G. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
C.M. and J.L. were supported by fellowships from the French Research Ministry and from the « Ecole Universitaire de Recherche Saclay Plant Sciences (EUR SPS) Graduate School of Research », respectively. MN was supported by an individual ‘Invited-researcher’ fellowship from Université de Paris in 2019 and 2021. This work was supported by fundings from the Plant Biology and Breeding (BAP) department of French National Research Institute for Agriculture, Food, and Environment (INRAE) (HiT-VIRUSRESIST project), from the French National Research Agency (HiPATH project, Grant Agreement No. ANR-17-CE20-0025), and from the European Union’s Horizon 2020 research and innovation program (BRESOV project, Grant Agreement No. 774244). IPS2 benefits from the support of Saclay Plant Sciences-SPS (ANR-17-EUR-0007).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
We thank Séverine Domenichini and Gautier Bernal from IPS2 for their implication in the microscope work. We also thank Chunquan Zhang and Steven Whitham at Iowa State University, USA, for sharing the set of BPMV vectors and for providing the seeds of P. vulgaris cv. Black Valentine, Juan Camilo Alvarez Diaz and Etienne Delannoy for advices with RT-qPCR analysis and quantification of BPMV virus titer, Manon Richard for managing the construction of the F2 population, Holger Ornstrup and all the greenhouse team of IPS2 for precious help with plant growing, and Benoît Moury and Cécile Desbiez of INRAE Avignon, France, for helpful discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Abreviations
| Avr factor | avirulence factor |
| CO2 | carbon dioxide |
| dpi | day(s) post-inoculation |
| ETI | effector-triggered immunity |
| HR | hypersensitive reaction |
| NLR | nucleotide-binding domain leucin-rich repeat containing receptors |
| ppm | parts per million |
| R | resistance gene/protein |
References
- Anderson, P.K.; Cunningham, A.A.; Patel, N.G.; Morales, F.J.; Epstein, P.R.; Daszak, P. Emerging infectious diseases of plants: Pathogen pollution, climate change and agrotechnology drivers. Trends Ecol. Evol. 2004, 19, 535–544. [Google Scholar] [CrossRef]
- Sastry, K.S.; Zitter, T.A. Plant Virus and Viroid Diseases in the Tropics. Volume 2: Epidemiology and Management; Springer: Dordrecht, The Netherlands, 2014. [Google Scholar]
- Amari, K.; Niehl, A. Nucleic acid-mediated PAMP-triggered immunity in plants. Curr. Opin. Virol. 2020, 42, 32–39. [Google Scholar] [CrossRef]
- Ratcliff, F.; Harrison, B.D.; Baulcombe, D.C. A similarity between viral defense and gene silencing in plants. Science 1997, 276, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Kørner, C.; Klauser, D.; Niehl, A.; Domínguez-Ferreras, A.; Chinchilla, D.; Boller, T.; Heinlein, M.; Hann, D.R. The immunity regulator BAK1 contributes to resistance against diverse RNA viruses. Mol. Plant Microbe Interact. 2013, 26, 1271–1280. [Google Scholar] [CrossRef]
- Lee, B.; Park, Y.S.; Lee, S.; Song, G.C.; Ryu, C.M. Bacterial RNAs activate innate immunity in Arabidopsis. New Phytol. 2016, 209, 785–797. [Google Scholar] [CrossRef]
- Niehl, A.; Wyrsch, I.; Boller, T.; Heinlein, M. Double-stranded RNAs induce a pattern-triggered immune signaling pathway in plants. New Phytol. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Kontra, L.; Burgyán, J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Zvereva, A.S.; Golyaev, V.; Turco, S.; Gubaeva, E.G.; Rajeswaran, R.; Schepetilnikov, M.V.; Srour, O.; Ryabova, L.A.; Boller, T.; Pooggin, M.M. Viral protein suppresses oxidative burst and salicylic acid-dependent autophagy and facilitates bacterial growth on virus-infected plants. New Phytol. 2016, 211, 1020–1034. [Google Scholar] [CrossRef]
