Clinical Course of Infection and Cross-Species Detection of Equine Parvovirus-Hepatitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Serum Sample Collection
2.2. Detection of EqPV-H DNA
2.3. Detection of Anti-EqPV-H Antibodies
2.4. Histology
2.5. Measurement of Liver Specific Biochemical Analytes
2.6. Sequence Analysis
2.7. Illustrations
3. Results
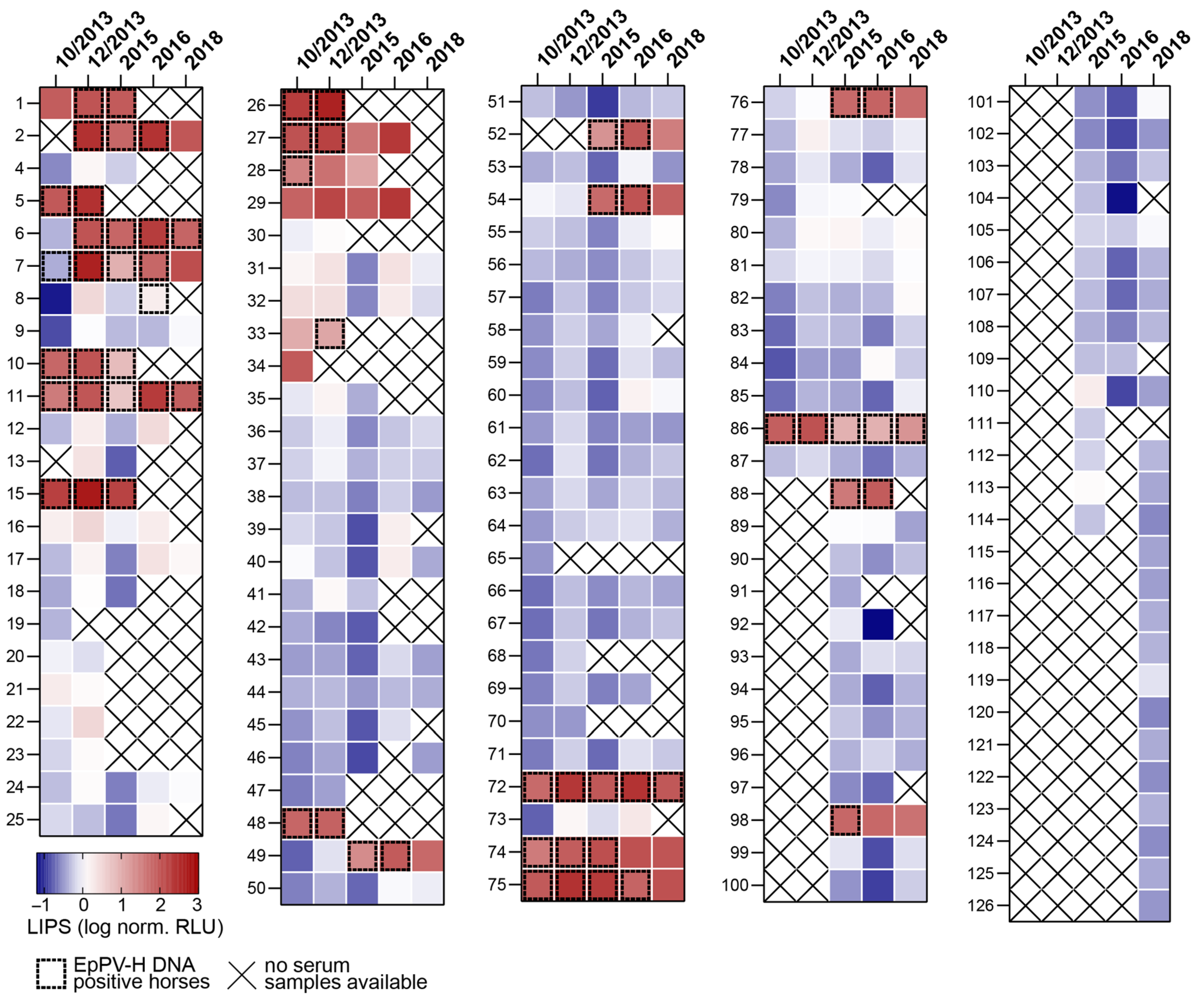
3.1. Evidence of Persistent and Chronic EqPV-H Infection in Horses from Germany
3.2. Pathology of Chronic Horses
3.3. Biochemical Analysis of Liver Enzymes in Viremic and Non-Viremic Horses
3.4. Cross-Species Detection of EqPV-H
3.5. Sequence and Phylogenetic Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Theiler, A. Acute Liver-Atrophy and Parenchymatous Hepatitis in Horses. In Union of South Africa, 5th and 6th Reports of the Director of Veterinary Research; The Government Printing and Stationery Office: Pretoria, South Africa, 1919; pp. 7–165. [Google Scholar]
- Divers, T.J.; Tennant, B.C.; Kumar, A.; McDonough, S.; Cullen, J.; Bhuva, N.; Jain, K.; Chauhan, L.S.; Scheel, T.K.H.; Lipkin, W.I.; et al. New Parvovirus Associated with Serum Hepatitis in Horses after Inoculation of Common Biological Product. Emerg. Infect. Dis. 2018, 24, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Aleman, M.; Nieto, J.E.; Carr, E.A.; Carlson, G.P. Serum Hepatitis Associated with Commercial Plasma Transfusion in Horses. J. Vet. Intern. Med. 2005, 19, 120–122. [Google Scholar] [CrossRef] [PubMed]
- Thomsett, L.R. Acute Hepatic Failure in the Horse. Equine Vet. J. 1971, 3, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Chandriani, S.; Skewes-Cox, P.; Zhong, W.; Ganem, D.E.; Divers, T.J.; Van Blaricum, A.J.; Tennant, B.C.; Kistler, A.L. Identification of a previously undescribed divergent virus from the Flaviviridae family in an outbreak of equine serum hepatitis. Proc. Natl. Acad. Sci. USA 2013, 110, E1407–E1415. [Google Scholar] [CrossRef] [Green Version]
- Tomlinson, J.E.; Tennant, B.C.; Struzyna, A.; Mrad, D.; Browne, N.; Whelchel, D.; Johnson, P.J.; Jamieson, C.; Löhr, C.V.; Bildfell, R.; et al. Viral testing of 10 cases of Theiler’s disease and 37 in-contact horses in the absence of equine biologic product administration: A prospective study (2014–2018). J. Vet. Intern. Med. 2019, 33, 258–265. [Google Scholar] [CrossRef]
- Kapoor, A.; Simmonds, P.; Gerold, G.; Qaisar, N.; Jain, K.; Henriquez, J.A.; Firth, C.; Hirschberg, D.L.; Rice, C.M.; Shields, S.; et al. Characterization of a canine homolog of hepatitis C virus. Proc. Natl. Acad. Sci. USA 2011, 108, 11608–11613. [Google Scholar] [CrossRef] [Green Version]
