Influence of Herbicide Exposure and Ranavirus Infection on Growth and Survival of Juvenile Red-Eared Slider Turtles (Trachemys scripta elegans)
Abstract
:1. Introduction
2. Materials and Methods
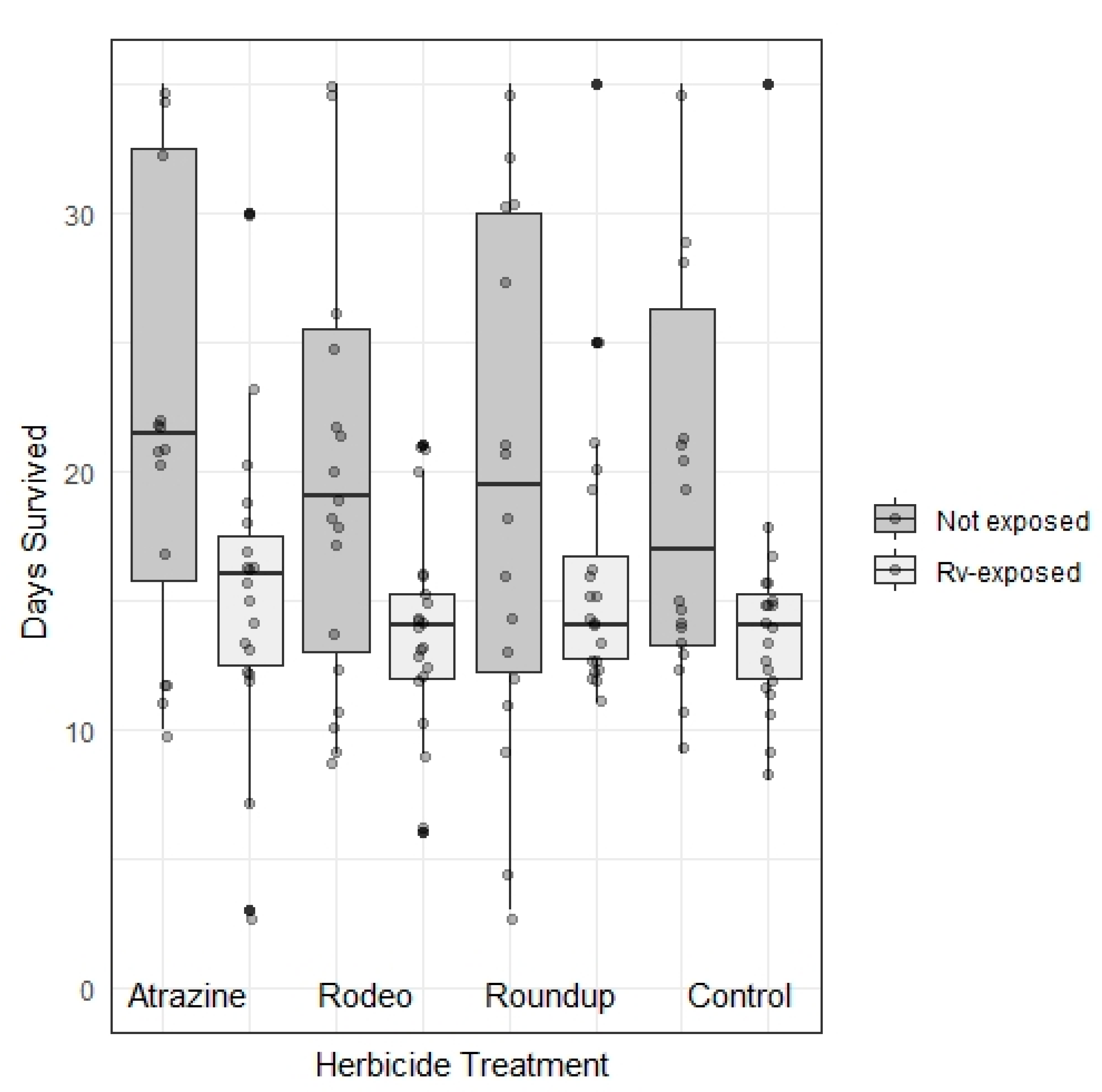
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gray, M.J.; Chinchar, V.G. History and future of ranaviruses. In Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates; Gray, M.J., Chinchar, V.G., Eds.; Springer: Berlin, Germany, 2015; pp. 1–7. [Google Scholar] [CrossRef]
- Chinchar, V.G. Ranaviruses (family Iridoviridae): Emerging cold-blooded killers. Arch. Virol. 2002, 147, 447–470. [Google Scholar] [CrossRef]
- Daszak, P.; Berger, L.; Cunningham, A.A.; Hyatt, A.D.; Green, D.E.; Speare, R. Emerging infectious diseases and amphibian population declines. Emerg. Infect. Dis. 1999, 5, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Hoverman, J.T.; Gray, M.J.; Miller, D.L. Anuran susceptibilities to ranaviruses: Role of species identity, exposure route, and a novel virus isolate. Dis. Aquat. Org. 2010, 89, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Forson, D.D.; Storfer, A. Atrazine increases ranavirus susceptibility in the Tiger Salamander, Ambystoma tigrinum. Ecol. Appl. 2006, 16, 2325–2332. [Google Scholar] [CrossRef] [Green Version]
- Schock, D.M.; Bollinger, T.K.; Collins, J.P. Mortality rates differ among amphibian populations exposed to three strains of a lethal ranavirus. Ecohealth 2009, 6, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Brunner, J.L.; Richards, K.; Collins, J.P. Dose and host characteristics influence virulence of ranavirus infections. Oecologia 2005, 144, 399–406. [Google Scholar] [CrossRef]
- Brand, M.D.; Hill, R.D.; Brenes, R.; Chaney, J.C.; Wilkes, R.P.; Grayfer, L.; Miller, D.L.; Gray, M.J. Water temperature affects susceptibility to ranavirus. Ecohealth 2016, 13, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Ahne, W.; Bremont, M.; Hedrick, R.P.; Hyatt, A.D.; Whittington, R.J. Iridoviruses associated with epizootic haematopoietic necrosis (EHN) in aquaculture. World J. Microbiol. Biotechnol. 1997, 13, 367–373. [Google Scholar] [CrossRef]
- Haislip, N.A.; Gray, M.J.; Hoverman, J.T.; Miller, D.L. Development and disease: How susceptibility to an emerging pathogen changes through anuran development. PLoS ONE 2011, 6, e22307. [Google Scholar] [CrossRef]
- Whittington, R.J.; Becker, J.A.; Dennis, M.M. Iridovirus infections in finfish—Critical review with emphasis on ranaviruses. J. Fish. Dis. 2010, 33, 95–122. [Google Scholar] [CrossRef]
- Duffus, A.L.J.; Waltzek, T.B.; Stöhr, A.C.; Allender, M.C.; Gotesman, M.; Whittington, R.J.; Hick, P.; Hines, M.K.; Marschang, R.E. Distribution and host range of ranaviruses. In Ranaviruses: Lethal Pathogens of Ectothermic Vertebrates; Gray, M.J., Chinchar, V.G., Eds.; Springer: Berlin, Germany, 2015; pp. 9–57. ISBN 9783319137551. [Google Scholar]
- Green, D.E.; Converse, K.A.; Schrader, A.K. Epizootiology of sixty-four amphibian morbidity and mortality events in the USA, 1996-2001. Ann. N. Y. Acad. Sci. 2002, 969, 323–339. [Google Scholar] [CrossRef]
- Daszak, P.; Cunningham, A.A.; Consortium, A.D.H. Infectious disease and amphibian population declines. Divers. Distrib. 2003, 9, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Hyatt, A.D.; Williamson, M.; Coupar, B.E.H.; Middleton, D.; Hengstberger, S.G.; Gould, R.; Selleck, P.; Wise, T.G.; Kattenbelt, J.; Cunningham, A.A.; et al. First identification of a ranavirus from green pythons (Chondropython viridis). J. Wildl. Dis. 2002, 38, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Stöhr, A.C.; Blahak, S.; Heckers, K.O.; Wiechert, J.; Behncke, H.; Mathes, K.; Günther, P.; Zwart, P.; Ball, I.; Rüschoff, B.; et al. Ranavirus infections associated with skin lesions in lizards. Vet. Res. 2013, 44, 84. [Google Scholar] [CrossRef] [Green Version]