- Nicaise, V.; Candresse, T. Plum pox virus capsid protein suppresses plant pathogen-associated molecular pattern (PAMP)-triggered immunity. Mol. Plant Pathol. 2017, 18, 878–886. [Google Scholar] [CrossRef] [PubMed]
- Kong, J.; Wei, M.; Li, G.; Lei, R.; Qiu, Y.; Wang, C.; Li, Z.H.; Zhu, S. The cucumber mosaic virus movement protein suppresses PAMP-triggered immune responses in Arabidopsis and tobacco. Biochem. Biophys. Res. Commun. 2018, 498, 395–401. [Google Scholar] [CrossRef]
- Palukaitis, P.; Yoon, J.Y. R gene mediated defense against viruses. Curr. Opin. Virol. 2020, 45, 1–7. [Google Scholar] [CrossRef]
- Flor, H.H. Current status of the gene-for-gene concept. Annu. Rev. Phytopathol. 1955, 1971, 275–296. [Google Scholar] [CrossRef]
- Gouveia, B.C.; Calil, I.P.; Machado, J.P.B.; Santos, A.A.; Fontes, E.P.B. Immune Receptors and Co-receptors in Antiviral Innate Immunity in Plants. Front. Microbiol. 2017, 7, 2139. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Butterbach, P.; Kormelink, R. Dominant resistance against plant viruses. Front. Plant Sci. 2014, 5, 307. [Google Scholar] [CrossRef]
- Kou, Y.; Wang, S. Broad-spectrum and durability: Understanding of quantitative disease resistance. Curr. Opin. Plant Biol. 2010, 13, 181–185. [Google Scholar] [CrossRef]
- Schmitt-Keichinger, C. Manipulating cellular factors to combat viruses: A case study from the plant eukaryotic translation initiation factors eIF4. Front. Microbiol. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, J.; Kang, B.C. Current views on temperature-modulated R gene-mediated plant defense responses and tradeoffs between plant growth and immunity. Curr. Opin. Plant Biol. 2019, 50, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Trebicki, P. Climate change and plant virus epidemiology. Virus Res. 2020, 286, 198059. [Google Scholar] [CrossRef]
- Tenllado, F.; Canto, T. Effects of a changing environment on the defenses of plants to viruses. Curr. Opin. Virol. 2020, 42, 40–46. [Google Scholar] [CrossRef]
- Desaint, H.; Aoun, N.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Fight hard or die trying: When plants face pathogens under heat stress. New Phytol. 2021, 229, 712–734. [Google Scholar] [CrossRef] [PubMed]
- Weststeijn, E. Lesion growth and virus localization in leaves of Nicotiana tabacum cv. Xanthi nc. after inoculation with tobacco mosaic virus and incubation alternately at 22 °C and 32 °C. Physiol. Plant Pathol. 1981, 18, 357–368. [Google Scholar] [CrossRef]
- Chung, B.N.; Lee, J.H.; Kang, B.C.; Koh, S.W.; Joa, J.H.; Choi, K.S.; Ahn, J.J. HR-mediated defense response is overcome at high temperatures in capsicum species. Plant Pathol. J. 2018, 34, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Richard, M.M.S.; Knip, M.; Aalders, T.; Beijaert, M.S.; Takken, F.L.W. Unlike Many Disease Resistances, Rx1-Mediated Immunity to Potato Virus X Is Not Compromised at Elevated Temperatures. Front. Genet. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Kabbage, M.; Kessens, R.; Bartholomay, L.C.; Williams, B. The Life and Death of a Plant Cell. Annu. Rev. Plant Biol. 2017, 68, 375–404. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.S.; del Toro, F.; Tenllado, F.; Canto, T.; Chung, B.N. A model to explain temperature dependent systemic infection of potato plants by Potato virus Y. Plant Pathol. J. 2017, 33, 206–211. [Google Scholar] [CrossRef]
