Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Virus Infection
2.2. Reagents and Antibodies
2.3. Western Blotting
2.4. Real-Time PCR
2.5. Cloning of Viperin and NSP4
2.6. Knockdown of Viperin
2.7. Confocal Microscopy
2.8. Estimation of Infectious Virus Particle by Plaque Assay
2.9. Isolation of the ER Fraction
2.10. Isolation of Mitochondrial and Cytosolic Fractions
2.11. Co-Immunoprecipitation
2.12. MTT Assay
2.13. Statistical Analysis
3. Results
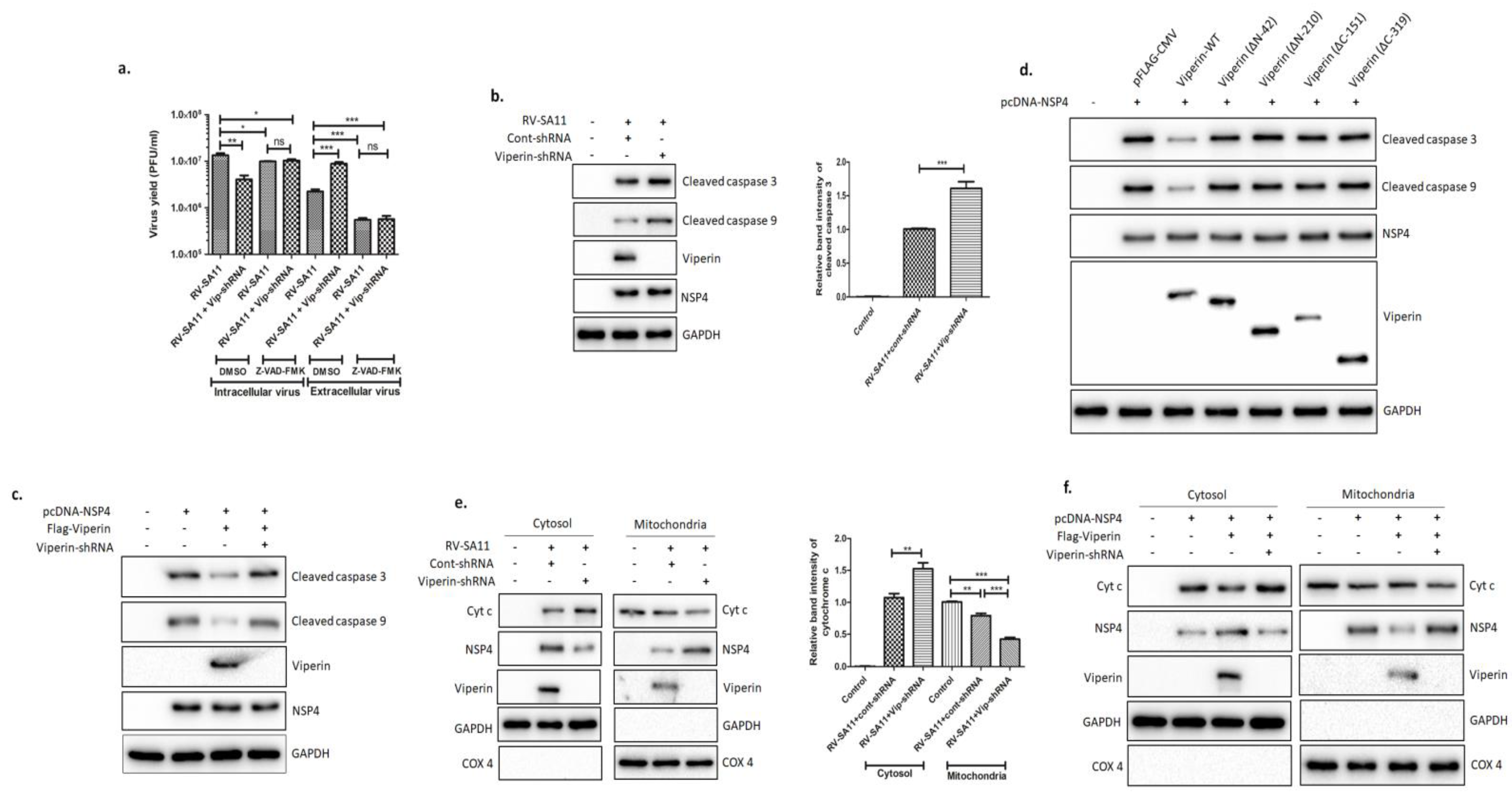
3.1. Rotavirus Infection Induced the Expression of Viperin
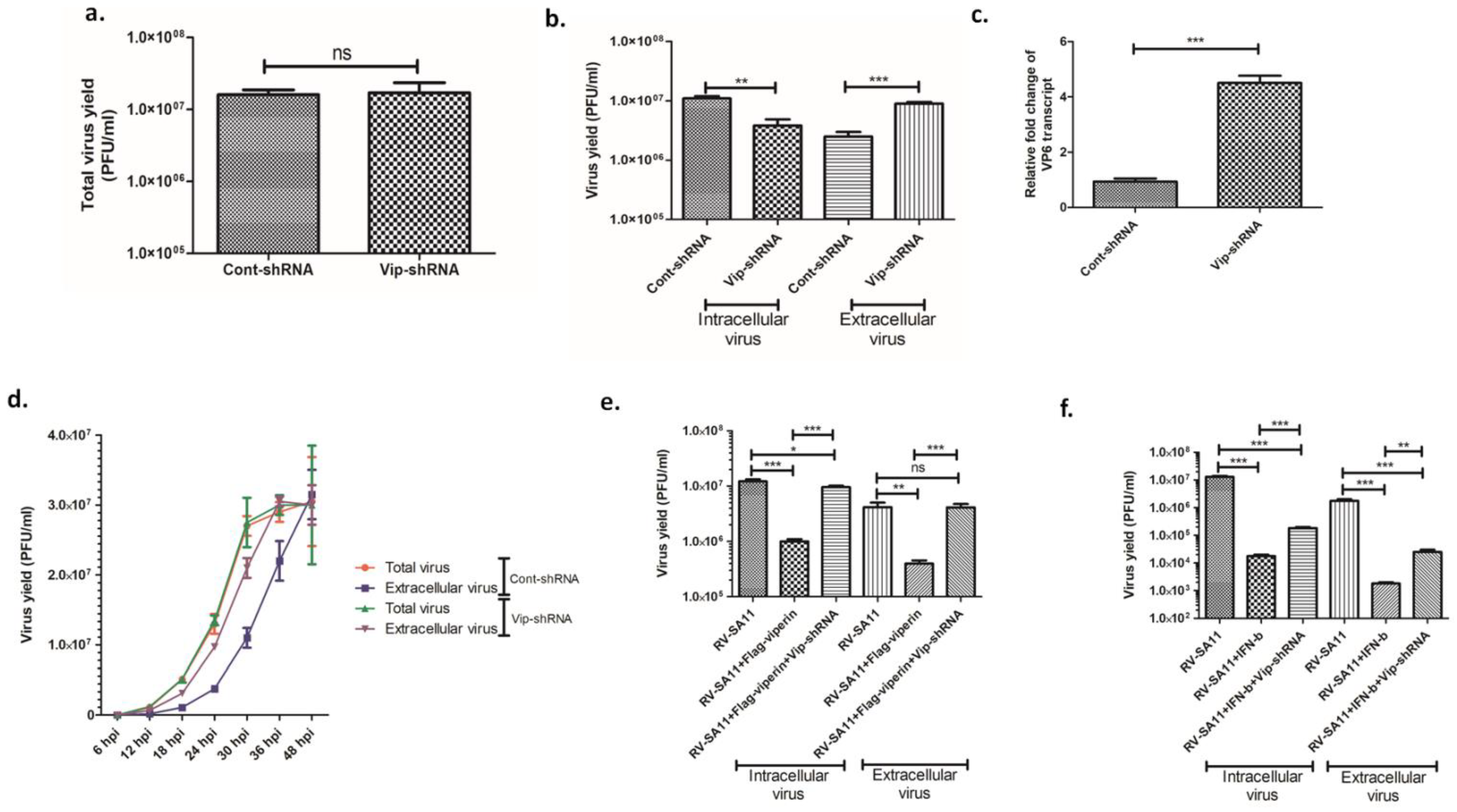
3.2. Viperin Delayed the Release of Rotavirus from the Infected Host Cell
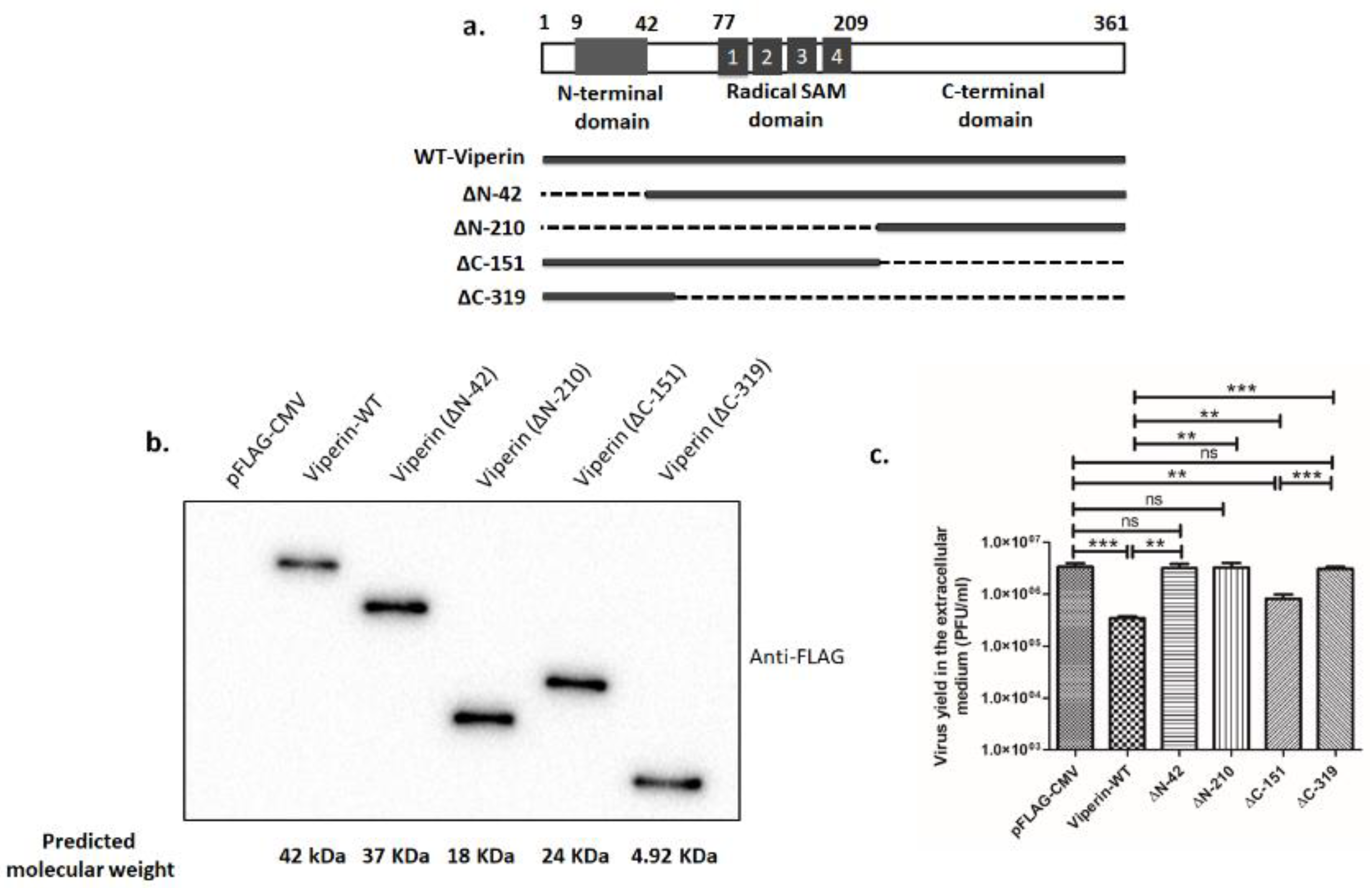
3.3. Full-Length Viperin Was Essential for Anti-Rotaviral Function
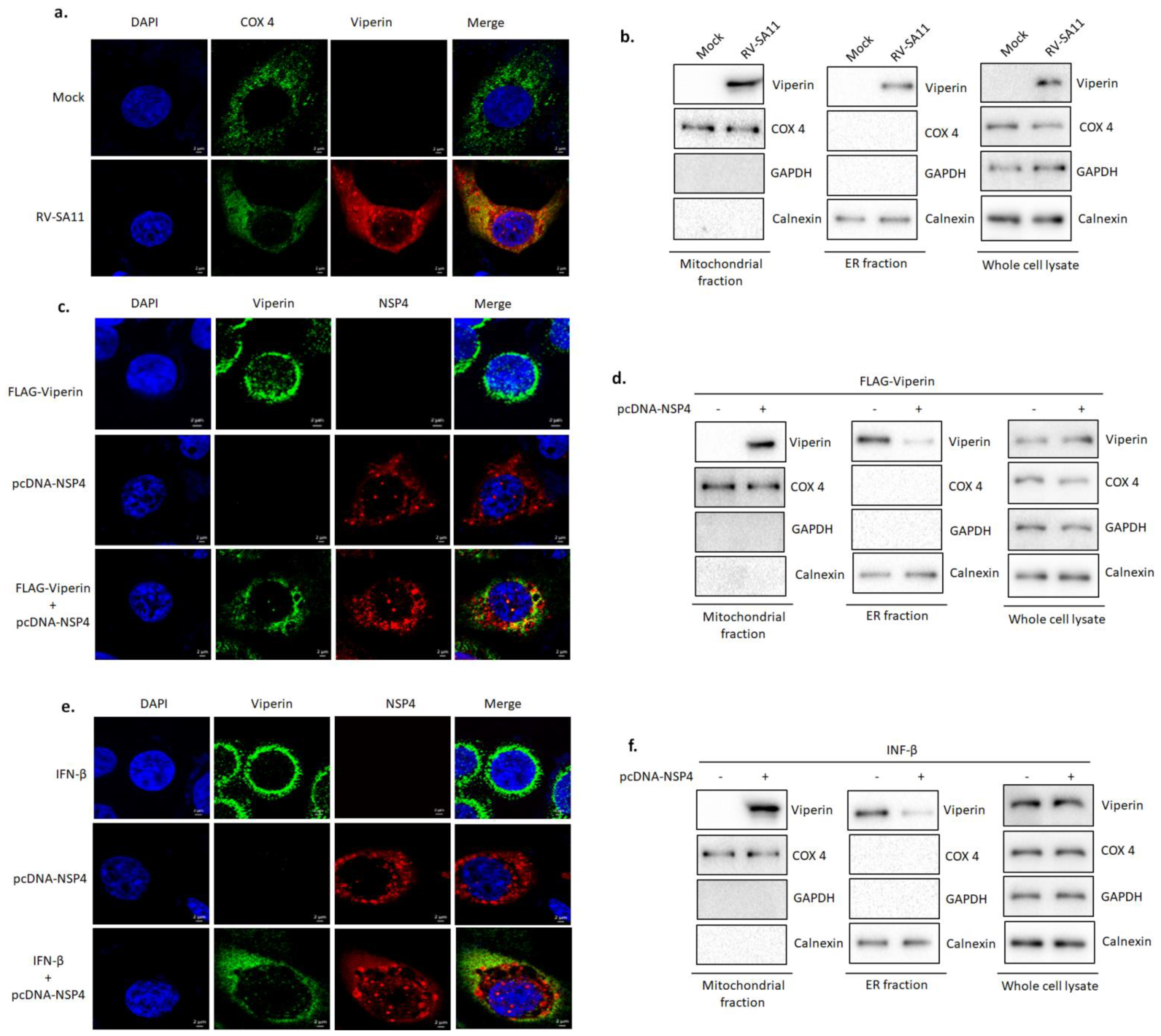
3.4. NSP4 Triggered Viperin Relocalization from the ER to the Mitochondria during Rotavirus Infection
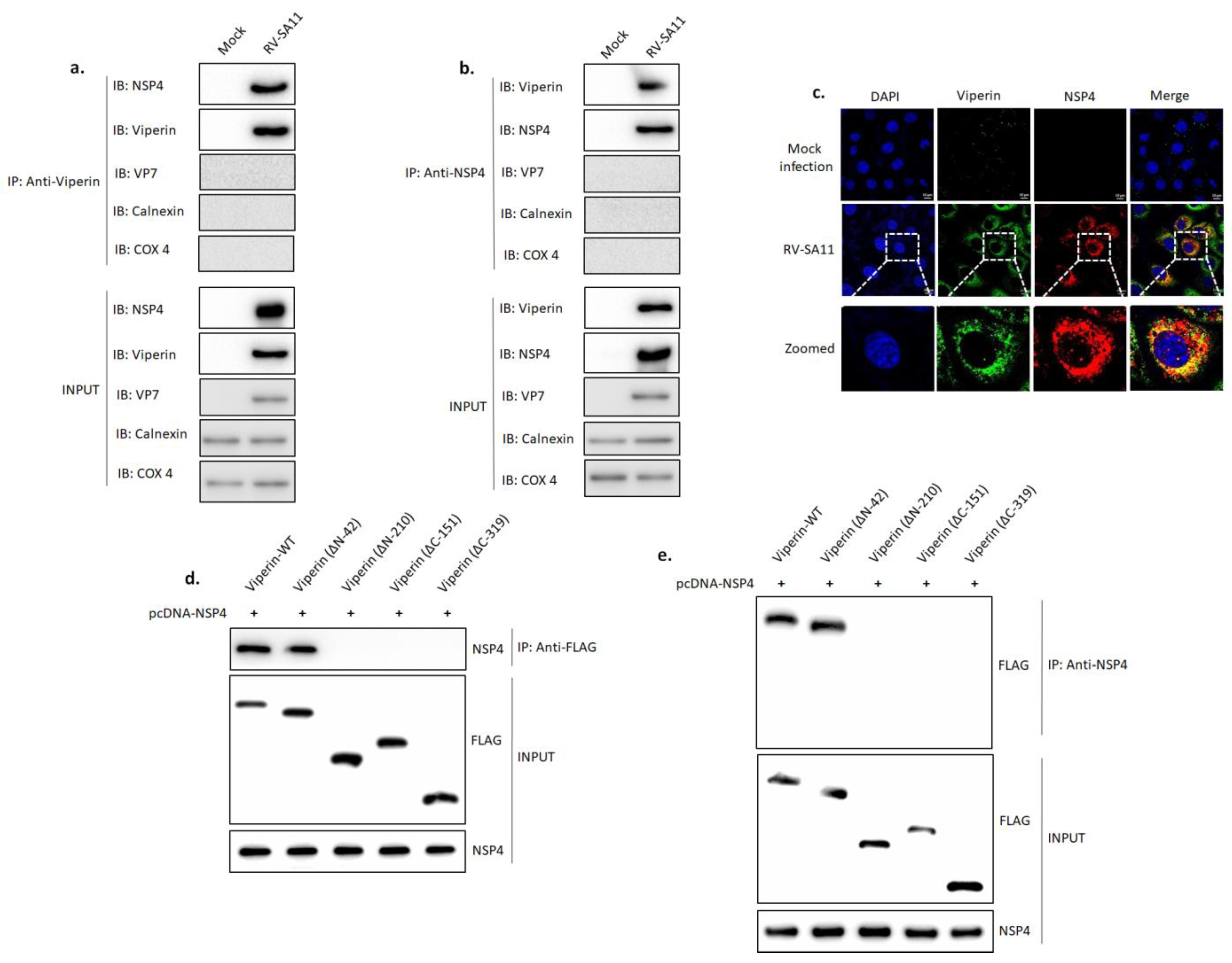
3.5. Viperin Associated with NSP4
3.6. Viperin Inhibited the NSP4-Induced Intrinsic Apoptotic Pathway by Restricting the Translocation of NSP4 to the Mitochondria
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Desselberger, U. Rotavirus infection. Nat. Rev. Dis. Prim. 2017, 3, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lawton, J.A.; Estes, M.K.; Prasad, B.V.V. Three-dimensional visualization of mRNA release from actively transcribing rotavirus particles. Nat. Genet. 1997, 4, 118–121. [Google Scholar] [CrossRef]
- Estes, M.K.; Kapikian, A.Z. Rotaviruses. In Fields Virology, 5th ed.; Knipe, D.M., Howley, P.M., Griffin, D.E., Lamb, R.A., Martin, M.A., Roizman, B., Straus, S.E., Eds.; Kluwer/Lippincott, Williams and Wilkins: Philadelphia, PA, USA, 2007; Volume 2, pp. 1917–1974. [Google Scholar]
- Piron, M.; Vende, P.; Cohen, J.; Poncet, D. Rotavirus RNA-binding protein NSP3 interacts with eIF4GI and evicts the poly(A) binding protein from eIF4F. EMBO J. 1998, 17, 5811–5821. [Google Scholar] [CrossRef] [PubMed]
- Groft, C.M.; Burley, S.K. Recognition of eIF4G by rotavirus NSP3 reveals a basis for mRNA circularization. Mol. Cell 2002, 9, 1273–1283. [Google Scholar] [CrossRef]
- Montero, H.; Arias, C.F.; Lopez, S. Rotavirus Nonstructural Protein NSP3 Is Not Required for Viral Protein Synthesis. J. Virol. 2006, 80, 9031–9038. [Google Scholar] [CrossRef] [PubMed]
- Barro, M.; Patton, J.T. Rotavirus NSP1 inhibits expression of type I interferon by antagonizing the function of interferon regulatory factors IRF3, IRF5, and IRF7. J. Virol. 2007, 81, 4473–4481. [Google Scholar] [CrossRef]