- De Voe, R.; Geissler, K.; Elmore, S.; Rotstein, D.; Lewbart, G.; Guy, J. Ranavirus-associated morbidity and mortality in a group of captive eastern box turtles (Terrapene carolina carolina). J. Zoo Wildl. Med. 2004, 35, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Sim, R.R.; Allender, M.C.; Crawford, L.K.; Wack, A.N.; Murphy, K.J.; Mankowski, J.L.; Bronson, E. Ranavirus epizootic in captive Eastern Box Turtles (Terrapene carolina carolina) with concurrent herpesvirus and mycoplasma infection: Management and monitoring. J. Zoo Wildl. Med. 2016, 47, 256–270. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, C.M.; Piczak, M.L.; Snyman, H.N.; Joseph, T.; Theijin, C.; Chow-Fraser, P.; Jardine, C.M. First report of ranavirus mortality in a common snapping turtle Chelydra serpentina. Dis. Aquat. Org. 2019, 132, 221–227. [Google Scholar] [CrossRef]
- Brenes, R.; Gray, M.J.; Waltzek, T.B.; Wilkes, R.P.; Miller, D.L. Transmission of ranavirus between ectothermic vertebrate hosts. PLoS ONE 2014, 9, e92476. [Google Scholar] [CrossRef]
- Johnson, A.J.; Pessier, A.P.; Jacobson, E.R. Experimental transmission and induction of ranaviral disease in Western Ornate box turtles (Terrapene ornata ornata) and Red-eared Sliders (Trachemys scripta elegans). Vet. Pathol. 2007, 44, 285–297. [Google Scholar] [CrossRef] [Green Version]
- Ariel, E.; Wirth, W.; Burgess, G.; Scott, J.; Owens, L. Pathogenicity in six Australian reptile species following experimental inoculation with Bohle iridovirus. Dis. Aquat. Org. 2015, 115, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Wirth, W.; Schwarzkopf, L.; Skerratt, L.F.; Tzamouzaki, A.; Ariel, E. Dose-dependent morbidity of freshwater turtle hatchlings, Emydura macquarii krefftii, inoculated with Ranavirus isolate (Bohle iridovirus, Iridoviridae). J. Gen. Virol. 2019, 100, 1431–1441. [Google Scholar] [CrossRef]
- Allender, M.C.; Barthel, A.C.; Rayl, J.M.; Terio, K.A. Experimental transmission of frog virus 3–like ranavirus in juvenile chelonians at two temperatures. J. Wildl. Dis. 2018, 54, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Goodman, R.M.; Miller, D.L.; Ararso, Y.T. Prevalence of ranavirus in Virginia turtles as detected by tail-clip sampling versus oral-cloacal swabbing. Northeast. Nat. 2013, 20, 325–332. [Google Scholar] [CrossRef]
- Goodman, R.M.; Hargadon, K.M.; Carter, E.D. Detection of ranavirus in eastern fence lizards and eastern box turtles in central Virginia. Northeast. Nat. 2018, 25, 391–398. [Google Scholar] [CrossRef]
- Sutton, W.B.; Gray, M.J.; Hoverman, J.T.; Secrist, R.G.; Super, P.E.; Hardman, R.H.; Tucker, J.L.; Miller, D.L. Trends in ranavirus prevalence among Plethodontid salamanders in the Great Smoky Mountains National Park. Ecohealth 2014, 12, 320–329. [Google Scholar] [CrossRef]
- Allender, M.; Mitchell, M.; Torres, T.; Sekowska, J.; Driskell, E.A. Pathogenicity of frog virus 3-like virus in red-eared slider turtles (Trachemys scripta elegans) at two environmental temperatures. J. Comp. Pathol. 2013, 149, 356–367. [Google Scholar] [CrossRef]
- Powell, R.; Roger, C.; Collins, J.T. Peterson Field Guide to Reptiles and Amphibians of Eastern and Central North America, 4th ed.; Houghton Mifflin Co.: Boston, MA, USA, 2016. [Google Scholar]
- Rhodin, A.G.; Stanford, C.B.; Van Dijk, P.P.; Eisemberg, C.; Luiselli, L.; Mittermeier, R.A.; Hudson, R.; Horne, B.D.; Goode, E.V.; Kuchling, G.; et al. Global conservation status of turtles and tortoises (order Testudines). Chelonian Conserv. Biol. 2018, 17, 135–161. [Google Scholar] [CrossRef]
- Atwood, D.; Paisley-Jones, C. Pesticides Industry Sales and Usage: 2008–2012 Market Estimates; EPA: Washington, DC, USA, 2017.
