Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case and Control Definitions
2.2. Specimen Collection and Laboratory Testing
2.3. Statistical Analysis
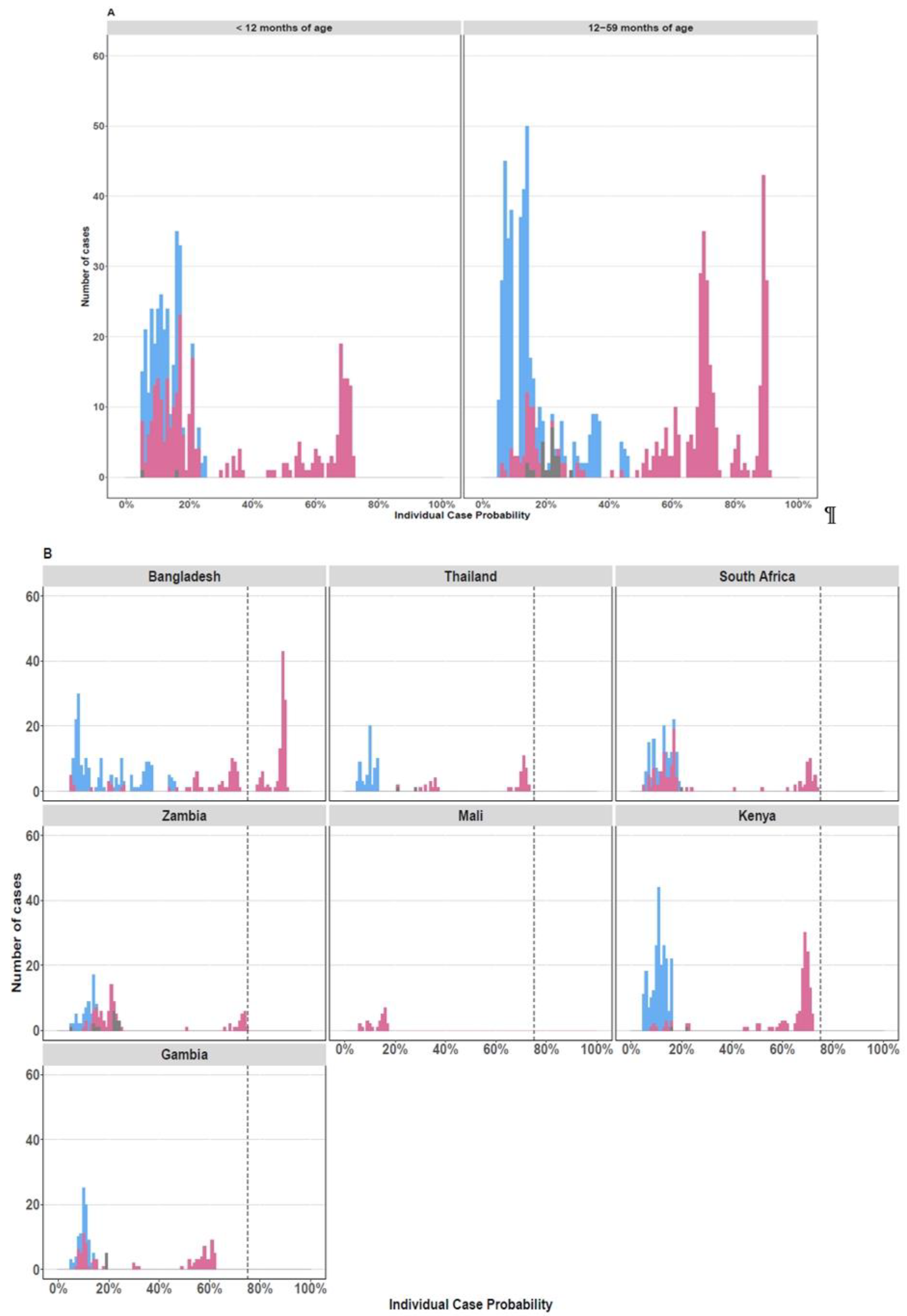
3. Results
3.1. Characteristics of Community Controls by RV Status
3.2. RV infection Among Pneumonia Cases
3.3. Case-Control Comparison of RV Infection in Children
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
- Study Site: South Africa conducted by the Respiratory and Meningeal Pathogens Research Unit, Wits Health Consortium, based at Chris Hani Baragwanath Academic HospitalWits HREC Approval number: M10M101129Principal investigator: Prof Shabir A. Madhi
- Study Site: Kilifi, Kenya conducted by the Kilifi-KEMRI Wellcome Trust InstituteApproval number from the Kenya Medical Research Institute: KEMRI/RES/7/3/1Oxtrec Approval number: 60-09Principal investigator: Dr Laura Hammitt
- Study site: Basse in The Gambia at the MRC institutionApproval number from The Gambia Government/MRC joint Ethics Committee: L2010.105Principal investigators: Dr Stephen Howie
- Study Site: Bamako, Mali at the University of Maryland InsitutionApproval number from FMPOS Ethics committee: 2011/07/FMPOSApproval number from The University of Maryland Institutional Review Board (IRB): HP00048100Principal Investigator: Dr Karen Kotloff
- Study Site: Lusaka, Zambia at the Boston University InstitutionApproval number from the Blue Panel IRB: HP-29860Approval from the ERES Converge IRB: 2010-Dec-001Principal Investigator: Dr Donald Thea
- Study site: Dhaka, Bangladesh at the ICDDR and JHSPH institutionApproval number from ICDDR,B Ethics Review Committee: PR-11012Principal Investigators: Dr Abdullah Brooks
- Study site: Sa Kaeo and Nakhon Phanom in Thailand at the CDC-ThailandApproval number from the Ethical Review Committee for Research in Human Subjects-Ministry of Public Health, Thailand: 17/2554Principal Investigators: Dr Pasakorn AkarasewiApproval to conduct the clinical and molecular subtyping analysis of HRV in the PERCH project was obtained from the PERCH executive committee on the 18 June 2014 and ethical approval was granted by the University of the Witwatersrand Human Rights and Ethics Board (HREC number: M140906).