- Nancarrow, N.; Constable, F.E.; Finlay, K.J.; Freeman, A.J.; Rodoni, B.C.; Trebicki, P.; Vassiliadis, S.; Yen, A.L.; Luck, J.E. The effect of elevated temperature on barley yellow dwarf virus-PAV in wheat. Virus Res. 2014, 186, 97–103. [Google Scholar] [CrossRef]
- Kido, K.; Tanaka, C.; Mochizuki, T.; Kubota, K.; Ohki, T.; Ohnishi, J.; Knight, L.M.; Tsuda, S. High temperatures activate local viral multiplication and cell-to-cell movement of Melon necrotic spot virus but restrict expression of systemic symptoms. Phytopathology 2008, 98, 181–186. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chung, B.N.; Choi, K.S.; Ahn, J.J.; Joa, J.H.; Do, K.S.; Park, K.S. Effects of temperature on systemic infection and symptom expression of turnip mosaic virus in Chinese cabbage (Brassica campestris). Plant Pathol. J. 2015, 31, 363–370. [Google Scholar] [CrossRef]
- Del Toro, F.J.; Aguilar, E.; Hernández-Walias, F.J.; Tenllado, F.; Chung, B.N.; Canto, T. High temperature, high ambient CO2 affect the interactions between three positive-sense RNA viruses and a compatible host differentially, but not their silencing suppression efficiencies. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef]
- Dahal, G.; Hughes, J.D.; Thottappilly, G.; Lockhart, B.E.L. Effect of temperature on symptom expression and reliability of banana streak badnavirus detection in naturally infected plantain and banana (Musa spp.). Plant Dis. 1998, 82, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Chellappan, P.; Vanitharani, R.; Ogbe, F.; Fauquet, C.M. Effect of temperature on geminivirus-induced RNA silencing in plants. Plant Physiol. 2005, 138, 1828–1841. [Google Scholar] [CrossRef] [PubMed]
- Broughton, W.J.; Hernandez, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Beebe, S.; Ramirez, J.; Jarvis, A.; Rao, I.M.; Mosquera, G.; Bueno, J.M.; Blair, M.W. Genetic Improvement of Common Beans and the Challenges of Climate Change. Crop Adapt. Clim. Chang. 2011, 356–369. [Google Scholar] [CrossRef]
- Julian R—Climate Change and Bean Production. Available online: https://pt.slideshare.net/ciatdapa/julian-r-climate-change-and-bean-production/2 (accessed on 25 April 2021).
- Omae, H.; Kumar, A.; Kashiwaba, K.; Shono, M. Adaptation to high temperature and water deficit in the common bean (Phaseolus vulgaris L.) during the reproductive period. J. Bot. 2012. [Google Scholar] [CrossRef]
- Suarez, J.C.; Polania, J.A.; Contreras, A.T.; Rodriguez, L.; Machado, L.; Ordonez, C.; Beebe, S.; Rao, I. Adaptation of common bean lines to high temperature conditions: Genotypic differences in phenological and agronomic performance Adaptation of common bean lines to high temperature conditions: Genotypic differences in phenological and agronomic performan. Euphytica 2020, 216, 28. [Google Scholar] [CrossRef]
- Meziadi, C.; Blanchet, S.; Geffroy, V.; Pflieger, S. Genetic resistance against viruses in Phaseolus vulgaris L.: State of the art and future prospects. Plant Sci. 2017, 265, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Morales, F.J.; Castano, M. Increased disease severity induced by some comoviruses in bean genotypes possessing monogenic dominant resistance to Bean common mosaic potyvirus. Plant Dis. 1992, 76, 570–573. [Google Scholar] [CrossRef]
