Antibodies Elicited in Response to a Single Cycle Glycoprotein D Deletion Viral Vaccine Candidate Bind C1q and Activate Complement Mediated Neutralization and Cytolysis
Abstract
:1. Introduction
2. Methods
2.1. Cells, Virus and Vaccines
2.2. Murine Vaccinations
2.3. C1q Binding ELISA
2.4. Viral Neutralization in the Absence or Presence of Complement
2.5. Complement Dependent Cytotoxicity
2.6. Passive Transfer Studies
2.7. Statistical Analysis
3. Results
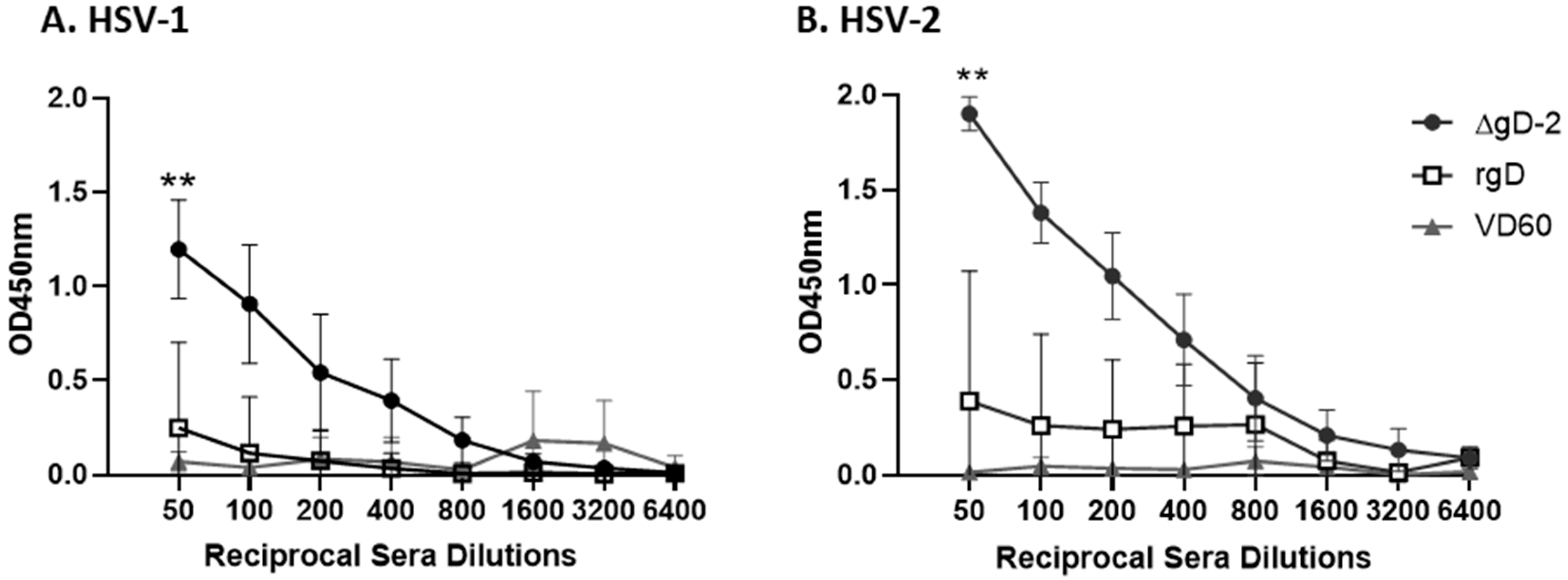
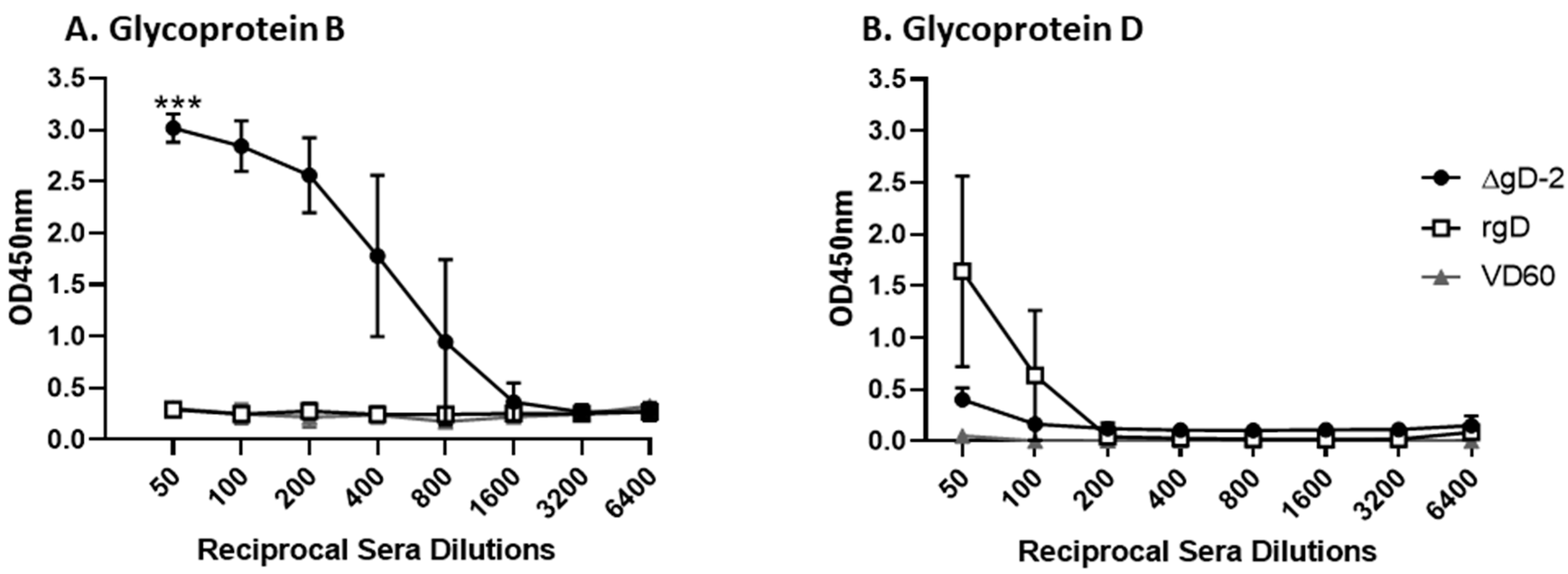
3.1. Immune Serum from ΔgD-2, but Not rgD-2Alum-MPL Vaccinated Mice Binds C1q
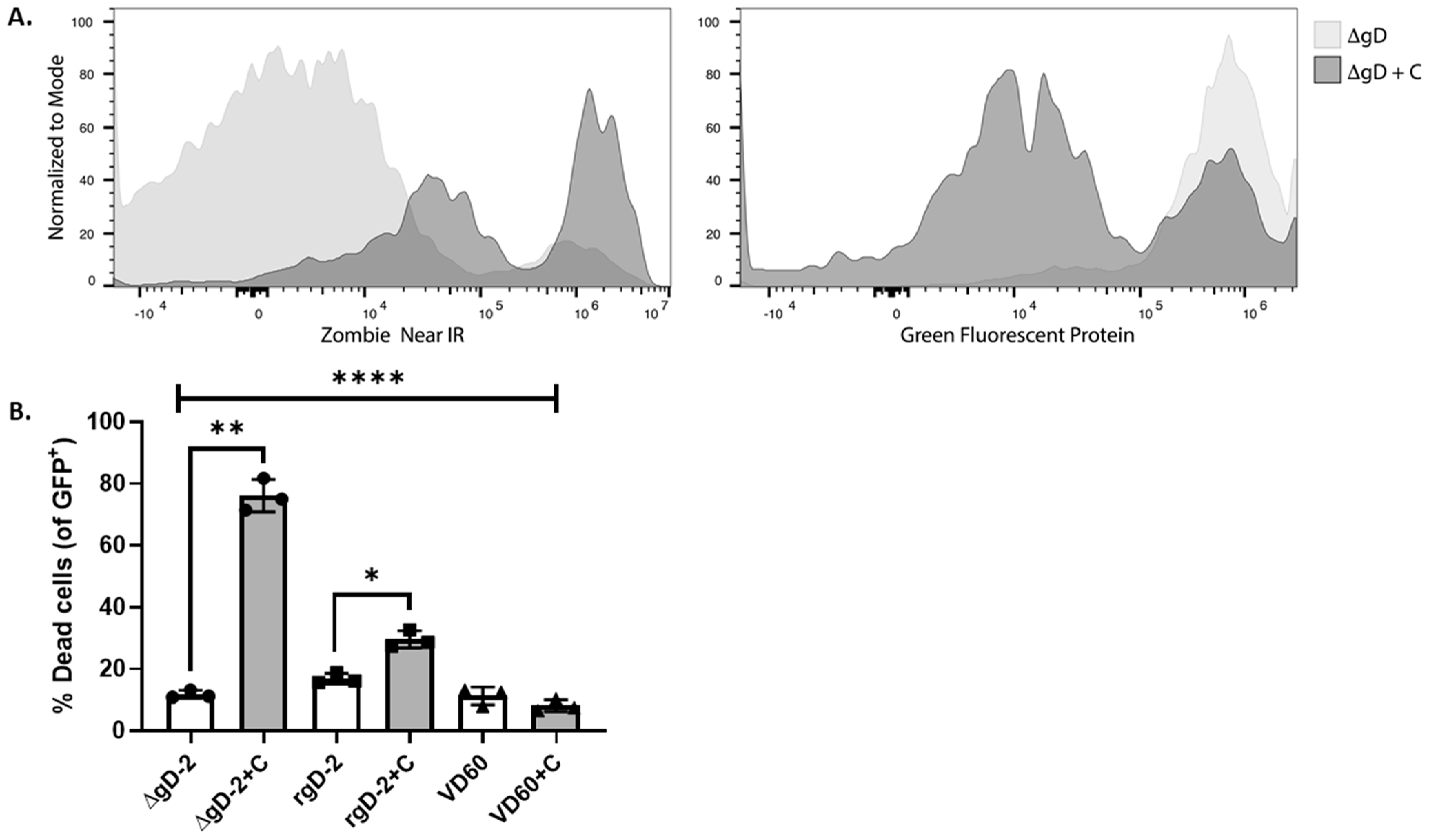
3.2. gD-2 Immune Serum Mediates Complement Dependent Cytotoxicity
3.3. Complement Enhances Neutralization Potency of ΔgD-2 Immune Serum
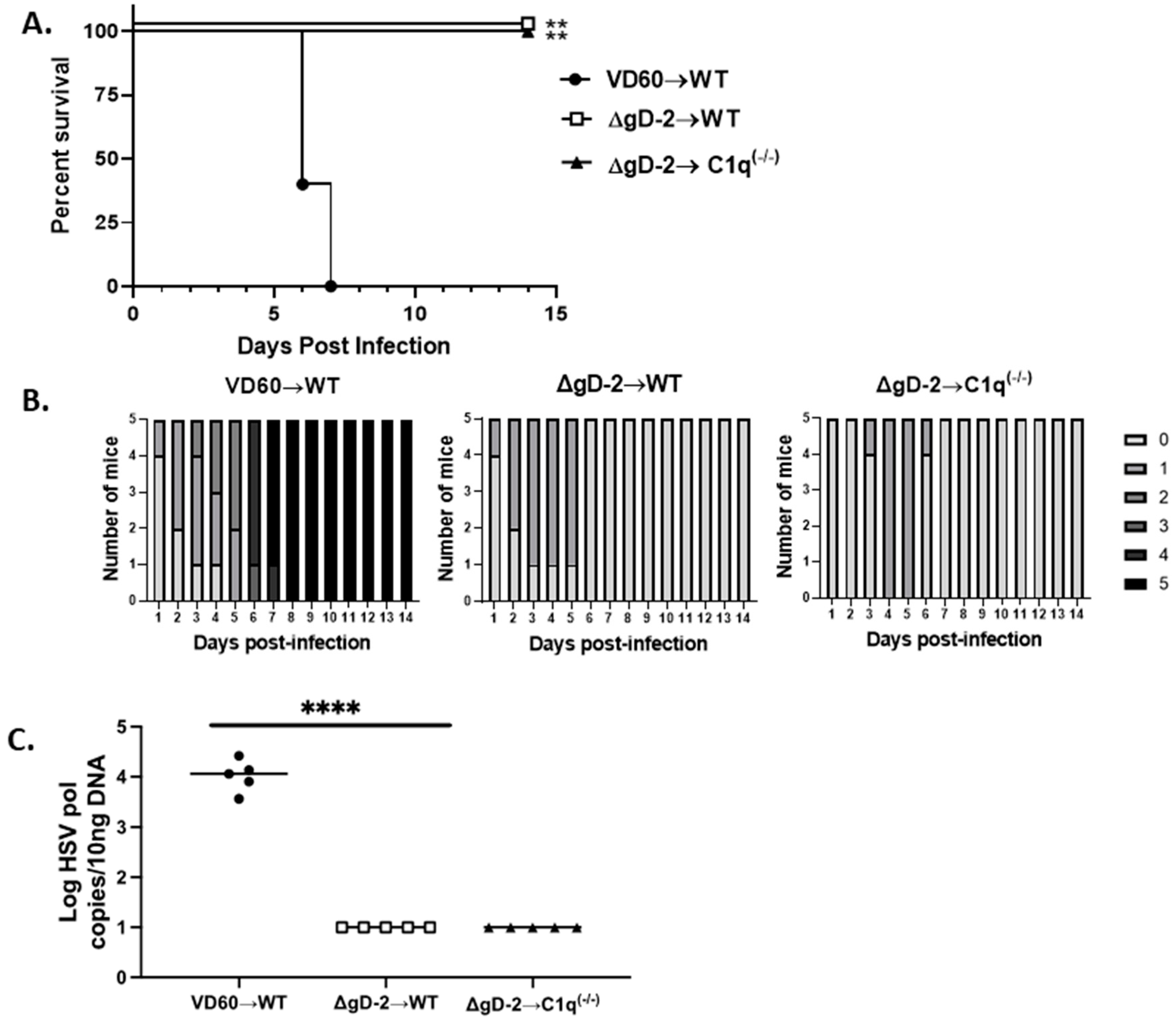
3.4. C1q Is Not Required for Passive Protection
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE. 2015, 10, e0140765. [Google Scholar] [CrossRef] [Green Version]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE. 2015, 10, e114989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corey, L. Synergistic copathogens--HIV-1 and HSV-2. N. Engl. J. Med. 2007, 356, 854–856. [Google Scholar] [CrossRef]
- Abu-Raddad, L.J.; Magaret, A.S.; Celum, C.; Wald, A.; Longini, I.M.; Self, S.G.; Corey, L. Genital herpes has played a more important role than any other sexually transmitted infection in driving HIV prevalence in Africa. PLoS ONE 2008, 3, e2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottlieb, S.L.; Giersing, B.K.; Hickling, J.; Jones, R.; Deal, C.; Kaslow, D.C. Meeting report: Initial World Health Organization consultation on herpes simplex virus (HSV) vaccine preferred product characteristics, March 2017. Vaccine 2019, 37, 7408–7418. [Google Scholar] [CrossRef]