- Fabbretti, E.; Afrikanova, I.; Vascotto, F.; Burrone, O.R. Two non-structural rotavirus proteins, NSP2 and NSP5, form viroplasm-like structures in vivo. J. Gen. Virol. 1999, 80, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Chnaiderman, J.; Barro, M.; Spencer, E. NSP5 phosphorylation regulates the fate of viral mRNA in rotavirus infected cells. Arch. Virol. 2002, 147, 1899–1911. [Google Scholar] [CrossRef] [PubMed]
- López, T.; Rojas, M.; Ayala-Bretón, C.; Lopez, S.; Arias, C.F. Reduced expression of the rotavirus NSP5 gene has a pleiotropic effect on virus replication. J. Gen. Virol. 2005, 86, 1609–1617. [Google Scholar] [CrossRef]
- Patton, J.T.; Silvestri, L.S.; Tortorici, M.A.; Vasquez-Del Carpio, R.; Taraporewala, Z.F. Rotavirus genome replication and morphogenesis: Role of the viroplasm. In Reoviruses: Entry, Assembly and Morphogenesis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 169–187. [Google Scholar]
- Eichwald, C.; Rodriguez, J.F.; Burrone, O.R. Characterization of rotavirus NSP2/NSP5 interactions and the dynamics of viroplasm formation. J. Gen. Virol. 2004, 85, 625–634. [Google Scholar] [CrossRef]
- González, R.A.; Espinosa, R.; Romero, P.; Lopez, S.; Arias, C.F. Relative localization of viroplasmic and endoplasmic reticulum-resident rotavirus proteins in infected cells. Arch. Virol. 2000, 145, 1963–1973. [Google Scholar] [CrossRef]
- Jourdan, N.; Maurice, M.; Delautier, D.; Quero, A.M.; Servin, A.L.; Trugnan, G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the Golgi apparatus. J. Virol. 1997, 71, 8268–8278. [Google Scholar] [CrossRef]
- Hyser, J.M.; Collinson-Pautz, M.R.; Utama, B.; Estes, M.K. Rotavirus disrupts calcium homeostasis by NSP4 vi-roporin activity. MBio 2010, 1, e00265-10. [Google Scholar] [CrossRef] [PubMed]
- Hyser, J.M.; Estes, M.K. Pathophysiological Consequences of Calcium-Conducting Viroporins. Annu. Rev. Virol. 2015, 2, 473–496. [Google Scholar] [CrossRef] [PubMed]
- Sen, A.; Sen, N.; Mackow, E.R. The Formation of Viroplasm-Like Structures by the Rotavirus NSP5 Protein Is Calcium Regulated and Directed by a C-Terminal Helical Domain. J. Virol. 2007, 81, 11758–11767. [Google Scholar] [CrossRef]
- Crawford, S.E.; Estes, M.K. Viroporin-mediated calcium-activated autophagy. Autophagy 2013, 9, 797–798. [Google Scholar] [CrossRef][Green Version]
- Abdoli, A.; Alirezaei, M.; Mehrbod, P.; Forouzanfar, F. Autophagy: The multi-purpose bridge in viral infections and host cells. Rev. Med. Virol. 2018, 28, e1973. [Google Scholar] [CrossRef] [PubMed]