- Morales, F.J.; Singh, S.P. Inheritance of the mosaic and necroses reactions induced by bean severe mosaic comoviruses in Phaseolus vulgaris L. Euphytica 1997, 93, 223–226. [Google Scholar] [CrossRef]
- Pflieger, S.; Blanchet, S.; Meziadi, C.; Richard, M.M.S.; Thareau, V.; Mary, F.; Mazoyer, C.; Geffroy, V. The “ one-step ” Bean pod mottle virus (BPMV)—Derived vector is a functional genomics tool for efficient overexpression of heterologous protein, virus-induced gene silencing and genetic mapping of BPMV R-gene in common bean ( Phaseolus vulgaris L.). BMC Plant Biol. 2014, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Provvidenti, R. Two useful selections of the bean cultivar Black Turtle Soup for viral identification. Bean Improv. Coop. Annu. Rep. 1983, 26, 73–75. [Google Scholar]
- Zhang, C.; Bradshaw, J.D.; Whitham, S.A.; Hill, J.H. The Development of an Efficient Multipurpose Bean Pod Mottle Virus Viral Vector Set for Foreign Gene Expression and RNA silencing. Plant Physiol. 2010, 153, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Pflieger, S.; Blanchet, S.; Meziadi, C.; Richard, M.M.S.; Geffroy, V. Bean pod mottle virus (BPMV) viral inoculation procedure in common bean (Phaseolus vulgaris L.). Bio-Protocol 2015, 5, e1524. [Google Scholar] [CrossRef]
- Richard, M.M.S.; Gratias, A.; Alvarez Diaz, J.C.; Thareau, V.; Pflieger, S.; Meziadi, C.; Blanchet, S.; Marande, W.; Bitocchi, E.; Papa, R.; et al. A common bean truncated CRINKLY4 kinase controls gene-for-gene resistance to the fungus Colletotrichum lindemuthianum. J. Exp. Bot. 2021, 72, 3569–3581. [Google Scholar] [CrossRef]
- Ali, M.A. Genetics of resistance to the common Bean mosaic virus in the bean (Phaseolus vulgaris L). Phytopathology 1950, 40, 69–79. [Google Scholar]
- Bos, L. Bean common mosaic virus. C. Descr. Plant Viruses 1971, 73. [Google Scholar]
- Drijfhout, E. Genetic Interaction between Phaseolus vulgaris and bean Common Mosaic Virus with Implications for Strain Identification and Breeding for Resistance; Centre for Agricultural Publishing and Documentation: Wageningen, The Netherlands, 1978. [Google Scholar]
- Whitham, S.; McCormick, S.; Baker, B. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc. Natl. Acad. Sci. USA 1996, 93, 8776–8781. [Google Scholar] [CrossRef] [PubMed]
- Collmer, C.W.; Marston, M.F.; Taylor, J.C.; Jahn, M. The I gene of bean: A dosage-dependent allele conferring extreme resistance, hypersensitive resistance, or spreading vascular necrosis in response to the potyvirus Bean common mosaic virus. Mol. Plant Microbe Interact. 2000, 13, 1266–1270. [Google Scholar] [CrossRef]
- Cadle-Davidson, M.M.; Jahn, M.M. Resistance conferred against bean common mosaic virus by the incompletely dominant I locus of Phaseolus vulgaris is active at the single cell level. Arch. Virol. 2005, 150, 2601–2608. [Google Scholar] [CrossRef]
- Cadle-Davidson, M.M.; Jahn, M.M. Patterns of accumulation of Bean common mosaic virus in Phaseolus vulgaris genotypes nearly isogenic for the I locus. Ann. Appl. Biol. 2006, 148, 179–185. [Google Scholar] [CrossRef]
- Tomita, R.; Sekine, K.-T.; Mizumoto, H.; Sakamoto, M.; Murai, J.; Kiba, A.; Hikichi, Y.; Suzuki, K.; Kobayashi, K. Genetic Basis for the Hierarchical Interaction between Tobamovirus spp. and L Resistance Gene Alleles from Different Pepper Species. Mol. Plant Microbe Interact. 2011, 24, 108–117. [Google Scholar] [CrossRef]