- Aschner, C.B.; Herold, B.C. Alphaherpesvirus Vaccines. Curr. Issues Mol. Biol. 2021, 41, 469–508. [Google Scholar] [CrossRef]
- Belshe, R.B.; Heineman, T.C.; Bernstein, D.I.; Bellamy, A.R.; Ewell, M.; Van Der Most, R.; Deal, C.D. Correlate of immune protection against HSV-1 genital disease in vaccinated women. J. Infect. Dis. 2014, 209, 828–836. [Google Scholar] [CrossRef] [Green Version]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.A.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, D.I.; Aoki, F.Y.; Tyring, S.K.; Stanberry, L.R.; Pierre, C.S.; Shafran, S.D.; Roels, G.L.; Van Herck, K.; Bollaerts, A.; Dubin, G.; et al. Safety and immunogenicity of glycoprotein D-adjuvant genital herpes vaccine. Clin. Infect. Dis. 2005, 40, 1271–1281. [Google Scholar] [CrossRef] [PubMed]
- Stanberry, L.R.; Spruance, S.L.; Cunningham, A.L.; Bernstein, D.I.; Mindel, A.; Sacks, S.; Tyring, S.; Aoki, F.Y.; Slaoui, M.; Denis, M.; et al. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N. Engl. J. Med. 2002, 347, 1652–1661. [Google Scholar] [CrossRef]
- Corey, L.; Langenberg, A.G.; Ashley, R.; Sekulovich, R.E.; Izu, A.E.; Douglas, J.M., Jr.; Handsfield, H.H.; Warren, T.; Marr, L.; Tyring, S.; et al. Recombinant glycoprotein vaccine for the prevention of genital HSV-2 infection: Two randomized controlled trials. Chiron HSV Vaccine Study Group. JAMA 1999, 282, 331–340. [Google Scholar] [CrossRef]
- Aschner, C.B.; Pierce, C.; Knipe, D.M.; Herold, B.C. Vaccination Route as a Determinant of Protective Antibody Responses against Herpes Simplex Virus. Vaccines 2020, 8, 277. [Google Scholar] [CrossRef]