- Stone, W.W.; Gilliom, R.J.; Ryberg, K.R. Pesticides in US streams and rivers: Occurrence and trends during 1992–2011. Environ. Sci. Technol. 2014, 48, 11025–11030. [Google Scholar] [CrossRef]
- Grube, A.; Donaldson, D.; Kiely, T.; Wu, L. Pesticide Industry Sales and Usage Report: 2006 and 2007 Market Estimates; EPA: Washington, DC, USA, 2007.
- Holland, J.; Sinclair, P. Environmental fate of pesticides and the consequences for residues in food and drinking water. In Pesticide Residues in Food and Drinking Water: Human Exposure and Risks; Hamilton, D., Crossley, S., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2003; pp. 27–62. [Google Scholar]
- Relyea, R.A. The impact of insecticides and herbicides on the biodiversity and productivity of aquatic communities. Ecol. Appl. 2011, 15, 618–627. [Google Scholar] [CrossRef]
- Brodkin, M.A.; Madhoun, H.; Rameswaran, M.; Vatnick, I. Atrazine is an immune disruptor in adult Northern Leopard Frogs (Rana pipiens). Environ. Toxicol. Chem. 2007, 26, 80. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.J.; Miller, D.L.; Hoverman, J.T. Ecology and pathology of amphibian ranaviruses. Dis. Aquat. Org. 2009, 87, 243–266. [Google Scholar] [CrossRef]
- Sures, B. How parasitism and pollution affect the physiological homeostasis of aquatic hosts. J. Helminthol. 2006, 80, 151–157. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G. Stress responses and disease resistance in salmonid fish: Effects of chronic elevation of plasma cortisol. Fish. Physiol. Biochem. 1989, 7, 253–258. [Google Scholar] [CrossRef]
- Galloway, T.S.; Depledge, M.H. Immunotoxicity in invertebrates: Measurement and ecotoxicological relevance. Ecotoxicology 2001, 10, 5–23. [Google Scholar] [CrossRef]
- Christin, M.S.; Gendron, A.D.; Brousseau, P.; Ménard, L.; Marcogliese, D.J.; Cyr, D.; Ruby, S.; Fournier, M. Effects of agricultural pesticides on the immune system of Rana pipiens and on its resistance to parasitic infection. Environ. Toxicol. Chem. Int. J. 2003, 22, 1127–1133. [Google Scholar] [CrossRef]
- Coors, A.; Decaestecker, E.; Jansen, M.; De Meester, L. Pesticide exposure strongly enhances parasite virulence in an invertebrate host model. Oikos 2008, 117, 1840–1846. [Google Scholar] [CrossRef]