- Veremis, J.C.; van Heusden, A.W.; Roberts, P.A. Mapping a novel heat-stable resistance to Meloidogyne in Lycopersicon peruvianum. Theor. Appl. Genet. 1999, 98, 274–280. [Google Scholar] [CrossRef]
- Ammiraju, J.S.S.; Veremis, J.C.; Huang, X.; Roberts, P.A.; Kaloshian, I. The heat-stable root-knot nematode resistance gene Mi-9 from Lycopersicon peruvianum is localized on the short arm of chromosome 6. Theor. Appl. Genet. 2003, 106, 478–484. [Google Scholar] [CrossRef]
- Jablonska, B.; Ammiraju, J.S.S.; Bhattarai, K.K.; Mantelin, S.; de Ilarduya, O.M.; Roberts, P.A.; Kaloshian, I. The Mi-9 gene from Solanum arcanum conferring heat-stable resistance to root-knot nematodes is a homolog of Mi-1. Plant Physiol. 2007, 143, 1044–1054. [Google Scholar] [CrossRef]
- Djian-Caporalino, C.; Pijarowski, L.; Januel, A.; Lefebvre, V.; Daubèze, A.; Palloix, A.; Dalmasso, A.; Abad, P. Spectrum of resistance to root-knot nematodes and inheritance of heat-stable resistance in in pepper (Capsicum annuum L.). Theor. Appl. Genet. 1999, 99, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Djian-Caporalino, C.; Pijarowski, L.; Fazari, A.; Samson, M.; Gaveau, L.; O’Byrne, C.; Lefebvre, V.; Caranta, C.; Palloix, A.; Abad, P. High-resolution genetic mapping of the pepper (Capsicum annuum L.) resistance loci Me3 and Me4 conferring heat-stable resistance to root-knot nematodes (Meloidogyne spp.). Theor. Appl. Genet. 2001, 103, 592–600. [Google Scholar] [CrossRef]
- Bradshaw, J.; Ramsey, G. Utilisation of the Commonwealth Potato Collection in potato breeding. Euphytica 2005, 146, 9–19. [Google Scholar] [CrossRef]
- Solomon-Blackburn, R.; Bradshaw, J. Resistance to Potato virus Y in a Multitrait Potato Breeding Scheme without Direct Selection in Each Generation. Potato Res. 2007, 50, 87–95. [Google Scholar] [CrossRef]
- Moury, B.; Selassie, K.G.; Marchoux, G.; Daub, A.; Palloix, A. High temperature effects on hypersensitive resistance to Tomato Spotted Wilt Tospovirus (TSWV) in pepper (Capsicum chinense Jacq.). Eur. J. Plant Pathol. 1998, 104, 489–498. [Google Scholar] [CrossRef]
- De Carvalho, L.; Benda, N.; Vaughan, M.; Cabrera, A.; Hung, K.; Cox, T.; Abdo, Z.; Allen, L.; Teal, P. Mi-1-Mediated Nematode Resistance in Tomatoes is Broken by Short-Term Heat Stress but Recovers Over Time. J. Nematol. 2015, 47, 133–140. [Google Scholar]
- Romero, A.M.; Kousik, C.S.; Ritchie, D.F. Temperature Sensitivity of the Hypersensitive Response of Bell Pepper to Xanthomonas axonopodis pv. vesicatoria. Phytopathology 2002, 92, 197–203. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cai, X.; Takken, F.; Joosten, M.; de Wit, P. Specific recognition of AVR4 and AVR9 results in distinct patterns of hypersensitive cell death in tomato, but similar patterns of defence-related gene expression. Mol. Plant Pathol. 2001, 2, 77–86. [Google Scholar] [CrossRef] [PubMed]
- De Jong, C.F.; Takken, F.L.; Cai, X.; de Wit, P.J.; Joosten, M.H. Attenuation of Cf-Mediated Defense Responses at Elevated Temperatures Correlates With a Decrease in Elicitor-Binding Sites. Mol. Plant Microbe Interact. 2002, 15, 1040–1049. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, X.; Feng, B.; Sheen, J.; Shan, L.; He, P. Differential temperature operation of plant immune responses. Nat. Commun. 2013, 4, 2530. [Google Scholar] [CrossRef] [PubMed]