- Ramsey, N.L.M.; Visciano, M.; Hunte, R.; Loh, L.N.; Aschner, C.B.; Jacobs, W.R.; Herold, B.C. A Single-Cycle Glycoprotein D Deletion Viral Vaccine Candidate, DeltagD-2, Elicits Polyfunctional Antibodies That Protect against Ocular Herpes Simplex Virus. J. Virol. 2020, 94, e00335-20. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.M.; Goymer, J.; Loh, L.N.; Mahant, A.; Burn Aschner, C.; Herold, B.C. Murine Model of Maternal Immunization Demonstrates Protective Role for Antibodies That Mediate Antibody-Dependent Cellular Cytotoxicity in Protecting Neonates From Herpes Simplex Virus Type 1 and Type 2. J. Infect. Dis. 2020, 221, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Burn, C.; Ramsey, N.; Garforth, S.J.; Almo, S.; Jacobs, W.R., Jr.; Herold, B.C. A Herpes Simplex Virus (HSV)-2 Single-Cycle Candidate Vaccine Deleted in Glycoprotein D Protects Male Mice from Lethal Skin Challenge with Clinical Isolates of HSV-1 and HSV-2. J. Infect. Dis. 2018, 217, 754–758. [Google Scholar]
- Petro, C.D.; Weinrick, B.; Khajoueinejad, N.; Burn, C.; Sellers, R.; Jacobs, W.R., Jr.; Herold, B.C. HSV-2 DeltagD elicits FcgammaR-effector antibodies that protect against clinical isolates. JCI Insight. 2016, 1, e88529. [Google Scholar] [CrossRef] [Green Version]
- Petro, C.; González, P.A.; Cheshenko, N.; Jandl, T.; Khajoueinejad, N.; Bénard, A.; Sengupta, M.; Herold, B.C.; Jacobs, W.R. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 2015, 4, e06054. [Google Scholar] [CrossRef] [PubMed]
- Burn Aschner, C.; Loh, L.N.; Galen, B.; Delwel, I.; Jangra, R.K.; Garforth, S.J.; Chandran, K.; Almo, S.; Jacobs, W.R., Jr.; Ware, C.F.; et al. HVEM signaling promotes protective antibody-dependent cellular cytotoxicity (ADCC) vaccine responses to herpes simplex viruses. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Mehlhop, E.; Nelson, S.; Jost, C.A.; Gorlatov, S.; Johnson, S.; Fremont, D.H.; Diamond, M.S.; Pierson, T.C. Complement protein C1q reduces the stoichiometric threshold for antibody-mediated neutralization of West Nile virus. Cell Host Microbe 2009, 6, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Ligas, M.W.; Johnson, D.C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J. Virol. 1988, 62, 1486–1494. [Google Scholar] [CrossRef] [Green Version]