- Taylor, S.K.; Williams, E.S.; Mills, K.W. Effects of malathion on disease susceptibility in Woodhouse’s toads. J. Wildl. Dis. 1999, 35, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, C.M.; Berrill, M.; Pauli, B.D.; Helbing, C.C.; Werry, K.; Veldhoen, N. Toxicity of glyphosate-based pesticides to four North American frog species. Environ. Toxicol. Chem. 2004, 23, 1928–1938. [Google Scholar] [CrossRef]
- Hayes, T.B.; Anderson, L.L.; Beasley, V.R.; De Solla, S.R.; Iguchi, T.; Ingraham, H.; Kestemont, P.; Kniewald, J.; Kniewald, Z.; Langlois, V.S.; et al. Demasculinization and feminization of male gonads by atrazine: Consistent effects across vertebrate classes. J. Steroid Biochem. Mol. Biol. 2001, 127, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Pochini, K.M.; Hoverman, J.T. Reciprocal effects of pesticides and pathogens on amphibian hosts: The importance of exposure order and timing. Environ. Pollut. 2017, 221, 359–366. [Google Scholar] [CrossRef]
- Relyea, R.A.; Hoverman, J.T. Interactive effects of predators and a pesticide on aquatic communities. Oikos 2008, 117, 1647–1658. [Google Scholar] [CrossRef]
- Kerby, J.L.; Storfer, A. Combined effects of atrazine and chlorpyrifos on susceptibility of the tiger salamander to Ambystoma tigrinum virus. Ecohealth 2009, 6, 91–98. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianessi, L.P. Economic and herbicide use impacts of glyphosate-resistant crops. Pest Manag. Sci. 2005, 61, 241–245. [Google Scholar] [CrossRef]
- Wan, M.T.; Watts, R.G.; Moul, D.J. Effects of different dilution water types on the acute toxicity to juvenile Pacific salmonids and rainbow trout of glyphosate and its formulated products. Bull. Environ. Contam. Toxicol. 1989, 43, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.L.; Vera, M.S.; Miranda, L.A. Effects of herbicide glyphosate and glyphosate-based formulations on aquatic ecosystems. In Herbicides and Environment; Kortekamp, A., Ed.; IntechOpen: London, UK, 2011; Volume 16, pp. 343–368. [Google Scholar]
- Battaglin, W.A.; Rice, K.C.; Focazio, M.J.; Salmons, S.; Barry, R.X. The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005–2006. Environ. Monit. Assess. 2009, 155, 281–307. [Google Scholar] [CrossRef] [PubMed]