- Menna, A.; Nguyen, D.; Guttman, D.S.; Desveaux, D. Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front. Plant Sci. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Aoun, N.; Tauleigne, L.; Lonjon, F.; Deslandes, L.; Vailleau, F.; Roux, F.; Berthomé, R. Quantitative disease resistance under elevated temperature: Genetic basis of new resistance mechanisms to Ralstonia solanacearum. Front. Plant Sci. 2017, 8, 1387. [Google Scholar] [CrossRef]
- Yang, S.; Hua, J. A haplotype-specific Resistance gene regulated by BONZAI1 mediates temperature-dependent growth control in Arabidopsis. Plant Cell 2004, 16, 1060–1071. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, W.; Hua, J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog. 2010, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mang, H.G.; Qian, W.; Zhu, Y.; Qian, J.; Kang, H.G.; Klessig, D.F.; Hua, J. Abscisic acid deficiency antagonizes high-temperature inhibition of disease resistance through enhancing nuclear accumulation of resistance proteins SNC1 and RPS4 in Arabidopsis. Plant Cell 2012, 24, 1271–1284. [Google Scholar] [CrossRef] [PubMed]
- Fesenko, I.; Spechenkova, N.; Mamaeva, A.; Makhotenko, A.V.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Role of the methionine cycle in the temperature-sensitive responses of potato plants to potato virus Y. Mol. Plant Pathol. 2021, 22, 77–91. [Google Scholar] [CrossRef]
- Amari, K.; Huang, C.; Heinlein, M. Potential Impact of Global Warming on Virus Propagation in Infected Plants and Agricultural Productivity. Front. Plant Sci. 2021, 12, 649768. [Google Scholar] [CrossRef]
- Whitham, S.A.; Yang, C.; Goodin, M.M. Global impact: Elucidating plant responses to viral infection. Mol. Plant Microbe Interact. 2006, 19, 1207–1215. [Google Scholar] [CrossRef]
- Lewsey, M.; Palukaitis, P.; Carr, J. Plant–Virus Interactions: Defence and Counter-Defence. In Annual Plant Reviews Volume 34: Molecular Aspects of Plant Disease Resistance; Wiley-Blackwell: Oxford, UK, 2009; pp. 134–176. [Google Scholar] [CrossRef]
- Baebler, Š.; Witek, K.; Petek, M.; Stare, K.; Tušek-Žnidarič, M.; Pompe-Novak, M.; Renaut, J.; Szajko, K.; Strzelczyk-Zyta, D.; Marczewski, W.; et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus y infection in potato. J. Exp. Bot. 2014, 65, 1095–1109. [Google Scholar] [CrossRef]
- Chandra-Shekara, A.; Navarre, D.; Kachroo, A.; Kang, H.; Klessig, D.; Kachroo, P. Signaling requirements and role of salicylic acid in HRT—And rrt -mediated resistance to turnip crinkle virus in Arabidopsis. Plant J. 2004, 40, 647–659. [Google Scholar] [CrossRef]
- Lukan, T.; Baebler, S.; Pompe-Novak, M.; Gucek, K.; Zagoršcak, M.; Coll, A.; Gruden, K. Cell Death Is Not Sufficient for the Restriction of Potato Virus Y Spread in Hypersensitive Response-Conferred Resistance in Potato. Front. Plant Sci. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yanan, L.; Lijuan, C.; Lisha, Z.; Han, R.; Honghui, L.; Dehui, X. Temperature dependent defence of Nicotiana tabacum against Cucumber mosaic virus and recovery occurs with the formation of dark green islands. J. Plant Biol. 2016, 59, 293–301. [Google Scholar] [CrossRef]