- Burbelo, P.D.; Dubovi, E.J.; Simmonds, P.; Medina, J.L.; Henriquez, J.A.; Mishra, N.; Wagner, J.; Tokarz, R.; Cullen, J.M.; Iadarola, M.J.; et al. Serology-Enabled Discovery of Genetically Diverse Hepaciviruses in a New Host. J. Virol. 2012, 86, 6171–6178. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.B.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, A.S.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; et al. Proposed update to the taxonomy of the genera Hepacivirus and Pegivirus within the Flaviviridae family. J. Gen. Virol. 2016, 97, 2894–2907. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Wolfisberg, R.; Fahnøe, U.; Sharma, H.; Renshaw, R.W.; Nielsen, L.; Nishiuchi, E.; Holm, C.; Dubovi, E.; Rosenberg, B.R.; et al. Equine pegiviruses cause persistent infection of bone marrow and are not associated with hepatitis. PLoS Pathog. 2020, 16, e1008677. [Google Scholar] [CrossRef]
- Pfaender, S.; Cavalleri, J.M.V.; Walter, S.; Doerrbecker, J.; Campana, B.; Brown, R.J.P.; Burbelo, P.D.; Postel, A.; Hahn, K.; Anggakusuma; et al. Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology 2015, 61, 447–459. [Google Scholar] [CrossRef]
- Divers, T.J.; Tomlinson, J.E. Theiler’s disease. Equine Vet. Educ. 2020, 32, 63–65. [Google Scholar] [CrossRef]
- Tomlinson, J.E.; Jager, M.; Struzyna, A.; Laverack, M.; Fortier, L.A.; Dubovi, E.; Foil, L.D.; Burbelo, P.D.; Divers, T.J.; Van de Walle, G.R. Tropism, pathology, and transmission of equine parvovirus-hepatitis. Emerg. Microbes Infect. 2020, 9, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meister, T.L.; Tegtmeyer, B.; Postel, A.; Cavalleri, J.-M.V.; Todt, D.; Stang, A.; Steinmann, E. Equine Parvovirus-Hepatitis Frequently Detectable in Commercial Equine Serum Pools. Viruses 2019, 11, 461. [Google Scholar] [CrossRef] [Green Version]
- Baird, J.; Tegtmeyer, B.; Arroyo, L.; Stang, A.; Brüggemann, Y.; Hazlett, M.; Steinmann, E. The association of Equine Parvovirus-Hepatitis (EqPV-H) with cases of non-biologic-associated Theiler’s disease on a farm in Ontario, Canada. Vet. Microbiol. 2020, 242, 108575. [Google Scholar] [CrossRef]
- Vengust, M.; Jager, M.C.; Zalig, V.; Cociancich, V.; Laverack, M.; Renshaw, R.W.; Dubovi, E.; Tomlinson, J.E.; Van de Walle, G.R.; Divers, T.J. First report of equine parvovirus-hepatitis-associated Theiler’s disease in Europe. Equine Vet. J. 2020, 52, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Sun, L.; Ou, J.; Xu, H.; Wu, L.; Li, S. Identification and genetic characterization of a novel parvovirus associated with serum hepatitis in horses in China. Emerg. Microbes Infect. 2018, 7, 170. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Wu, L.; Ou, J.; Li, S. Equine Parvovirus-Hepatitis in China: Characterization of Its Genetic Diversity and Evidence for Natural Recombination Events Between the Chinese and American Strains. Front. Vet. Sci. 2020, 7, 121. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Woo, P.C.Y.; Yeung, H.C.; Teng, J.L.L.; Wu, Y.; Bai, R.; Fan, R.Y.Y.; Chan, K.-H.; Yuen, K.-Y. Identification and characterization of bocaviruses in cats and dogs reveals a novel feline bocavirus and a novel genetic group of canine bocavirus. J. Gen. Virol. 2012, 93, 1573–1582. [Google Scholar] [CrossRef]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family Parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef]
- Palinski, R.M.; Mitra, N.; Hause, B.M. Discovery of a novel Parvovirinae virus, porcine parvovirus 7, by metagenomic sequencing of porcine rectal swabs. Virus Genes 2016, 52, 564–567. [Google Scholar] [CrossRef]
- Walter, S.; Rasche, A.; Moreira-Soto, A.; Pfaender, S.; Bletsa, M.; Corman, V.M.; Aguilar-Setien, A.; García-Lacy, F.; Hans, A.; Todt, D.; et al. Differential Infection Patterns and Recent Evolutionary Origins of Equine Hepaciviruses in Donkeys. J. Virol. 2017, 91, e01711-16. [Google Scholar] [CrossRef] [Green Version]
- Burbelo, P.D.; Ching, K.H.; Klimavicz, C.M.; Iadarola, M.J. Antibody Profiling by Luciferase Immunoprecipitation Systems (LIPS). JoVE J. Vis. Exp. 2009, 7, 1549. [Google Scholar] [CrossRef] [Green Version]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Nei, M.; Kumar, S. Molecular Evolution and Phylogenetics; Oxford University Press: Oxford, UK; New York, NY, USA, 2000; ISBN 9780195350517. [Google Scholar]
- Tamura, K.; Nei, M.; Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc. Natl. Acad. Sci. USA 2004, 101, 11030–11035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orlando, L.; Ginolhac, A.; Zhang, G.; Froese, D.; Albrechtsen, A.; Stiller, M.; Schubert, M.; Cappellini, E.; Petersen, B.; Moltke, I.; et al. Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature 2013, 499, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Meister, T.L.; Tegtmeyer, B.; Brüggemann, Y.; Sieme, H.; Feige, K.; Todt, D.; Stang, A.; Cavalleri, J.V.; Steinmann, E. Characterization of Equine Parvovirus in Thoroughbred Breeding Horses from Germany. Viruses 2019, 11, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badenhorst, M.; Heus, P.; Auer, A.; Tegtmeyer, B.; Stang, A.; Dimmel, K.; Tichy, A.; Kubacki, J.; Bachofen, C.; Steinmann, E.; et al. Active equine parvovirus-hepatitis infection is most frequently detected in Austrian horses of advanced age. Equine Vet. J. 2021. [Google Scholar] [CrossRef]
- Mittel, L.D.; Norton, P.; Tomlinson, J.E.; Divers, T.J. Equine Parvovirus Hepatitis Virus Control Guidelines. American Association of Equine Practitioners. Available online: https://aaep.org/sites/default/files/2021-03/Equine_Parvovirus_Hepatitis_Virus_Control_Guidelines.pdf (accessed on 25 March 2021).