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Price, W.H. The isolation of a new virus associated with respiratory clinical disease in humans. Proc. Natl. Acad. Sci. USA 1956, 42, 892–896. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, N.G. Do rhinoviruses cause pneumonia in children? Paediatr. Respir. Rev. 2004, 5, S191–S195. [Google Scholar] [CrossRef]
- Louie, J.K.; Roy-Burman, A.; Guardia-LaBar, L.; Boston, E.J.; Kiang, D.; Padilla, T.; Yagi, S.; Messenger, S.; Petru, A.M.; Glaser, C.A.; et al. Rhinovirus associated with severe lower respiratory tract infections in children. Pediatr. Infect. Dis. J. 2009, 28, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Esposito, S.; Daleno, C.; Prunotto, G.; Scala, A.; Tagliabue, C.; Borzani, I.; Fossali, E.; Pelucchi, C.; Principi, N. Impact of viral infections in children with community-acquired pneumonia: Results of a study of 17 respiratory viruses. Influenza Other Respir. Viruses 2012, 7, 18–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, Z.; Vázquez, M.; Shapiro, E.D.; Weibel, C.; Ferguson, D.; Landry, M.L.; Kahn, J.S. Rhinoviruses are a major cause of wheezing and hospitalization in children less than 2 years of age. Pediatr. Infect. Dis. J. 2009, 28, 25–29. [Google Scholar] [CrossRef] [Green Version]
- Renwick, N.; Schweiger, B.; Kapoor, V.; Liu, Z.; Villari, J.; Bullmann, R.; Miething, R.; Briese, T.; Lipkin, W.I. A recently identified rhinovirus genotype is associated with severe respirato-ry-tract infection in children in Germany. J. Infect. Dis. 2007, 196, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, A.; Leitch, E.M.; Gaunt, E.; Harvala, H.; Simmonds, P. Screening respiratory samples for detection of human rhinovirus-es (HRVs) and enteroviruses: Comprehensive VP4-VP2 typing reveals high incidence and genetic diversity of HRV species C. J. Clin. Microbiol. 2009, 47, 3958–3967. [Google Scholar] [CrossRef] [Green Version]
- E Smuts, H.; Workman, L.J.; Zar, H.J. Human rhinovirus infection in young African children with acute wheezing. BMC Infect. Dis. 2011, 11, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnston, S.L.; Sanderson, G.; Pattemore, P.K.; Smith, S.; Bardin, P.G.; Bruce, C.B.; Lambden, P.R.; Tyrrell, D.A.; Holgate, S.T. Use of polymerase chain reaction for diagnosis of picornavirus infection in subjects with and without respiratory symptoms. J. Clin. Microbiol. 1993, 31, 111–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khetsuriani, N.; Lu, X.; Teague, W.G.; Kazerouni, N.; Anderson, L.J.; Erdman, D.D. Novel human rhinoviruses and exacerbation of asthma in children1. Emerg. Infect. Dis. 2008, 14, 1793–1796. [Google Scholar] [CrossRef]
- Arden, K.E.; Mackay, I.M. Newly identified human rhinoviruses: Molecular methods heat up the cold viruses. Rev. Med. Virol. 2010, 20, 156–176. [Google Scholar] [CrossRef] [PubMed]
- Hansbro, N.G.; Horvat, J.C.; Wark, P.; Hansbro, P. Understanding the mechanisms of viral induced asthma: New therapeutic directions. Pharmacol. Ther. 2008, 117, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Jartti, T.; Jartti, L.; Peltola, V.; Waris, M.; Ruuskanen, O. Identification of respiratory viruses in asymptomatic subjects: Asympto-matic respiratory viral infections. Pediatric Infect. Dis. J. 2008, 27, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.M. Human rhinoviruses: The cold wars resume. J. Clin. Virol. 2008, 42, 297–320. [Google Scholar] [CrossRef] [PubMed]
- Calvo, C.; Casas, I.; Garcia-Garcia, M.L.; Pozo, F.; Reyes, N.; Cruz, N.; García-Cuenllas, L.; Pérez-Breña, P. Role of Rhinovirus c respiratory infections in sick and healthy children in Spain. Pediatr. Infect. Dis. J. 2010, 29, 717–720. [Google Scholar] [CrossRef]
- Jartti, T.; Lehtinen, P.; Vuorinen, T.; Koskenvuo, M.; Ruuskanen, O. Persistence of rhinovirus and enterovirus RNA after acute respiratory illness in children. J. Med. Virol. 2004, 72, 695–699. [Google Scholar] [CrossRef]