- Au, K.S.; Chan, W.K.; Burns, J.W.; Estes, M.K. Receptor activity of rotavirus nonstructural glycoprotein NS28. J. Virol. 1989, 63, 4553–4562. [Google Scholar] [CrossRef]
- Meyer, J.C.; Bergmann, C.; Bellamy, A. Interaction of rotavirus cores with the nonstructural glycoprotein NS28. Virology 1989, 171, 98–107. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patra, U.; Bhowmick, R.; Chawla-Sarkar, M. Rotaviral nonstructural protein 4 triggers dynamin-related protein 1-dependent mitochondrial fragmentation during infection. Cell. Microbiol. 2018, 20, e12831. [Google Scholar] [CrossRef]
- Broquet, A.; Hirata, Y.; McAllister, C.S.; Kagnoff, M.F. RIG-I/MDA5/MAVS Are Required to Signal a Protective IFN Response in Rotavirus-Infected Intestinal Epithelium. J. Immunol. 2010, 186, 1618–1626. [Google Scholar] [CrossRef]
- Holloway, G.; Truong, T.T.; Coulson, B.S. Rotavirus Antagonizes Cellular Antiviral Responses by Inhibiting the Nuclear Accumulation of STAT1, STAT2, and NF-κB. J. Virol. 2009, 83, 4942–4951. [Google Scholar] [CrossRef]
- Graff, J.W.; Ettayebi, K.; Hardy, M.E. Rotavirus NSP1 inhibits NFκB activation by inducing proteasome-dependent degradation of β-TrCP: A novel mechanism of IFN antagonism. PLoS Pathog. 2009, 5, e1000280. [Google Scholar] [CrossRef]
- Arnold, M.M.; Barro, M.; Patton, J.T. Rotavirus NSP1 Mediates Degradation of Interferon Regulatory Factors through Targeting of the Dimerization Domain. J. Virol. 2013, 87, 9813–9821. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y.; Broquet, A.; Menchén, L.; Kagnoff, M.F. Activation of Innate Immune Defense Mechanisms by Signaling through RIG-I/IPS-1 in Intestinal Epithelial Cells. J. Immunol. 2007, 179, 5425–5432. [Google Scholar] [CrossRef]
- Bagchi, P.; Nandi, S.; Chattopadhyay, S.; Bhowmick, R.; Halder, U.C.; Nayak, M.K.; Chawla-Sarkar, M. Identifi-cation of common human host genes involved in pathogenesis of different rotavirus strains: An attempt to recognize probable antiviral targets. Virus Res. 2012, 169, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cong, J.-P.; Mamtora, G.; Gingeras, T.; Shenk, T. Cellular gene expression altered by human cytomegalovirus: Global monitoring with oligonucleotide arrays. Proc. Natl. Acad. Sci. USA 1998, 95, 14470–14475. [Google Scholar] [CrossRef]
- Chin, K.C.; Cresswell, P. Viperin (cig5), an IFN-inducible antiviral protein directly induced by human cytomeg-alovirus. Proc. Natl. Acad. Sci. USA 2001, 98, 15125–15130. [Google Scholar] [CrossRef]
- Zhang, Y.; Burke, C.W.; Ryman, K.D.; Klimstra, W.B. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 2007, 81, 11246–11255. [Google Scholar] [CrossRef]