- Cheshenko, N.; Trepanier, J.B.; Stefanidou, M.; Buckley, N.; Gonzalez, P.; Jacobs, W.; Herold, B.C. HSV activates Akt to trigger calcium release and promote viral entry: Novel candidate target for treatment and suppression. FASEB J. 2013, 27, 2584–2599. [Google Scholar] [CrossRef] [PubMed]
- Ejercito, P.M.; Kieff, E.D.; Roizman, B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J. Gen. Virol. 1968, 2, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Nixon, B.; Stefanidou, M.; Mesquita, P.M.M.; Fakioglu, E.; Segarra, T.; Rohan, L.; Halford, W.; Palmer, K.; Herold, B.C. Griffithsin Protects Mice from Genital Herpes by Preventing Cell-to-Cell Spread. J. Virol. 2013, 87, 6257–6269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segarra, T.J.; Fakioglu, E.; Cheshenko, N.; Wilson, S.S.; Mesquita, P.M.M.; Doncel, G.F.; Herold, B.C. Bridging the gap between preclinical and clinical microbicide trials: Blind evaluation of candidate gels in murine models of efficacy and safety. PLoS ONE 2011, 6, e27675. [Google Scholar] [CrossRef] [PubMed]
- Nemerow, G.R.; Jensen, F.C.; Cooper, N.R. Neutralization of Epstein-Barr virus by nonimmune human serum. Role of cross-reacting antibody to herpes simplex virus and complement. J. Clin. Investig. 1982, 70, 1081–1091. [Google Scholar] [CrossRef] [Green Version]
- Cairns, T.M.; Huang, Z.-Y.; Gallagher, J.R.; Lin, Y.; Lou, H.; Whitbeck, J.C.; Wald, A.; Cohen, G.H.; Eisenberg, R.J. Patient-Specific Neutralizing Antibody Responses to Herpes Simplex Virus Are Attributed to Epitopes on gD, gB, or Both and Can Be Type Specific. J. Virol. 