- Fry, A.M.; Lu, X.; Olsen, S.J.; Chittaganpitch, M.; Sawatwong, P.; Chantra, S.; Baggett, H.C.; Erdman, D. Human rhinovirus infections in rural thailand: Epidemiological evidence for rhinovirus as both pathogen and bystander. PLoS ONE 2011, 6, e17780. [Google Scholar] [CrossRef] [Green Version]
- Iwane, M.K.; Prill, M.M.; Lu, X.; Miller, E.K.; Edwards, K.M.; Hall, C.B.; Griffin, M.R.; Staat, M.A.; Anderson, L.J.; Williams, J.; et al. Human Rhinovirus Species Associated with Hospitalizations for Acute Respiratory Illness in Young US Children. J. Infect. Dis. 2011, 204, 1702–1710. [Google Scholar] [CrossRef] [Green Version]
- Venter, M.; Lassaunière, R.; Kresfelder, T.L.; Westerberg, Y.; Visser, A. Contribution of common and recently described respira-tory viruses to annual hospitalizations in children in South Africa. J. Med. Virol. 2011, 83, 1458–1468. [Google Scholar] [CrossRef]
- Baillie, V.L.; Olwagen, C.P.; Madhi, S.A. Review on clinical and molecular epidemiology of human rhinovirus–associated lower respiratory tract infections in African and southeast Asian children. Pediatr. Infect. Dis. J. 2018, 37, e185–e194. [Google Scholar] [CrossRef]
- O’Brien, K.L.; Baggett, H.C.; Brooks, W.A.; Feikin, D.R.; Hammitt, L.L.; Higdon, M.M.; Howie, S.R.; Knoll, M.D.; Kotloff, K.L.; Levine, O.S.; et al. Causes of severe pneumonia requiring hospital admission in children without HIV infection from Africa and Asia: The PERCH multi-country case-control study. Lancet 2019, 394, 757–779. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Acute Respiratory Infections in Children: Case Management in Small Hospitals in Developing Countries, a Manual for Doctors and Other Senior Health Workers; World Health Organization: Geneva, Switzerland, 1990. [Google Scholar]
- World Health Organization. Technical Bases for the WHO Recommendations on the Management of Pneumonia in Children at First-Level Health Facilities; World Health Organization: Geneva, Switzerland, 1991. [Google Scholar]
- Murdoch, D.R.; O’Brien, K.; Driscoll, A.J.; Karron, R.A.; Bhat, N. The Pneumonia Methods Working Group; Perch core the perch core team laboratory methods for determining pneumonia etiology in children. Clin. Infect. Dis. 2012, 54, S146–S152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deloria-Knoll, M.; Feikin, D.R.; Scott, J.A.G.; O’Brien, K.L.; DeLuca, A.N.; Driscoll, A.J.; Levine, O.S.; Pneumonia Methods Working Group. Identification and selection of cases and controls in the Pneumonia Eti-ology Research for Child Health project. Clin. Infect. Dis. 2012, 54 (Suppl. S2), S117–S123. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Deloria-Knoll, M.; Zeger, S.L. Nested partially latent class models for dependent binary data; estimating disease etiol-ogy. Biostatistics 2016, 18, 200–213. [Google Scholar]
- Deloria Knoll, M.; Fu, W.; Shi, Q.; Prosperi, C.; Wu, Z.; Hammitt, L.L.; Feikin, D.R.; Baggett, H.C.; Howie, S.R.; Scott, J.A.G.; et al. Bayesian estimation of pneumonia etiology: Epidemiologic considerations and applications to the pneumonia etiology research for child health study. Clin. Infect. Dis. 2017, 64 (Suppl. S3), S213–S227. [Google Scholar] [CrossRef] [Green Version]
- Principi, N.; Zampiero, A.; Gambino, M.; Scala, A.; Senatore, L.; Lelii, M.; Ascolese, B.; Pelucchi, C.; Esposito, S. Prospective evaluation of rhinovirus infection in healthy young children. J. Clin. Virol. 2015, 66, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, M.; Tempia, S.; Treurnicht, F.K.; Walaza, S.; Cohen, A.L.; Moyes, J.; Hellferscee, O.; Variava, E.; Dawood, H.; Chhagan, M.; et al. Genetic diversity and molecular epidemiology of human rhinoviruses in South Africa. Influenza Other Respir. Viruses 2014, 8, 567–573. [Google Scholar] [CrossRef]
- Loeffelholz, M.J.; Trujillo, R.; Pyles, R.B.; Miller, A.L.; Alvarez-Fernandez, P.; Pong, D.L.; Chonmaitree, T. Duration of Rhinovirus Shedding in the Upper Respiratory Tract in the First Year of Life. Pediatrics 2014, 134, 1144–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfister, A.M. Air Pollution: Mass Killer in Bangladesh. 2001. Available online: https://www.who.int/docstore/peh/ceh/articles/airpollution.htm (accessed on 11 May 2020).