- Sass, J.B.; Colangelo, A. European Union bans atrazine, while the United States negotiates continued use. Int. J. Occup. Environ. Health 2006, 12, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Carr, J.A.; Du Preez, L.H.; Giesy, J.P.; Kendall, R.J.; Smith, E.E.; Van Der Kraak, G.J. Effects of atrazine on fish, amphibians, and aquatic reptiles: A critical review. Crit. Rev. Toxicol. 2008, 38, 721–772. [Google Scholar] [CrossRef]
- Van Der Kraak, G.J.; Hosmer, A.J.; Hanson, M.L.; Kloas, W.; Solomon, K.R. Effects of atrazine in fish, amphibians, and reptiles: An analysis based on quantitative weight of evidence. Crit. Rev. Toxicol. 2014, 44, 1–66. [Google Scholar] [CrossRef] [Green Version]
- Rohr, J.R.; McCoy, K.A. A qualitative meta-analysis reveals consistent effects of atrazine on freshwater fish and amphibians. Environ. Health Perspect. 2010, 118, 20–32. [Google Scholar] [CrossRef] [Green Version]
- Solomon, K.R.; Thompson, D.G. Ecological risk assessment for aquatic organisms from over-water uses of glyphosate. J. Toxicol. Environ. Health Part B 2003, 6, 289–324. [Google Scholar] [CrossRef]
- Giesy, J.P.; Dobson, S.; Solomon, K.R. Ecotoxicological risk assessment for Roundup (R) herbicide. Rev. Environ. Contam. Toxicol. 2000, 167, 35–120. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Thurman, E.M.; Kalkhoff, S.J.; Porter, S.D. Herbicides and transformation products in surface waters of the Midwestern United States. J. Am. Water Resour. Assoc. 2004, 80225, 743–756. [Google Scholar] [CrossRef]
- Solomon, K.R.; Baker, D.B.; Richards, R.P.; Dixon, K.R.; Klaine, S.J.; La Point, T.W.; Kendall, R.J.; Weisskopf, C.P.; Giddins, J.M.; Giesy, J.P.; et al. Ecological risk assessment of atrazine in North American surface waters. Environ. Toxicol. Chem. 1996, 15, 31–76. [Google Scholar] [CrossRef]
- Brenes, R.; Miller, D.L.; Waltzek, T.B.; Wilkes, R.P.; Tucker, J.L.; Chaney, J.C.; Hardman, R.H.; Brand, M.D.; Huether, R.R.; Gray, M.J. Susceptibility of fish and turtles to three ranaviruses isolated from different ectothermic vertebrate classes. J. Aquat. Anim. Health 2014, 26, 118–126. [Google Scholar] [CrossRef]