| Primer/Probe | Sequence |
|---|---|
| Forward primer | ATGCAGATGCTTTCCGACC |
| Reverse primer | GCCCCAGAAACATATGGAAA |
| Probe | [6-FAM]ACCGTAGCGGATTCGGGATCTGC[BHQ1a-6FAM] |
| Horse | EqPV-H DNA in Serum | EqPV-H DNA in Biopsy |
|---|---|---|
| 6 | 6.4 × 103 copies/mL | 6.2 × 102 copies/µg DNA |
| 11 | <2.5 × 103 copies/mL | Not detected |
| 72 | 1.6 × 104 copies/mL | 2.3 × 103 copies/µg DNA |
| Sample ID | Species | Country | NCBI Accession Number |
|---|---|---|---|
| BG128 | donkey | Bulgaria | MW828692 |
| BG147 | donkey | Bulgaria | MW828693 |
| BG153 | donkey | Bulgaria | MW828694 |
| BG155 | donkey | Bulgaria | MW828695 |
| I | donkey | Italy | MW828696 |
| 6_2018 | horse | Germany | MW828697 |
| 11_2018 | horse | Germany | MW828698 |
| 72_2018 | horse | Germany | MW828699 |
| 2_2015 | horse | Germany | MW828700 |
| 2_12/2013 | horse | Germany | MW828701 |
| 6_2016 | horse | Germany | MW828702 |
| 6_2015 | horse | Germany | MW828703 |
| 6_12/2013 | horse | Germany | MW828704 |
| 7_2015 | horse | Germany | MW828705 |
| 7_12/2013 | horse | Germany | MW828706 |
| 7_10/2013 | horse | Germany | MW828707 |
| 11_2015 | horse | Germany | MW828708 |
| 11_2016 | horse | Germany | MW828709 |
| 11_12/2013 | horse | Germany | MW828710 |
| 11_10/2013 | horse | Germany | MW828711 |
| 15_2015 | horse | Germany | MW828712 |
| 15_12/2013 | horse | Germany | MW828713 |
| 15_10/2013 | horse | Germany | MW828714 |
| 27_12/2013 | horse | Germany | MW828715 |
| 27_10/2013 | horse | Germany | MW828716 |
| 49_2015 | horse | Germany | MW828717 |
| 49_2016 | horse | Germany | MW828718 |
| 52_2015 | horse | Germany | MW828719 |
| 54_2016 | horse | Germany | MW828720 |
| 72_2016 | horse | Germany | MW828721 |
| 72_12/2013 | horse | Germany | MW828722 |
| 72_10/2013 | horse | Germany | MW828723 |
| 74_2015 | horse | Germany | MW828724 |
| 74_12/2013 | horse | Germany | MW828725 |
| 75_12/2013 | horse | Germany | MW828726 |
| 75_10/2013 | horse | Germany | MW828727 |
| 86_10/2013 | horse | Germany | MW828728 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reinecke, B.; Klöhn, M.; Brüggemann, Y.; Kinast, V.; Todt, D.; Stang, A.; Badenhorst, M.; Koeppel, K.; Guthrie, A.; Groner, U.; et al. Clinical Course of Infection and Cross-Species Detection of Equine Parvovirus-Hepatitis. Viruses 2021, 13, 1454. https://doi.org/10.3390/v13081454
Reinecke B, Klöhn M, Brüggemann Y, Kinast V, Todt D, Stang A, Badenhorst M, Koeppel K, Guthrie A, Groner U, et al. Clinical Course of Infection and Cross-Species Detection of Equine Parvovirus-Hepatitis. Viruses. 2021; 13(8):1454. https://doi.org/10.3390/v13081454
Chicago/Turabian StyleReinecke, Birthe, Mara Klöhn, Yannick Brüggemann, Volker Kinast, Daniel Todt, Alexander Stang, Marcha Badenhorst, Katja Koeppel, Alan Guthrie, Ursula Groner, and et al. 2021. "Clinical Course of Infection and Cross-Species Detection of Equine Parvovirus-Hepatitis" Viruses 13, no. 8: 1454. https://doi.org/10.3390/v13081454
APA StyleReinecke, B., Klöhn, M., Brüggemann, Y., Kinast, V., Todt, D., Stang, A., Badenhorst, M., Koeppel, K., Guthrie, A., Groner, U., Puff, C., de le Roi, M., Baumgärtner, W., Cavalleri, J.-M. V., & Steinmann, E. (2021). Clinical Course of Infection and Cross-Species Detection of Equine Parvovirus-Hepatitis. Viruses, 13(8), 1454. https://doi.org/10.3390/v13081454