- Esposito, S.; Daleno, C.; Scala, A.; Castellazzi, L.; Terranova, L.; Papa, S.S.; Longo, M.R.; Pelucchi, C.; Principi, N. Impact of rhinovirus nasopharyngeal viral load and viremia on severity of respiratory infections in children. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 41–48. [Google Scholar] [CrossRef]
- Bruning, A.H.; Thomas, X.V.; van der Linden, L.; Wildenbeest, J.G.; Minnaar, R.P.; Jansen, R.R.; de Jong, M.D.; Sterk, P.J.; van der Schee, M.P.; Wolthers, K.C.; et al. Clinical, virological and epidemiological characteristics of rhinovirus infections in early childhood: A comparison between non-hospitalised and hospitalised children. J. Clin. Virol. 2015, 73, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Takeyama, A.; Hashimoto, K.; Sato, M.; Sato, T.; Kanno, S.; Takano, K.; Ito, M.; Katayose, M.; Nishimura, H.; Kawasaki, Y.; et al. Rhinovirus load and disease severity in children with lower respiratory tract in-fections. J. Med. Virol. 2012, 84, 1135–1142. [Google Scholar] [CrossRef] [PubMed]
- Ambrosioni, J.; Bridevaux, P.-O.; Aubert, J.-D.; Soccal, P.; Wagner, G.; Kaiser, L. Role of rhinovirus load in the upper respiratory tract and severity of symptoms in lung transplant recipients. J. Clin. Virol. 2015, 64, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Sakthivel, S.K.; Whitaker, B.; Lu, X.; Oliveira, D.B.; Stockman, L.J.; Kamili, S.; Oberste, M.S.; Erdman, D.D. Comparison of fast-track diagnostics respiratory pathogens multiplex real-time RT-PCR assay with in-house singleplex assays for comprehensive detection of human respiratory viruses. J. Virol. Methods 2012, 185, 259–266. [Google Scholar] [CrossRef]
- Baillie, V.L.; Moore, D.P.; Mathunjwa, A.; Morailane, P.; Simões, E.A.; Madhi, S.A. Molecular subtyping of human rhinovirus in chil-dren from three sub-Saharan African countries. J. Clin. Microbiol. 2019, 57, e00723-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Number | Percentage | ||||||
|---|---|---|---|---|---|---|---|
| Characteristics | RV+ | RV− | RV+ | RV− | aOR a | 95%CI a | p-Value a |
| Demographic and health: | |||||||
| 12–59 months b | 409 | 1832 | 39 | 47 | 1.4 | 1.2–1.6 | <0.001 |
| Premature birth c | 130 | 376 | 12 | 10 | 1.3 | 1.1–1.7 | 0.01 |
| Never breast fed | 974 | 3583 | 93 | 92 | 1.2 | 0.9–1.7 | 0.16 |
| Underweight d | 127 | 471 | 12 | 12 | 1.03 | 0.8–1.3 | 0.79 |
| Male | 538 | 1960 | 51 | 50 | 0.95 | 0.8–1.1 | 0.50 |
| Day care attendance | 163 | 739 | 16 | 19 | 0.95 | 0.8–1.1 | 0.50 |
| Smoker in household | 392 | 1518 | 37 | 39 | 0.91 | 0.8–1.1 | 0.15 |
| Clinical: | |||||||
| ARI e | 299 | 880 | 28 | 22 | 1.6 | 1.3–1.9 | <0.001 |
| Rhinorrhea | 223 | 629 | 21 | 16 | 1.7 | 1.4–2.1 | <0.001 |
| Cough | 120 | 300 | 11 | 8 | 1.6 | 1.2–2.0 | <0.001 |
| Fever f | 56 | 212 | 5 | 5 | 0.97 | 0.7–1.3 | 0.86 |
| Tachypnea g | 103 | 454 | 10 | 12 | 0.8 | 0.6–1.0 | 0.05 |