- Schock, D.M.; Bollinger, T.K.; Gregory Chinchar, V.; Jancovich, J.K.; Collins, J.P. Experimental evidence that amphibian ranaviruses are multi-host. Copeia 2008, 2008, 133–143. [Google Scholar] [CrossRef]
- Picco, A.M.; Brunner, J.L.; Collins, J.P. Susceptibility of the endangered California tiger salamander, Ambystoma californiense, to ranavirus infection. J. Wildl. Dis. 2007, 43, 286–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutton, W.B.; Gray, M.J.; Hardman, R.H.; Wilkes, R.P.; Kouba, A.J.; Miller, D.L. High susceptibility of the endangered dusky gopher frog to ranavirus. Dis. Aquat. Org. 2014, 112, 9–16. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Willingham, E. Embryonic exposure to low-dose pesticides: Effects on growth rate in the hatchling Red-eared Slider Turtle. J. Toxicol. Environ. Health Part A 2001, 64, 257–272. [Google Scholar] [CrossRef] [PubMed]
- Sparling, D.W.; Matson, C.; Bickham, J.; Doelling-Brown, P. Toxicity of glyphosate as Glypro and LI700 to Red-eared Slider (Trachemys scripta elegans) embryos and early hatchlings. Environ. Toxicol. Chem. 2006, 25, 2768–2774. [Google Scholar] [CrossRef]
- Soltanian, S. Effect of atrazine on immunocompetence of Red-eared Slider Turtle (Trachemys scripta). J. Immunotoxicol. 2016, 13, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Gill, J.P.K.; Sethi, N.; Mohan, A.; Datta, S.; Girdhar, M. Glyphosate toxicity for animals. Environ. Chem. Lett. 2018, 16, 401–426. [Google Scholar] [CrossRef]
- Mensah, P.K.; Palmer, C.G.; Odume, O.N. Ecotoxicology of glyphosate and glyphosate-based herbicides—Toxicity to wildlife and humans. In Toxicity and Hazard of Agrochemicals; Larramendy, M.L., Soloneski, S., Eds.; IntechOpen: London, UK, 2015. [Google Scholar] [CrossRef] [Green Version]
- Cusaac, J.; Carter, E.; Woodhams, D.; Robert, J.; Spatz, J.; Howard, J.; Lillard, C.; Graham, A.; Hill, R.; Reinsch, S.; et al. Emerging pathogens and a current-use pesticide: Potential impacts on Eastern Hellbenders. J. Aquat. Anim. Health 2021, 33, 24–32. [Google Scholar] [CrossRef]