- Wang, X.; Hinson, E.R.; Cresswell, P. The Interferon-Inducible Protein Viperin Inhibits Influenza Virus Release by Perturbing Lipid Rafts. Cell Host Microbe 2007, 2, 96–105. [Google Scholar] [CrossRef]
- Boudinot, P.; Riffault, S.; Salhi, S.; Carrat, C.; Sedlik, C.; Mahmoudi, N.; Benmansour, A. Vesicular stomatitis virus and pseudorabies virus induce a vig1/cig5 homologue in mouse dendritic cells via different pathwaysThe GenBank accession number of the sequence reported in this paper is AF236064. J. General Virol. 2000, 81, 2675–2682. [Google Scholar] [CrossRef]
- Chan, Y.L.; Chang, T.H.; Liao, C.L.; Lin, Y.L. The cellular antiviral protein viperin is attenuated by pro-teasome-mediated protein degradation in Japanese encephalitis virus-infected cells. J. Virol. 2008, 82, 10455–10464. [Google Scholar] [CrossRef] [PubMed]
- Szretter, K.; Brien, J.D.; Thackray, L.B.; Virgin, H.W.; Cresswell, P.; Diamond, M.S. The Interferon-Inducible Gene viperin Restricts West Nile Virus Pathogenesis. J. Virol. 2011, 85, 11557–11566. [Google Scholar] [CrossRef]
- Helbig, K.J.; Lau, D.T.-Y.; Semendric, L.; Harley, H.A.J.; Beard, M.R. Analysis of ISG expression in chronic hepatitis C identifies viperin as a potential antiviral effector. Hepatology 2005, 42, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.J.; Eyre, N.; Yip, E.; Narayana, S.; Li, K.; Fiches, G.; McCartney, E.M.; Jangra, R.K.; Lemon, S.M.; Beard, M.R. The antiviral protein viperin inhibits hepatitis C virus replication via interaction with nonstructural protein 5A. Hepatology 2011, 54, 1506–1517. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, X.; Pan, T.; Song, W.; Wang, Y.; Zhang, F.; Yuan, Z. Viperin inhibits hepatitis C virus replication by interfering with binding of NS5A to host protein hVAP-33. J. Gen. Virol. 2012, 93, 83–92. [Google Scholar] [CrossRef]
- Teng, T.-S.; Foo, S.-S.; Simamarta, D.; Lum, F.-M.; Teo, T.-H.; Lulla, A.; Yeo, N.K.; Koh, E.G.; Chow, A.; Leo, Y.-S.; et al. Viperin restricts chikungunya virus replication and pathology. J. Clin. Investig. 2012, 122, 4447–4460. [Google Scholar] [CrossRef] [PubMed]
- Carissimo, G.; Teo, T.-H.; Chan, Y.-H.; Lee, C.Y.-P.; Lee, B.; Torres-Ruesta, A.; Tan, J.J.; Chua, T.-K.; Fong, S.-W.; Lum, F.-M.; et al. Viperin controls chikungunya virus–specific pathogenic T cell IFNγ Th1 stimulation in mice. Life Sci. Alliance 2019, 2, e201900298. [Google Scholar] [CrossRef] [PubMed]