| Diarrhea | 13 | 82 | 1 | 2 | 0.6 | 0.3–1.04 | 0.07 |
| Respiratory viruses detected: | |||||||
| AdV | 134 | 458 | 13 | 12 | 1.2 | 0.98–1.5 | 0.07 |
| HMPV | 57 | 147 | 5 | 4 | 1.2 | 0.9–1.7 | 0.26 |
| HBoV | 138 | 521 | 13 | 13 | 1.02 | 0.8–1.3 | 0.80 |
| PIV | 68 | 246 | 6 | 6 | 0.9 | 0.7–1.2 | 0.63 |
| HCoV | 93 | 406 | 9 | 10 | 0.8 | 0.6–1.0 | 0.05 |
| RSV | 24 | 115 | 2 | 3 | 0.8 | 0.5–1.2 | 0.30 |
| InFV A-C | 9 | 104 | 1 | 3 | 0.3 | 0.1–0.5 | <0.001 |
| Any viral co-infection | 400 | 183 | 38 | 47 | 0.7 | 0.6–0.8 | <0.001 |
| Bacterial infections in the NP/OP: | |||||||
| H. influenzae type b | 23 | 59 | 2 | 2 | 1.6 | 0.96–2.6 | 0.07 |
| M. catarrhalis | 836 | 2857 | 79 | 73 | 1.5 | 1.2–1.7 | <0.001 |
| H. influenzae | 603 | 1958 | 57 | 50 | 1.4 | 1.2–1.6 | <0.001 |
| S. pneumoniae | 860 | 2979 | 81 | 76 | 1.4 | 1.2–1.7 | <0.001 |
| C. pneumoniae | 16 | 50 | 2 | 1 | 1.3 | 0.7–2.3 | 0.39 |
| M. pneumoniae | 18 | 52 | 2 | 1 | 1.3 | 0.8–2.3 | 0.31 |
| S. aureus | 135 | 542 | 13 | 14 | 0.9 | 0.7–1.1 | 0.16 |
| B. pertussis | 1 | 10 | 0 | 0 | 0.3 | 0.0–2.6 | 0.29 |
| Any bacterial co-infection | 1015 | 3687 | 96 | 94 | 1.7 | 1.2–2.3 | 0.003 |
| Number | Percentage | ||||||
|---|---|---|---|---|---|---|---|
| Mono-Rv Infections | Mixed-Rv Infections a | Mono-Rv Infections | Mixed-Rv Infections a | aOR b | 95%CI b | p-Value b | |
| Demographic and health: | |||||||
| 12–59 months of age c | 228 | 181 | 35 | 45 | 1.6 | 1.3–1.9 | <0.001 |
| Smoker in household | 243 | 149 | 37 | 38 | 1.2 | 0.9–1.5 | 0.31 |
| Male | 335 | 206 | 51 | 51 | 1.01 | 0.8–1.3 | 0.92 |
| Never breast fed | 52 | 25 | 8 | 6 | 1.01 | 0.6–1.8 | 0.97 |
| Underweight d | 75 | 52 | 11 | 13 | 0.98 | 0.7–1.5 | 0.91 |
| Premature birthe | 92 | 38 | 14 | 10 | 0.95 | 0.9–1.1 | 0.44 |
| Day care attendance | 86 | 77 | 13 | 19 | 0.9 | 0.7–1.2 | 0.35 |
| Clinical Features: | |||||||
| ARI f | 176 | 124 | 27 | 31 | 0.96 | 0.7–1.3 | 0.77 |
| Rhinorrhea | 132 | 733 | 20 | 17 | 1.08 | 0.9–1.2 | 0.22 |
| Fever g | 32 | 24 | 5 | 6 | 1.06 | 0.6–1.9 | 0.85 |
| Cough | 68 | 52 | 10 | 13 | 0.93 | 0.8–1.1 | 0.38 |
| Tachypnea h | 62 | 41 | 10 | 10 | 0.9 | 0.6–1.4 | 0.68 |
| Diarrhea | 10 | 3 | 2 | 1 | 0.8 | 0.5–1.1 | 0.20 |
| Bacterial infections in the NP/OP: | |||||||
| H. influenzae type b | 13 | 8 | 2 | 2 | 1.4 | 0.7–2.5 | 0.32 |
| S. pneumoniae | 536 | 304 | 82 | 76 | 1.4 | 1.2–1.8 | 0.001 |
| C. pneumoniae | 10 | 5 | 2 | 1 | 1.3 | 0.6–2.5 | 0.50 |
| M. catarrhalis | 502 | 296 | 77 | 74 | 1.2 | 0.95–1.4 | 0.12 |
| H. influenzae | 336 | 206 | 52 | 51 | 1.0 | 0.9–1.3 | 0.65 |
| S. aureus | 92 | 54 | 14 | 14 | 0.98 | 0.8–1.3 | 0.89 |
| B. pertussis | 1 | 1 | 0 | 0 | 0.5 | 0.1–4.3 | 0.57 |
| M. pneumoniae | 5 | 7 | 1 | 2 | 0.5 | 0.2–1.3 | 0.16 |
| Any bacterial co-infection | 628 | 370 | 96 | 92 | 2.2 | 1.5–3.2 | <0.001 |
| Number | Percentage | ||||||