- Lanctôt, C.; Robertson, C.; Navarro-Martín, L.; Edge, C.; Melvin, S.D.; Houlahan, J.; Trudeau, V.L. Effects of the glyphosate-based herbicide Roundup WeatherMax® on metamorphosis of wood frogs (Lithobates sylvaticus) in natural wetlands. Aquat. Toxicol. 2013, 140–141, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Schaumburg, L.; Siroski, P.; Poletta, G.; Mudry, M. Genotoxicity induced by Roundup® (Glyphosate) in tegu lizard (Salvator merianae) embryos. Pestic. Biochem. Physiol. 2016, 130, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Poletta, G.; Larriera, A.; Kleinsorge, E.; Mudry, M. Genotoxicity of the herbicide formulation Roundup® (glyphosate) in broad- snouted caiman (Caiman latirostris) evidenced by the Comet assay and the Micronucleus test. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2009, 672, 95–102. [Google Scholar] [CrossRef]
- González, E.; Latorre, M.; Larriera, A.; Siroski, P.; Poletta, G. Induction of micronuclei in broad snouted caiman (Caiman latirostris) hatchlings exposed in vivo to Roundup® (glyphosate) concentrations used in agriculture. Pestic. Biochem. Physiol. 2013, 105, 131–134. [Google Scholar] [CrossRef]
- Latorre, M.; López González, E.; Larriera, A.; Poletta, G.; Siroski, P. Effects of in vivo exposure to Roundup® on immune system of Caiman latirostris. J. Immunotoxicol. 2013, 10, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siroski, P.; Poletta, G.; Latorre, M.; Merchant, M.; Ortega, H.; Mudry, M. Immunotoxicity of commercial-mixed glyphosate in Broad Snouted Caiman (Caiman latirostris). Chem. Biol. Interact. 2016, 244, 64–70. [Google Scholar] [CrossRef]
- Duffus, A.; Bartlett, P.; Stilwell, N.; Goodman, R. Ranaviruses in Wild Reptiles in the USA. Available online: https://static1.squarespace.com/static/574455b9f8baf3fd9156d863/t/607dc2939e25a5085c7c3f34/1618854548016/Ranaviruses+in+Wild+North+American+Reptiles+-+17R1.pdf (accessed on 18 June 2021).
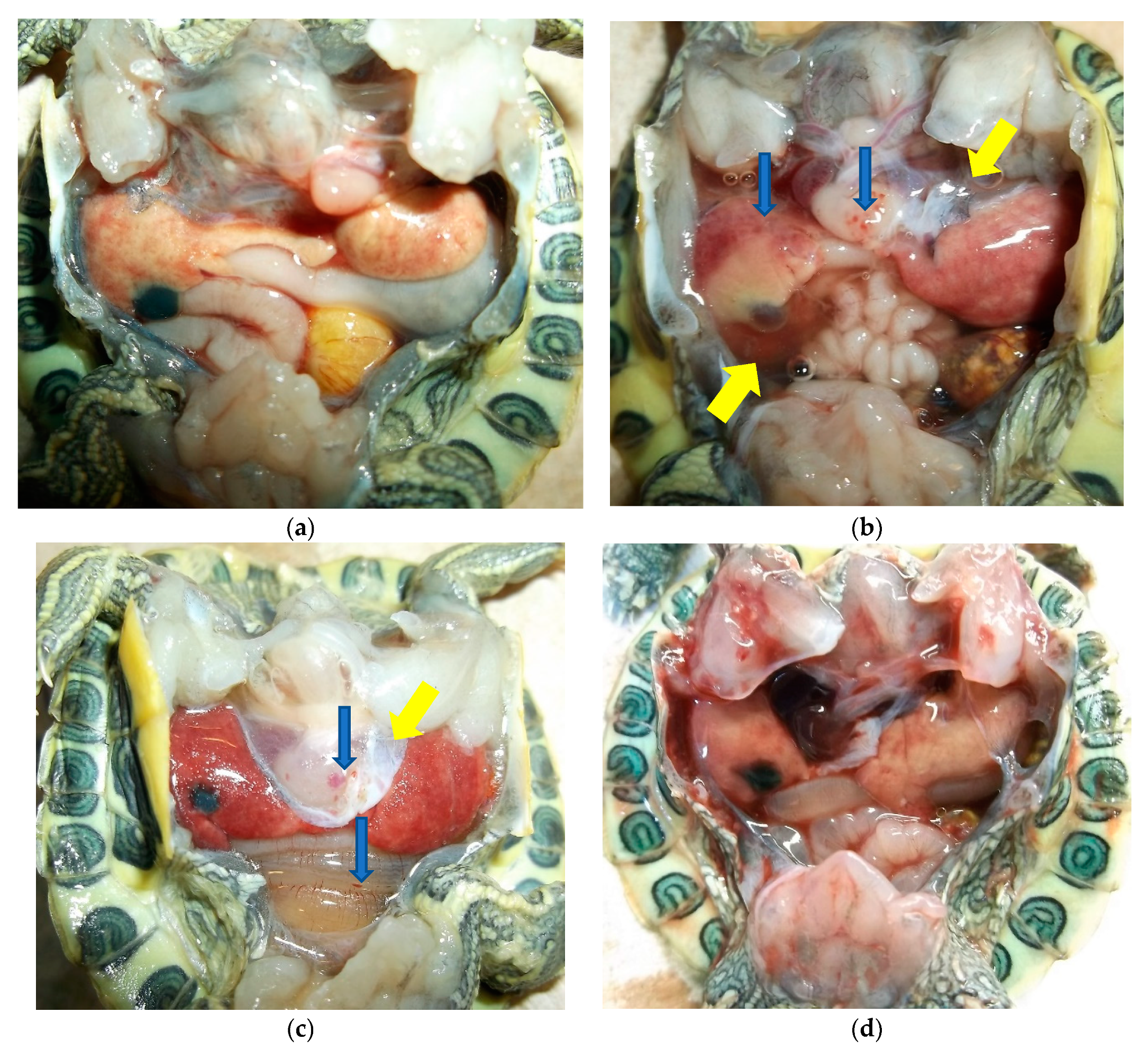


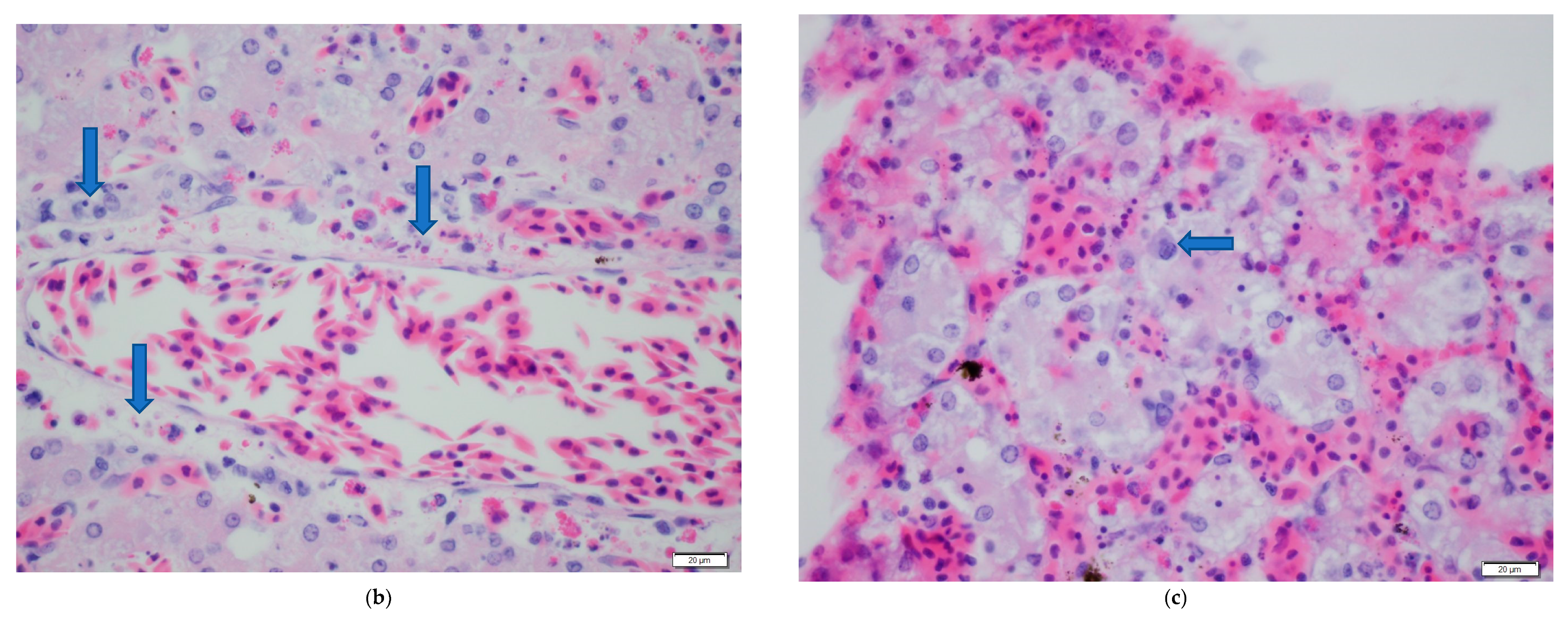
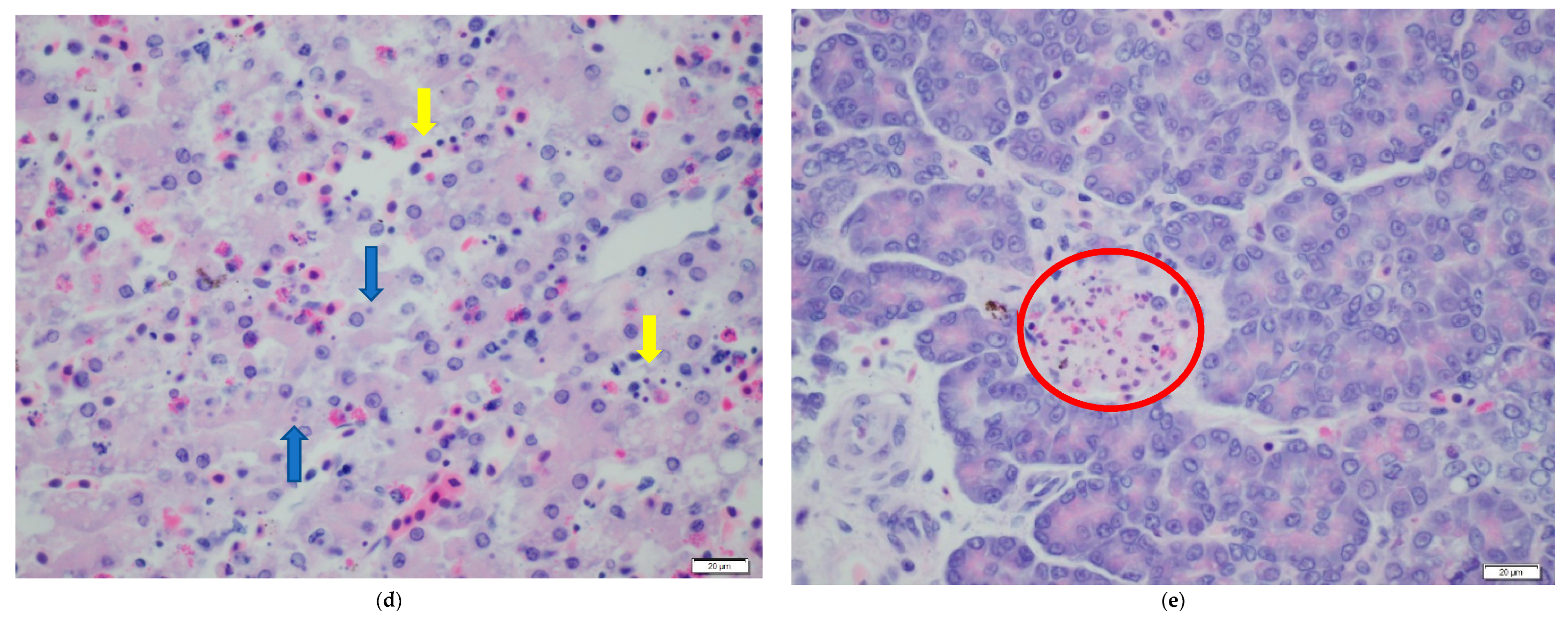

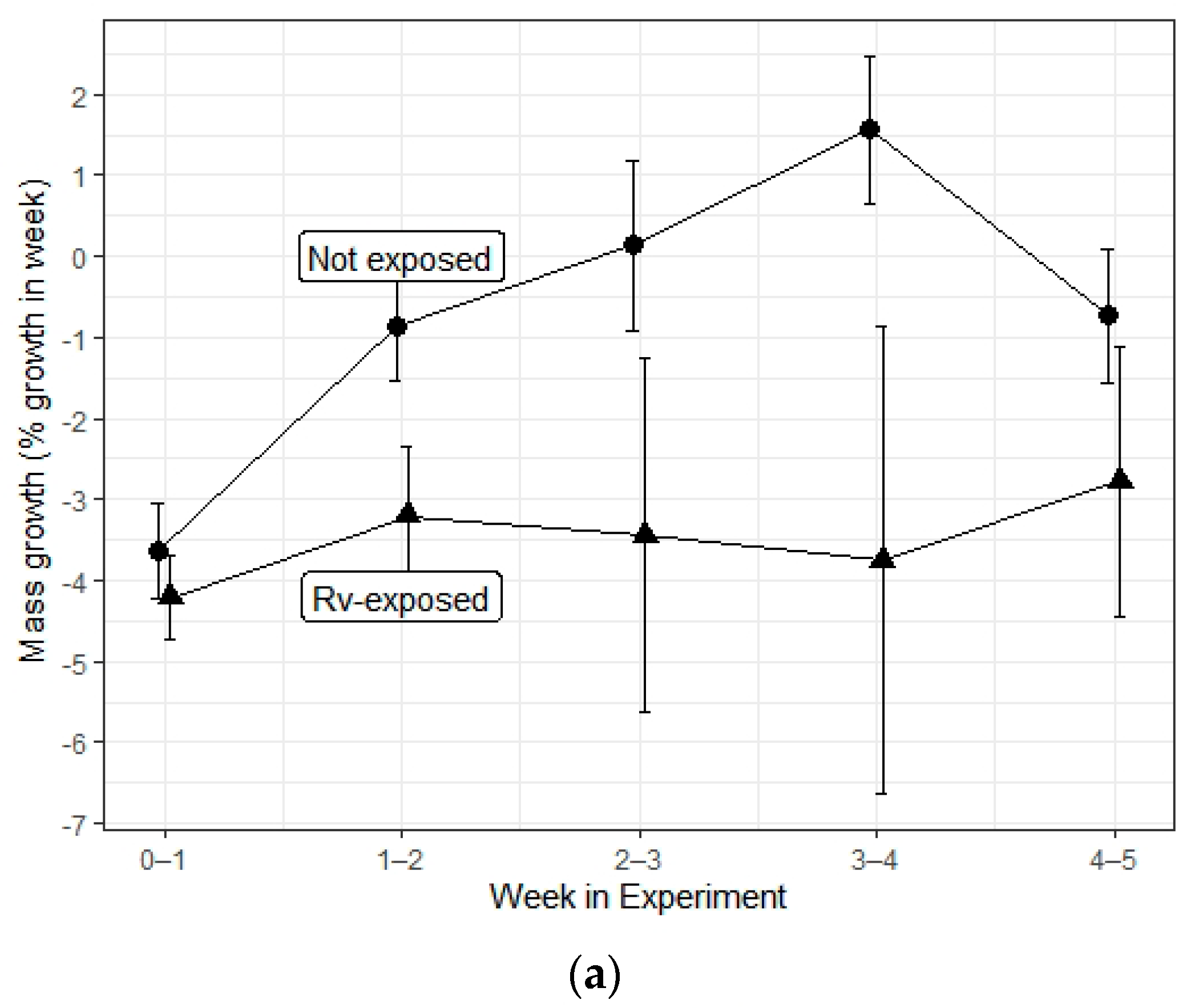
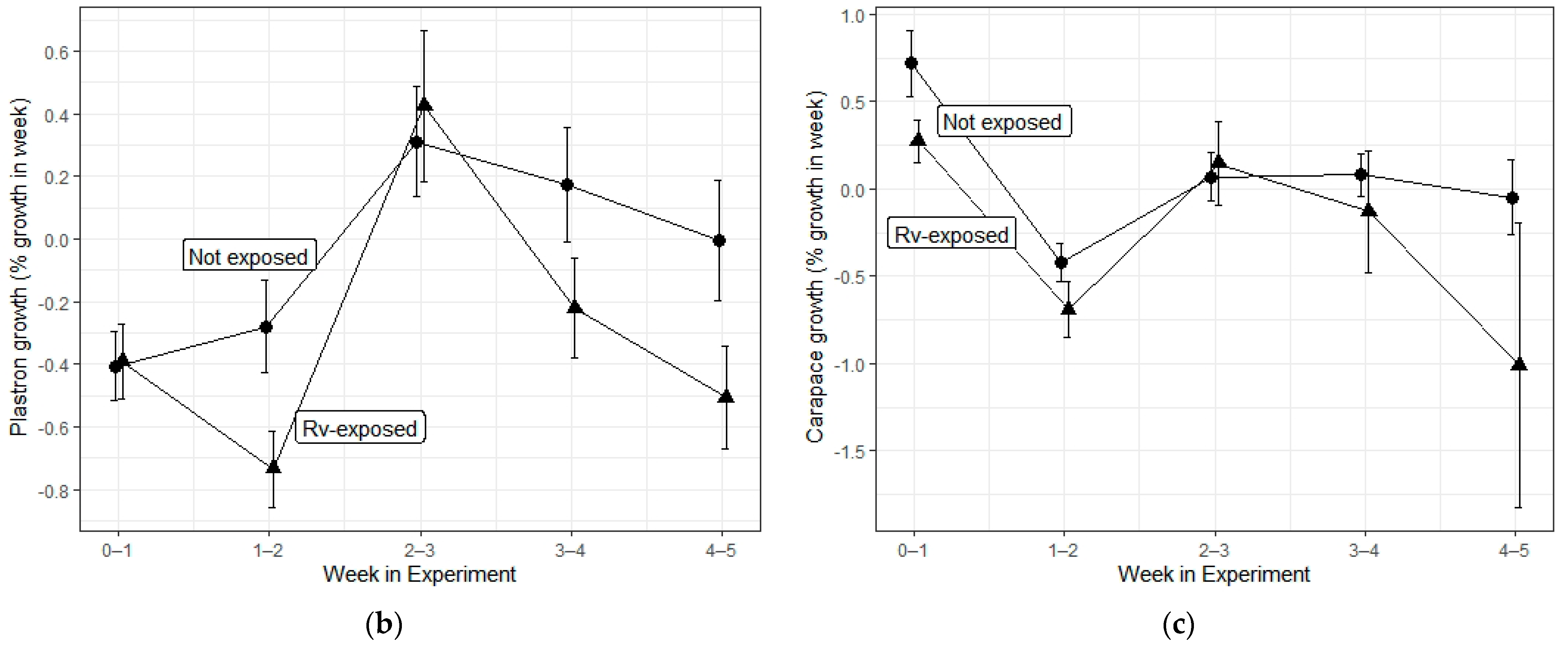
| Ranavirus Treatment | Herbicide Treatment | N | Incidence of Infection | Liver Discoloration | White Growths |
|---|---|---|---|---|---|