- Proud, D.; Turner, R.B.; Winther, B.; Wiehler, S.; Tiesman, J.P.; Reichling, T.D.; Poore, C.L. Gene expression profiles during in vivo human rhinovirus infection: Insights into the host response. Am. J. Respir. Crit. Care Med. 2008, 178, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Khaiboullina, S.F.; Rizvanov, A.A.; Holbrook, M.R.; Jeor, S.S. Yellow fever virus strains Asibi and 17D-204 infect human umbilical cord endothelial cells and induce novel changes in gene expression. Virology 2005, 342, 167–176. [Google Scholar] [CrossRef]
- Hinson, E.R.; Joshi, N.S.; Chen, J.H.; Rahner, C.; Jung, Y.W.; Wang, X.; Kaech, S.M.; Cresswell, P. Viperin Is Highly Induced in Neutrophils and Macrophages during Acute and Chronic Lymphocytic Choriomeningitis Virus Infection. J. Immunol. 2010, 184, 5723–5731. [Google Scholar] [CrossRef] [PubMed]
- Nasr, N.; Maddocks, S.; Turville, S.; Harman, A.N.; Woolger, N.; Helbig, K.J.; Wilkinson, J.; Bye, C.R.; Wright, T.K.; Rambukwelle, D.; et al. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood 2012, 120, 778–788. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.J.; Carr, J.; Calvert, J.K.; Wati, S.; Clarke, J.N.; Eyre, N.; Narayana, S.K.; Fiches, G.N.; McCartney, E.M.; Beard, M.R. Viperin Is Induced following Dengue Virus Type-2 (DENV-2) Infection and Has Anti-viral Actions Requiring the C-terminal End of Viperin. PLoS Neglected Trop. Dis. 2013, 7, e2178. [Google Scholar] [CrossRef]
- Kurokawa, C.; Iankov, I.D.; Galanis, E. A key anti-viral protein, RSAD2/VIPERIN, restricts the release of measles virus from infected cells. Virus Res. 2019, 263, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Van der Hoek, K.; Eyre, N.; Shue, B.; Khantisitthiporn, O.; Glab-Ampi, K.; Carr, J.; Gartner, M.; Jolly, L.A.; Thomas, P.Q.; Adikusuma, F.; et al. Viperin is an important host restriction factor in control of Zika virus infection. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Panayiotou, C.; Lindqvist, R.; Kurhade, C.; Vonderstein, K.; Pasto, J.; Edlund, K.; Upadhyay, A.S.; Överby, A.K. Viperin Restricts Zika Virus and Tick-Borne Encephalitis Virus Replication by Targeting NS3 for Proteasomal Degradation. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Zheng, C.; Su, C. Herpes simplex virus 1 infection dampens the immediate early antiviral innate immunity signaling from peroxisomes by tegument protein VP16. Virol. J. 2017, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shen, G.; Wang, K.; Wang, S.; Cai, M.; Li, M.-L.; Zheng, C. Herpes Simplex Virus 1 Counteracts Viperin via Its Virion Host Shutoff Protein UL41. J. Virol. 2014, 88, 12163–12166. [Google Scholar] [CrossRef]
- Seo, J.-Y.; Yaneva, R.; Cresswell, P. Viperin: A Multifunctional, Interferon-Inducible Protein that Regulates Virus Replication. Cell Host Microb. 2011, 10, 534–539. [Google Scholar] [CrossRef]