2015, 89, 9213–9231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Freed, D.C.; Tang, A.; Rustandi, R.R.; Troutman, M.C.; Espeseth, A.S.; Zhang, N.; An, Z.; McVoy, M.; Zhu, H.; et al. Complement enhances in vitro neutralizing potency of antibodies to human cytomegalovirus glycoprotein B (gB) and immune sera induced by gB/MF59 vaccination. NPJ Vaccines 2017, 2, 1–8. [Google Scholar] [CrossRef]
- Gerber, S.I.; Belval, B.J.; Herold, B.C. Differences in the role of glycoprotein C of HSV-1 and HSV-2 in viral binding may contribute to serotype differences in cell tropism. Virology 1995, 214, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Komala Sari, T.; Gianopulos, K.A.; Nicola, A.V. Glycoprotein C of Herpes Simplex Virus 1 Shields Glycoprotein B from Antibody Neutralization. J. Virol. 2020, 94, e01852-19. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Huang, J.; Shaw, C.; Friedman, H.M. Blocking herpes simplex virus 2 glycoprotein E immune evasion as an approach to enhance efficacy of a trivalent subunit antigen vaccine for genital herpes. J. Virol. 2014, 88, 8421–8432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awasthi, S.; Hook, L.M.; Pardi, N.; Wang, F.; Myles, A.; Cancro, M.P.; Cohen, G.H.; Weissman, D.; Friedman, H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 2019, 4. [Google Scholar] [CrossRef] [PubMed]
- Bergman, I.; Basse, P.H.; Barmada, M.A.; Griffin, J.A.; Cheung, N.K. Comparison of in vitro antibody-targeted cytotoxicity using mouse, rat and human effectors. Cancer Immunol. Immunother. 2000, 49, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Gunn, B.M.; Roy, V.; Karim, M.M.; Hartnett, J.N.; Suscovich, T.J.; Goba, A.; Momoh, M.; Sandi, J.D.; Kanneh, L.; Andersen, K.G.; et al. Survivors of Ebola Virus Disease Develop Polyfunctional Antibody Responses. J. Infect. Dis. 2020, 221, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, H.; Crowley, A.R.; Butler, S.E.; Xu, S.; Weiner, J.A.; Bloch, E.M.; Littlefield, K.; Wendy Wieland-Alter, R.I.C.; Connor, R.I.; Wright, P.F.; et al. Markers of Polyfunctional SARS-CoV-2 Antibodies in Convalescent Plasma. mBio 2021, 12, e00765-21. [Google Scholar] [CrossRef]
- Ackerman, M.E.; Mikhailova, A.; Brown, E.P.; Dowell, K.G.; Walker, B.D.; Bailey-Kellogg, C.; Suscovich, T.J.; Alter, G. Polyfunctional HIV-Specific Antibody Responses Are Associated with Spontaneous HIV Control. PLoS Pathog. 2016, 12, e1005315. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Visciano, M.L.; Mahant, A.M.; Pierce, C.; Hunte, R.; Herold, B.C. Antibodies Elicited in Response to a Single Cycle Glycoprotein D Deletion Viral Vaccine Candidate Bind C1q and Activate Complement Mediated Neutralization and Cytolysis. Viruses 2021, 13, 1284. https://doi.org/10.3390/v13071284
Visciano ML, Mahant AM, Pierce C, Hunte R, Herold BC. Antibodies Elicited in Response to a Single Cycle Glycoprotein D Deletion Viral Vaccine Candidate Bind C1q and Activate Complement Mediated Neutralization and Cytolysis. Viruses. 2021; 13(7):1284. https://doi.org/10.3390/v13071284
Chicago/Turabian StyleVisciano, Maria Luisa, Aakash Mahant Mahant, Carl Pierce, Richard Hunte, and Betsy C. Herold. 2021. "Antibodies Elicited in Response to a Single Cycle Glycoprotein D Deletion Viral Vaccine Candidate Bind C1q and Activate Complement Mediated Neutralization and Cytolysis" Viruses 13, no. 7: 1284. https://doi.org/10.3390/v13071284
APA StyleVisciano, M. L., Mahant, A. M., Pierce, C., Hunte, R., & Herold, B. C. (2021). Antibodies Elicited in Response to a Single Cycle Glycoprotein D Deletion Viral Vaccine Candidate Bind C1q and Activate Complement Mediated Neutralization and Cytolysis. Viruses, 13(7), 1284. https://doi.org/10.3390/v13071284