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y. Front. Microbiol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chu, M.; Desvoyes, B.; Turina, M.; Noad, R.; Scholthof, H.B. Genetic dissection of tomato bushy stunt virus p19-protein-mediated host-dependent symptom induction and systemic invasion. Virology 2000, 266, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Cawly, J.; Cole, A.B.; Király, L.; Qiu, W.; Schoelz, J.E. The plant gene CCD1 selectively blocks cell death during the hypersensitive response to cauliflower mosaic virus infection. Mol. Plant Microbe Interact. 2005, 18, 212–219. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ishibashi, K.; Masuda, K.; Naito, S.; Meshi, T.; Ishikawa, M. An inhibitor of viral RNA replication is encoded by a plant resistance gene. Proc. Natl. Acad. Sci. USA 2007, 104, 13833–13838. [Google Scholar] [CrossRef]
- Komatsu, K.; Hashimoto, M.; Ozeki, J.; Yamaji, Y.; Maejima, K.; Senshu, H.; Himeno, M.; Okano, Y.; Kagiwada, S.; Namba, S. Viral-Induced systemic necrosis in plants involves both programmed cell death and the inhibition of viral multiplication, which are regulated by independent pathways. Mol. Plant Microbe Interact. 2010, 23, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.P.; Bhattacharjee, S.; Klessig, D.F.; Moffett, P. Systemic acquired resistance is induced by R gene-mediated responses independent of cell death. Mol. Plant Pathol. 2010, 11, 155–160. [Google Scholar] [CrossRef]
- Hafez, Y.M.; Bacsó, R.; Király, Z.; Künstler, A.; Király, L. Up-regulation of antioxidants in tobacco by low concentrations of H 2O 2 suppresses necrotic disease symptoms. Phytopathology 2012, 102, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Ando, S.; Obinata, A.; Takahashi, H. WRKY70 interacting with RCY1 disease resistance protein is required for resistance to Cucumber mosaic virus in Arabidopsis thaliana. Physiol. Mol. Plant Pathol. 2014, 85, 8–14. [Google Scholar] [CrossRef]
- Harris, J.M.; Balint-kurti, P.; Bede, J.C.; Day, B.; Gold, S.; Goss, E.M.; Grenville-briggs, L.J.; Jones, K.M.; Wang, A.; Wang, Y.; et al. What are the Top 10 Unanswered Questions in Molecular Plant-Microbe Interactions? Mol. Plant Microbe Interact. 2020, X, 1–12. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, P.; Gergerich, R. Characterization of Resistance to Soybean mosaic virus in Diverse Soybean Germplasm. Crop Sci. 2005, 45, 2503–2509. [Google Scholar] [CrossRef]
- Meziadi, C.; (IPS2, Orsay, France). Personal communication, 2021.
- Vallejos, C.E.; Astua-monge, G.; Jones, V.; Plyler, T.R.; Sakiyama, N.S.; Mackenzie, S.A. Genetic and Molecular Characterization of the I Locus of Phaseolus vulgaris. Genetics 2006, 172, 1229–1242. [Google Scholar] [CrossRef]
- Nodari, R.O.; Tsai, S.M.; Gilbertson, R.L.; Gepts, P. Towards an integrated linkage map of common bean. 2. Development of an RFLP-based linkage map. Theor. Appl. Genet. 1993, 85, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Kyle, M.M.; Provvidenti, R. Inheritance of resistance to potato y viruses in Phaseolus vulgaris L: 1. Two independent genes for resistance to watermelon mosaic virus-2. Theor. Appl. Genet. 1987, 74, 595–600. [Google Scholar] [CrossRef]
- Provvidenti, R. Inheritance of resistance to watermelon mosaic virus 2 in Phaseolus vulgaris. Phytopathology 1974, 64, 1448–1450. [Google Scholar] [CrossRef]
- Provvidenti, R.; Gonsalves, D.; Taiwo, M.A. Inheritance of resistance to Blackeye cowpea mosaic and Cowpea aphid-borne mosaic viruses in Phaseolus vulgaris. J. Hered. 1983, 74, 60–61. [Google Scholar] [CrossRef][Green Version]
- Provvidenti, R.; Gonsalves, D.; Ranalli, P. Inheritance of resistance to soybean mosaic virus in Phaseolus vulgaris. J. Hered. 1982, 73, 302–303. [Google Scholar] [CrossRef]