- Frey, P.A.; Hegeman, A.; Ruzicka, F.J. The Radical SAM Superfamily. Crit. Rev. Biochem. Mol. Biol. 2008, 43, 63–88. [Google Scholar] [CrossRef]
- Nicolet, Y.; Drennan, C.L. AdoMet radical proteins—from structure to evolution—alignment of divergent protein sequences reveals strong secondary structure element conservation. Nucleic Acids Res. 2004, 32, 4015–4025. [Google Scholar] [CrossRef]
- Vey, J.L.; Yang, J.; Li, M.; Broderick, W.E.; Broderick, J.; Drennan, C.L. Structural basis for glycyl radical formation by pyruvate formate-lyase activating enzyme. Proc. Natl. Acad. Sci. USA 2008, 105, 16137–16141. [Google Scholar] [CrossRef]
- Sofia, H.J.; Chen, G.; Hetzler, B.G.; Reyes-Spindola, J.F.; Miller, N.E. Radical SAM, a novel protein superfamily linking unresolved steps in familiar biosynthetic pathways with radical mechanisms: Functional characterization using new analysis and information visualization methods. Nucleic Acids Res. 2001, 29, 1097–1106. [Google Scholar] [CrossRef]
- Fontecave, M.; Mulliez, E.; Ollagnier-De-Choudens, S. Adenosylmethionine as a source of 5′-deoxyadenosyl radicals. Curr. Opin. Chem. Biol. 2001, 5, 506–512. [Google Scholar] [CrossRef]
- Gizzi, A.S.; Grove, T.L.; Arnold, J.J.; Jose, J.; Jangra, R.K.; Garforth, S.J.; Du, Q.; Cahill, S.M.; Dulyaninova, N.G.; Love, J.D.; et al. A naturally occurring antiviral ribonucleotide encoded by the human genome. Nat. Cell Biol. 2018, 558, 610–614. [Google Scholar] [CrossRef]
- Moffat, J.; Grueneberg, D.A.; Yang, X.; Kim, S.Y.; Kloepfer, A.M.; Hinkle, G.; Piqani, B.; Eisenhaure, T.M.; Luo, B.; Grenier, J.K.; et al. A Lentiviral RNAi Library for Human and Mouse Genes Applied to an Arrayed Viral High-Content Screen. Cell 2006, 124, 1283–1298. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.M.; Estes, M.K.; Graham, D.Y.; Gerba, C.P. A Plaque Assay for the Simian Rotavirus SA11. J. Gen. Virol. 1979, 43, 513–519. [Google Scholar] [CrossRef]
- Wood-Allum, C.A.; Barber, S.C.; Kirby, J.; Heath, P.; Holden, H.; Mead, R.; Higginbottom, A.; Allen, S.; Beaujeux, T.; Alexson, S.E.; et al. Impairment of mitochondrial anti-oxidant defence in SOD1-related motor neuron injury and amelioration by ebselen. Brain 2006, 129, 1693–1709. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-Y.; Yaneva, R.; Hinson, E.R.; Cresswell, P. Human Cytomegalovirus Directly Induces the Antiviral Protein Viperin to Enhance Infectivity. Science 2011, 332, 1093–1097. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mukherjee, A.; Patra, U.; Bhowmick, R.; Basak, T.; Sengupta, S.; Chawla-Sarkar, M. Tyrosine phosphorylation modulates mitochondrial chaperonin Hsp60 and delays rotavirus NSP4-mediated apoptotic signaling in host cells. Cell. Microbiol. 2016, 19, e12670. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.; Perry, J.L.; Dosey, T.L.; Delcour, A.H.; Hyser, J.M. The rotavirus NSP4 viroporin domain is a calci-um-conducting ion channel. Sci. Rep. 2017, 7, 43487. [Google Scholar] [CrossRef]
- Munir, M.; Berg, M. The multiple faces of proteinkinase R in antiviral defense. Virulence 2013, 4, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Silverman, R.H. Viral Encounters with 2′,5′-Oligoadenylate Synthetase and RNase L during the Interferon Antiviral Response. J. Virol. 2007, 81, 12720–12729. [Google Scholar] [CrossRef]
- Skaug, B.; Chen, Z.J. Emerging Role of ISG15 in Antiviral Immunity. Cell 2010, 143, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Verhelst, J.; Parthoens, E.; Schepens, B.; Fiers, W.; Saelens, X. Interferon-Inducible Protein Mx1 Inhibits Influenza Virus by Interfering with Functional Viral Ribonucleoprotein Complex Assembly. J. Virol. 2012, 86, 13445–13455. [Google Scholar] [CrossRef]
- Helbig, K.J.; Beard, M.R. The Role of Viperin in the Innate Antiviral Response. J. Mol. Biol. 2014, 426, 1210–1219. [Google Scholar] [CrossRef] [PubMed]
- Rojas, M.; Arias, C.F.; López, S. Protein Kinase R Is Responsible for the Phosphorylation of eIF2α in Rotavirus Infection. J. Virol. 2010, 84, 10457–10466. [Google Scholar] [CrossRef]
- Sánchez-Tacuba, L.; Rojas, M.; Arias, C.F.; López, S. Rotavirus controls activation of the 2′-5′-oligoadenylate syn-thetase/RNase L pathway using at least two distinct mechanisms. J. Virol. 2015, 89, 12145–12153. [Google Scholar] [CrossRef]
- Jumat, M.R.; Huong, T.N.; Ravi, L.I.; Stanford, R.; Tan, B.H.; Sugrue, R.J. Viperin protein expression inhibits the late stage of respiratory syncytial virus morphogenesis. Antivir. Res. 2015, 114, 11–20. [Google Scholar] [CrossRef]
- Ruiz, M.C.; Leon, T.; Diaz, Y.; Michelangeli, F. Molecular Biology of Rotavirus Entry and Replication. Sci. World J. 2009, 9, 1476–1497. [Google Scholar] [CrossRef]
- Carlton-Smith, C.; Elliott, R.M. Viperin, MTAP44, and protein kinase R contribute to the interferon-induced inhi-bition of Bunyamwera Orthobunyavirus replication. J. Virol. 2012, 86, 11548–11557. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Guo, H.; Xu, C.; Chang, J.; Gu, B.; Wang, L.; Block, T.M.; Guo, J.-T. Identification of Three Interferon-Inducible Cellular Enzymes That Inhibit the Replication of Hepatitis C Virus. J. Virol. 2008, 82, 1665–1678. [Google Scholar] [CrossRef] [PubMed]