|---|---|---|---|---|---|---|---|
| RV+ | RV− | RV+ | RV− | aOR a | 95% CI a | p-Value a | |
| Demographic and health: | |||||||
| 12–59 months of age b | 393 | 1025 | 43 | 35 | 0.7 | 0.6–0.9 | <0.001 |
| Smoker in household | 340 | 969 | 37 | 33 | 1.1 | 0.96–1.4 | 0.12 |
| Male | 539 | 1701 | 59 | 58 | 1.1 | 0.9–1.2 | 0.49 |
| Day Care attendance | 126 | 517 | 14 | 17 | 0.96 | 0.7–1.3 | 0.77 |
| Underweight c | 277 | 916 | 30 | 31 | 0.9 | 0.8–1.1 | 0.41 |
| Premature birth d | 87 | 328 | 10 | 11 | 0.9 | 0.7–1.1 | 0.31 |
| Never breast fed | 83 | 330 | 9 | 11 | 0.9 | 0.7–1.1 | 0.29 |
| Clinical features: | |||||||
| Wheezing | 421 | 897 | 46 | 31 | 1.8 | 1.4–2.2 | <0.001 |
| Tachypnea e | 785 | 2379 | 86 | 81 | 1.5 | 1.1–1.9 | 0.01 |
| Very severe pneumonia | 291 | 955 | 32 | 32 | 1.1 | 0.96–1.4 | 0.13 |
| Deaths f | 47 | 185 | 5 | 6 | 1.0 | 0.7–1.4 | 0.98 |
| Diarrhea | 118 | 451 | 13 | 15 | 1.0 | 0.8–1.3 | 0.99 |
| Tachycardia g | 439 | 1512 | 48 | 51 | 0.98 | 0.8–1.2 | 0.83 |
| Hypoxic h | 297 | 1086 | 33 | 37 | 0.98 | 0.8–1.2 | 0.85 |
| Chest X-ray abnormal i | 365 | 1354 | 0 | 46 | 0.8 | 0.7–1.0 | 0.05 |
| Hospital stay > 3 days | 453 | 1729 | 50 | 58 | 0.8 | 0.7–0.97 | 0.02 |
| Convulsions | 43 | 201 | 5 | 7 | 0.7 | 0.5–1.04 | 0.08 |
| Any symptom | 909 | 2940 | 99 | 99 | 1.9 | 0.5–6.3 | 0.99 |
| Bacterial infection markers: | |||||||
| Leukocytosis j | 443 | 1133 | 51 | 41 | 1.3 | 1.1–1.5 | 0.01 |
| Blood culture positive k | 29 | 107 | 3 | 4 | 0.99 | 0.6–1.5 | 0.96 |
| Fever l | 707 | 2441 | 78 | 83 | 0.96 | 0.8–1.2 | 0.78 |
| CRP > 40 mg/mL m | 176 | 715 | 19 | 24 | 0.9 | 0.7–1.1 | 0.17 |
| Alveolar consolidation | 156 | 657 | 18 | 24 | 0.8 | 0.7–1.1 | 0.13 |
| MCPP n | 8 | 35 | 1 | 1 | 0.8 | 0.4–1.8 | 0.66 |
| Any bacterial marker | 827 | 2701 | 91 | 91 | 0.9 | 0.7–1.2 | 0.56 |
| Respiratory viral infections in the NP/OP: | |||||||
| AdV | 108 | 282 | 12 | 10 | 1.3 | 0.98–1.6 | 0.07 |
| HCoV | 56 | 232 | 6 | 8 | 0.8 | 0.6–1.1 | 0.14 |
| HMPV | 49 | 293 | 5 | 10 | 0.5 | 0.4–0.7 | <0.001 |
| PIV | 76 | 435 | 8 | 15 | 0.4 | 0.3–0.5 | <0.001 |
| HBoV | 138 | 364 | 15 | 12 | 0.3 | 0.2–0.5 | <0.001 |
| RSV | 121 | 832 | 13 | 28 | 0.3 | 0.2−0.4 | <0.001 |
| InFV A-C | 5 | 173 | 1 | 6 | 0.1 | 0.02–0.2 | <0.001 |
| Any viral co-infection | 431 | 2192 | 47 | 74 | 0.3 | 0.25–0.34 | <0.001 |
| Bacterial infections in the NP/OP: | |||||||
| H. influenzae type b | 23 | 57 | 3 | 2 | 1.4 | 0.8–2.3 | 0.19 |
| B. pertussis | 8 | 23 | 1 | 1 | 1.3 | 0.6–3.0 | 0.48 |
| C. pneumoniae | 9 | 26 | 1 | 1 | 1.2 | 0.5–2.5 | 0.73 |
| M. catarrhalis | 616 | 1944 | 68 | 66 | 1.1 | 0.96–1.3 | 0.15 |
| H. influenzae | 510 | 1567 | 56 | 53 | 1.1 | 0.9–1.3 | 0.23 |
| S. pneumoniae | 661 | 2117 | 72 | 72 | 1.04 | 0.9–1.2 | 0.66 |
| S. aureus | 129 | 494 | 14 | 17 | 0.86 | 0.7–1.1 | 0.18 |
| M. pneumoniae | 12 | 45 | 1 | 2 | 0.8 | 0.4–1.6 | 0.60 |