- Kyle, M.M.; Provvidenti, R. Inheritance of resistance to potyviruses in Phaseolus vulgaris L. 2. Linkage relations and utility of a dominant gene for lethal systemic necrosis to Soybean mosaic virus. Theor. Appl. Genet. 1993, 86, 189–196. [Google Scholar] [CrossRef]
- Provvidenti, R.; Chirco, E.M. Inheritance of resistance to peanut mottle virus in Phaseolus vulgaris. J. Hered. 1987, 78, 402–403. [Google Scholar]
- Li, R.H.; Zettler, F.W.; Elliott, M.S.; Petersen, M.A.; Still, P.E.; Baker, C.A.; Mink, G.I. A strain of Peanut Mottle Virus Seedborne in Bambarra Groundnut. Plant Dis. 1991, 75, 130–133. [Google Scholar] [CrossRef]
- Fisher, M.L.; Kyle, M.M. Inheritance of resistance to potyviruses in Phaseolus vulgaris L. III. Cosegregation of phenotypically similar dominant responses to nine potyviruses. Theor. Appl. Genet. 1994, 89, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Hart, J.P.; Griffiths, P.D. Genotyping-by-Sequencing Enabled Mapping and Marker Development for the By-2 Potyvirus Resistance Allele in Common Bean. Plant Genome 2015, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Dickson, M.H.; Natti, J.J. Inheritance of resistance of Phaseolus vulgaris to bean yellow mosaic virus. Phytopathology 1968, 58, 1450. [Google Scholar]
- Bello, M.H.; Moghaddam, S.M.; Massoudi, M.; Mcclean, P.E.; Cregan, P.B.; Miklas, P.N. Application of in silico bulked segregant analysis for rapid development of markers linked to Bean common mosaic virus resistance in common bean. BMC Genom. 2014, 15, 1–13. [Google Scholar] [CrossRef]
- Chung, B.N.; Canto, T.; Tenllado, F.; Choi, K.S.; Joa, J.H.; Ahn, J.J.; Kim, C.H.; Do, K.S. The effects of high temperature on infection by Potato virus Y, Potato virus A, and Potato leafroll virus. Plant Pathol. J. 2016, 32, 321–328. [Google Scholar] [CrossRef]
- Aguilar, E.; Allende, L.; Del Toro, F.J.; Chung, B.N.; Canto, T.; Tenllado, F. Effects of elevated CO2 and temperature on pathogenicity determinants and virulence of potato virus X/Potyvirus-Associated synergism. Mol. Plant-Microbe Interact. 2015, 28, 1364–1373. [Google Scholar] [CrossRef]
- Szittya, G.; Silhavy, D.; Molnar, A.; Havelda, Z.; Lovas, A.; Lakatos, L.; Banfaldi, Z.; Burgyan, J. Low temperature inhibits RNA silencing-mediated defence by the control of siRNA generation. EMBO J. 2003, 22, 633–640. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Razzaq, A.; Mehmood, S.S.; Zou, X.; Zhang, X.; Lv, Y.; Xu, J. Impact of Climate Change on Crops Adaptation and Strategies to Tackle Its Outcome: A Review. Plants 2019, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, F.; Rakhshandehroo, F.; Larruy, B.; Aguilar, E.; Tenllado, F.; Canto, T. Effects of simultaneously elevated temperature and CO2 levels on Nicotiana benthamiana and its infection by different positive-sense RNA viruses are cumulative and virus type-specific. Virology 2017, 511, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.N.; Koh, S.W.; Choi, K.S.; Joa, J.H.; Kim, C.H.; Selvakumar, G. Temperature and CO2 Level Influence Potato leafroll virus Infection in Solanum tuberosum. Plant Pathol. J. 2017, 33, 522–527. [Google Scholar] [CrossRef]
- Aguilar, E.; del Toro, F.J.; Figueira-Galán, D.; Hou, W.; Canto, T.; Tenllado, F. Virus infection induces resistance to Pseudomonas syringae and to drought in both compatible and incompatible bacteria—Host interactions, which are compromised under conditions of elevated temperature and CO2 levels. J. Gen. Virol. 2020, 101, 122–135. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).