- Martin-Latil, S.; Mousson, L.; Autret, A.; Colbère-Garapin, F.; Blondel, B. Bax Is Activated during Rotavirus-Induced Apoptosis through the Mitochondrial Pathway. J. Virol. 2007, 81, 4457–4464. [Google Scholar] [CrossRef] [PubMed]
- Díaz, Y.; Chemello, M.E.; Peña, F.; Aristimuño, O.C.; Zambrano, J.L.; Rojas, H.; Trugnan, G. Expression of non-structural rotavirus protein NSP4 mimics Ca2+ homeostasis changes induced by rotavirus infection in cultured cells. J. Virol. 2008, 82, 11331–11343. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primers | Restriction Enzyme | Expression Vector |
|---|---|---|---|
| Viperin (wild type) | Forward: 5’-GCCGCGAATTCAGATGTGGGTGCTTACACCTGCT-3′ | EcoR1 | pFLAG-CMV6b (Sigma) |
| Reverse: 5’-ATTAGGTCGACGTAGCAGCCAGAAGGTTGCCCT-3′ | Sal1 | ||
| Viperin (ΔN-42) | Forward: 5’-GCCGCGAATTCAGGCTACCAAGAGGAGAAAGCA-3′ | EcoR1 | pFLAG-CMV6b (Sigma) |
| Reverse: 5’-ATTAGGTCGACGTAGCAGCCAGAAGGTTGCCCT-3′ | Sal1 | ||
| Viperin (ΔN-210) | Forward: 5’-ATCCGGTCGACGCTACCAATCCAGCTTCAGATCA-3′ | EcoR1 | pFLAG-CMV6b (Sigma) |
| Reverse: 5’-ATTAGGTCGACGTAGCAGCCAGAAGGTTGCCCT-3′ | Sal1 | ||
| Viperin (ΔC-151) | Forward: 5’-GCCGCGAATTCAGATGTGGGTGCTTACACCTGCT-3′ | EcoR1 | pFLAG-CMV6b (Sigma) |
| Reverse: 5’-ATCAGGTCGACGCCACCTCCTCAGCTTTTGAAGG-3′ | Sal1 | ||
| Viperin (ΔC-319) | Forward: 5’-GCCGCGAATTCAGATGTGGGTGCTTACACCTGCT-3′ | EcoR1 | pFLAG-CMV6b (Sigma) |
| Reverse: 5′-ATCAGGTCGACGCCACCTCCTCAGCTTTTGAAGG-3′ | Sal1 | ||
| NSP4 | Forward: 5′-GAATTCATGGAAAAGCTTACCGACC-3′ | EcoR1 | pcDNATM6/V5-His B (Invitrogen) |
| Reverse: 5′-GATATCCACATTGCTGCAGTCACTTCT-3′ | EcoRV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sarkar, R.; Nandi, S.; Lo, M.; Gope, A.; Chawla-Sarkar, M. Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis. Viruses 2021, 13, 1324. https://doi.org/10.3390/v13071324
Sarkar R, Nandi S, Lo M, Gope A, Chawla-Sarkar M. Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis. Viruses. 2021; 13(7):1324. https://doi.org/10.3390/v13071324
Chicago/Turabian StyleSarkar, Rakesh, Satabdi Nandi, Mahadeb Lo, Animesh Gope, and Mamta Chawla-Sarkar. 2021. "Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis" Viruses 13, no. 7: 1324. https://doi.org/10.3390/v13071324
APA StyleSarkar, R., Nandi, S., Lo, M., Gope, A., & Chawla-Sarkar, M. (2021). Viperin, an IFN-Stimulated Protein, Delays Rotavirus Release by Inhibiting Non-Structural Protein 4 (NSP4)-Induced Intrinsic Apoptosis. Viruses, 13(7), 1324. https://doi.org/10.3390/v13071324