| Any bacterial co-infection | 845 | 2714 | 93 | 92 | 0.5 | 0.8–1.5 | 0.53 |
| Number | Percentage | ||||||
|---|---|---|---|---|---|---|---|
| Mono-RV Infections | Mixed-RV Infections a | Mono-RV Infections | Mixed-RV Infections a | aOR | 95%CI b | p-Value | |
| Demographic and health: | |||||||
| 12–59 months of age c | 231 | 162 | 48 | 38 | 0.6 | 0.5–0.8 | <0.001 |
| Never breast fed | 49 | 34 | 10 | 8 | 1.3 | 0.8–2.2 | 0.28 |
| Premature birth d | 57 | 30 | 12 | 7 | 1.3 | 0.8–1.95 | 0.28 |
| Underweight e | 156 | 121 | 32 | 28 | 1.1 | 0.9–1.5 | 0.38 |
| Male | 285 | 254 | 59 | 59 | 1.1 | 0.8–1.4 | 0.59 |
| Day Care attendance | 60 | 66 | 12 | 15 | 1.1 | 0.6–1.8 | 0.79 |
| Smoker in household | 163 | 177 | 34 | 41 | 0.95 | 0.7–1.3 | 0.76 |
| Clinical features: | |||||||
| Deaths f | 33 | 14 | 7 | 3 | 2.6 | 1.2–5.5 | 0.01 |
| Convulsions | 30 | 13 | 6 | 3 | 1.98 | 0.98–4.0 | 0.06 |
| Diarrhea | 67 | 51 | 14 | 12 | 1.2 | 0.8–1.9 | 0.31 |
| Tachycardia g | 199 | 240 | 46 | 50 | 1.1 | 0.8–1.4 | 0.57 |
| Very severe pneumonia | 163 | 128 | 34 | 30 | 1.1 | 0.8–1.5 | 0.71 |
| Wheezing | 214 | 207 | 45 | 48 | 0.99 | 0.7–1.4 | 0.95 |
| Chest X-ray abnormal h | 188 | 177 | 39 | 41 | 0.9 | 0.7–1.2 | 0.46 |
| Hospital stay >3 days | 233 | 220 | 48 | 51 | 0.9 | 0.7–1.2 | 0.45 |
| Hypoxic i | 156 | 141 | 33 | 33 | 0.8 | 0.6–1.1 | 0.22 |
| Tachypnea j | 380 | 405 | 89 | 84 | 0.8 | 0.5–1.1 | 0.18 |
| Any symptom | 480 | 429 | 100 | 100 | 2.2 | 0.2–24.8 | 0.51 |
| Bacterial co-infection markers: | |||||||
| MCPP k | 6 | 2 | 1 | 0 | 4.3 | 0.8–22.4 | 0.08 |
| Blood culture positive l | 18 | 11 | 4 | 3 | 1.9 | 0.9–4.3 | 0.12 |
| CRP ≥40 mg/mL m | 108 | 68 | 22 | 16 | 1.6 | 1.0–2.4 | 0.04 |
| Leukocytosis n | 256 | 187 | 55 | 47 | 1.3 | 0.9–1.7 | 0.13 |
| Fever o | 369 | 338 | 77 | 78 | 1.02 | 0.7–1.4 | 0.91 |
| Alveolar consolidation | 78 | 78 | 17 | 19 | 0.9 | 0.6–1.3 | 0.53 |
| Any marker of bacterial infection | 435 | 392 | 90 | 91 | 0.8 | 0.6–1.5 | 0.79 |
| Bacterial infections in the NP/OP: | |||||||
| B. pertussis | 6 | 2 | 1 | 0 | 3.0 | 0.6–15.2 | 0.18 |
| S. aureus | 76 | 53 | 16 | 12 | 1.4 | 0.98–2.1 | 0.06 |
| C. pneumoniae | 5 | 4 | 1 | 1 | 0.99 | 0.3–3.8 | 0.99 |
| M. catarrhalis | 313 | 303 | 65 | 70 | 0.7 | 0.5–0.98 | 0.04 |
| H. influenzae | 244 | 266 | 51 | 61 | 0.6 | 0.5–0.8 | 0.001 |
| S. pneumoniae | 338 | 323 | 70 | 75 | 0.6 | 0.6–1.01 | 0.07 |
| H. influenzae type b | 9 | 14 | 2 | 3 | 0.5 | 0.2–1.3 | 0.16 |
| M. pneumoniae | 4 | 8 | 1 | 2 | 0.4 | 0.1–1.5 | 0.18 |
| Any bacterial co-infection | 440 | 405 | 91 | 94 | 0.7 | 0.4–1.2 | 0.13 |
| Number | Percentage | ||||||
|---|---|---|---|---|---|---|---|
| RV+ cases | RV+ controls | RV+ cases | RV+ controls | aOR a | 95%CI a | p-Value a | |
| Demographic and health: | |||||||
| Underweight b | 140 | 66 | 36 | 16 | 2.8 | 2.0–4.0 | <0.001 |
| Male | 175 | 198 | 45 | 48 | 0.9 | 0.7–1.2 | 0.338 |