| Rv-exposed | Atrazine | 20 | 12 | 12 | 9 |
| Rv-exposed | Roundup | 20 | 9 | 6 | 8 |
| Rv-exposed | Rodeo | 20 | 9 | 8 | 11 |
| Rv-exposed | Control | 20 | 16 | 10 | 12 |
| Control | Atrazine | 20 | - | 9 | 9 |
| Control | Roundup | 20 | - | 7 | 3 |
| Control | Rodeo | 20 | - | 10 | 6 |
| Control | Control | 20 | - | 7 | 7 |
| Response Variables | Independent Variables | df | F | p |
|---|---|---|---|---|
| Days survived | Herbicide | 3, 347 | 1.181 | 0.319 |
| Rv exposure | 1, 347 | 31.366 | <0.001 * | |
| Interaction | 3, 347 | 0.235 | 0.872 | |
| Mass growth | Herbicide | 3, 347 | 0.68 | 0.565 |
| Rv exposure | 1, 347 | 7.933 | 0.005 * | |
| Interaction | 3, 347 | 0.582 | 0.627 | |
| Time | 1, 347 | 17.886 | <0.001 * | |
| Carapace growth | Herbicide | 3, 347 | 0.401 | 0.752 |
| Rv exposure | 1, 347 | 6.861 | 0.009 * | |
| Interaction | 3, 347 | 0.149 | 0.931 | |
| Time | 1, 347 | 13.539 | <0.001 * | |
| Plastron growth | Herbicide | 3, 347 | 0.286 | 0.836 |
| Rv exposure | 1, 347 | 2.761 | 0.098 | |
| Interaction | 3, 347 | 0.053 | 0.984 | |
| Time | 1, 347 | 8.529 | 0.004 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goodman, R.M.; Carter, E.D.; Miller, D.L. Influence of Herbicide Exposure and Ranavirus Infection on Growth and Survival of Juvenile Red-Eared Slider Turtles (Trachemys scripta elegans). Viruses 2021, 13, 1440. https://doi.org/10.3390/v13081440
Goodman RM, Carter ED, Miller DL. Influence of Herbicide Exposure and Ranavirus Infection on Growth and Survival of Juvenile Red-Eared Slider Turtles (Trachemys scripta elegans). Viruses. 2021; 13(8):1440. https://doi.org/10.3390/v13081440
Chicago/Turabian StyleGoodman, Rachel M., Edward Davis Carter, and Debra L. Miller. 2021. "Influence of Herbicide Exposure and Ranavirus Infection on Growth and Survival of Juvenile Red-Eared Slider Turtles (Trachemys scripta elegans)" Viruses 13, no. 8: 1440. https://doi.org/10.3390/v13081440
APA StyleGoodman, R. M., Carter, E. D., & Miller, D. L. (2021). Influence of Herbicide Exposure and Ranavirus Infection on Growth and Survival of Juvenile Red-Eared Slider Turtles (Trachemys scripta elegans). Viruses, 13(8), 1440. https://doi.org/10.3390/v13081440