| Never breast fed | 32 | 30 | 8 | 7 | 1.5 | 0.8–2.7 | 0.213 |
| Smoker in household | 161 | 162 | 41 | 40 | 1.1 | 0.8–1.4 | 0.673 |
| Day Care attendance | 54 | 85 | 14 | 21 | 0.7 | 0.4–1.2 | 0.216 |
| Premature birth c | 32 | 48 | 8 | 12 | 0.8 | 0.5–1.3 | 0.428 |
| RV epidemiology: | |||||||
| RV Co-infections d | 162 | 181 | 41 | 44 | 0.9 | 0.7–1.2 | 0.526 |
| RV Mono-infection e | 231 | 228 | 59 | 56 | 1.1 | 0.8–1.5 | 0.526 |
| Respiratory viral co-infections in the NP/OP: | |||||||
| RSV | 17 | 6 | 4 | 1 | 3.2 | 1.2–8.2 | 0.018 |
| PIV | 24 | 33 | 6 | 8 | 0.7 | 0.4–1.3 | 0.245 |
| HBoV | 70 | 68 | 18 | 17 | 1.1 | 0.8–1.7 | 0.507 |
| HMPV | 147 | 26 | 4 | 6 | 0.7 | 0.3–1.3 | 0.243 |
| AdV | 59 | 81 | 15 | 20 | 0.7 | 0.5–1.1 | 0.105 |
| InFV A-C | 2 | 1 | 1 | 0 | 2.1 | 0.2–23 | 0.549 |
| HCoV | 20 | 34 | 5 | 8 | 0.6 | 0.3–1.0 | 0.069 |
| Bacterial infections in the NP/OP: | |||||||
| B. pertussis | 2 | 0 | 1 | 0 | - | - | 0.149 |
| H. influenzae type b | 10 | 12 | 3 | 3 | 0.8 | 0.4–2.0 | 0.701 |
| S. aureus | 41 | 34 | 10 | 8 | 1.4 | 0.8–2.2 | 0.193 |
| H. influenzae | 222 | 246 | 56 | 60 | 0.8 | 0.6–1.1 | 0.150 |
| M. pneumoniae | 7 | 12 | 2 | 3 | 0.6 | 0.2–1.5 | 0.288 |
| C. pneumoniae | 7 | 7 | 2 | 2 | 1.1 | 0.4–3.2 | 0.874 |
| S. pneumoniae | 285 | 339 | 73 | 83 | 0.5 | 0.4–0.7 | <0.001 |
| M. catarrhalis | 261 | 330 | 66 | 81 | 0.4 | 0.3–0.6 | <0.001 |
| Any bacterial co-infection | 366 | 397 | 93 | 97 | 0.4 | 0.2–0.8 | 0.010 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baillie, V.L.; Moore, D.P.; Mathunjwa, A.; Baggett, H.C.; Brooks, A.; Feikin, D.R.; Hammitt, L.L.; Howie, S.R.C.; Knoll, M.D.; Kotloff, K.L.; et al. Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study. Viruses 2021, 13, 1249. https://doi.org/10.3390/v13071249
Baillie VL, Moore DP, Mathunjwa A, Baggett HC, Brooks A, Feikin DR, Hammitt LL, Howie SRC, Knoll MD, Kotloff KL, et al. Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study. Viruses. 2021; 13(7):1249. https://doi.org/10.3390/v13071249
Chicago/Turabian StyleBaillie, Vicky L., David P. Moore, Azwifarwi Mathunjwa, Henry C. Baggett, Abdullah Brooks, Daniel R. Feikin, Laura L. Hammitt, Stephen R. C. Howie, Maria Deloria Knoll, Karen L. Kotloff, and et al. 2021. "Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study" Viruses 13, no. 7: 1249. https://doi.org/10.3390/v13071249
APA StyleBaillie, V. L., Moore, D. P., Mathunjwa, A., Baggett, H. C., Brooks, A., Feikin, D. R., Hammitt, L. L., Howie, S. R. C., Knoll, M. D., Kotloff, K. L., Levine, O. S., O’Brien, K. L., Scott, A. G., Thea, D. M., Antonio, M., Awori, J. O., Driscoll, A. J., Fancourt, N. S. S., Higdon, M. M., ... Madhi, S. A. (2021). Epidemiology of the Rhinovirus (RV) in African and Southeast Asian Children: A Case-Control Pneumonia Etiology Study. Viruses, 13(7), 1249. https://doi.org/10.3390/v13071249






