Identification of Novel HBV/HDV Entry Inhibitors by Pharmacophore- and QSAR-Guided Virtual Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. NTCP-Expressing Cell Lines
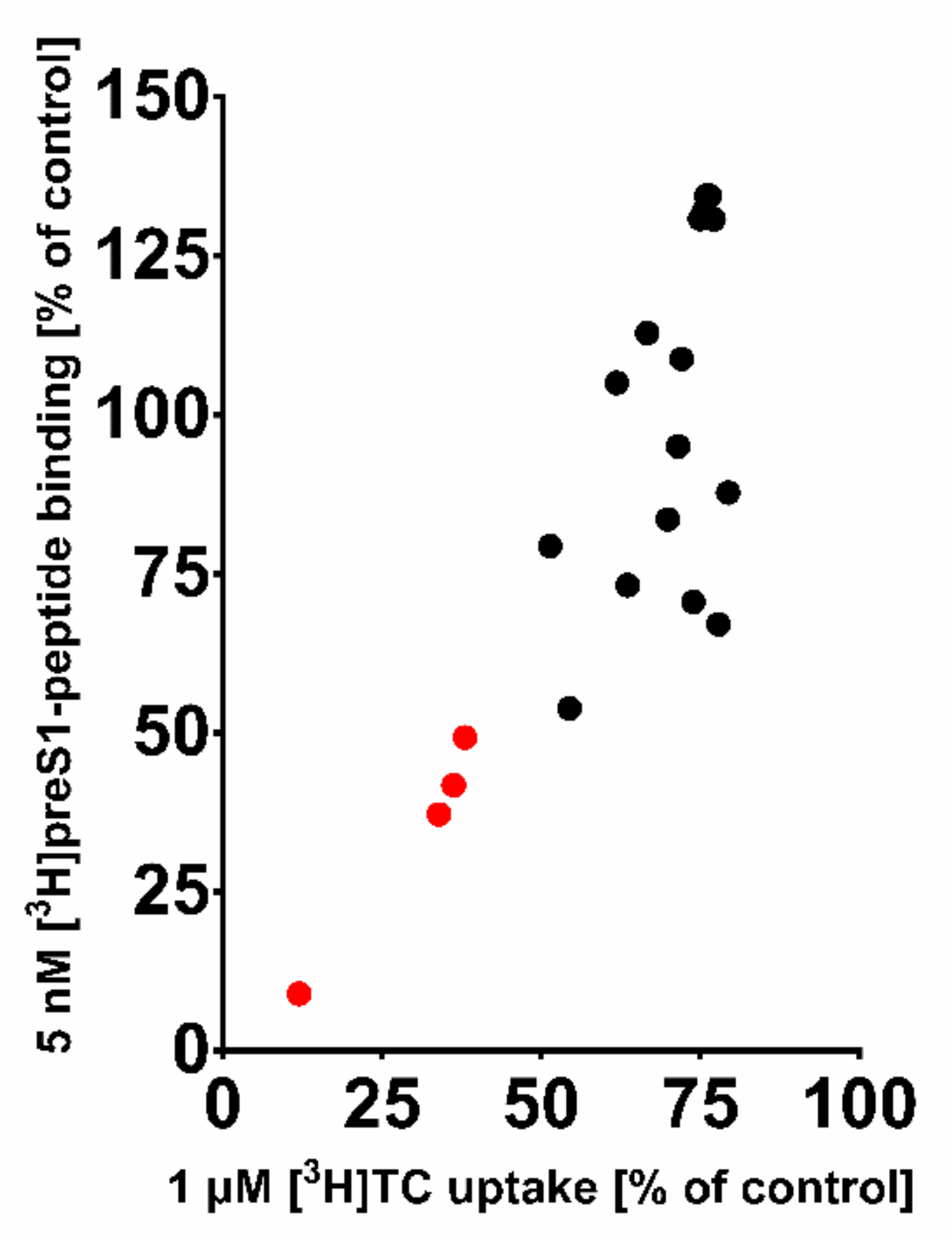
2.2. Inhibitory Concentrations (IC50) for [3H]preS1 Binding and [3H]TC Transport
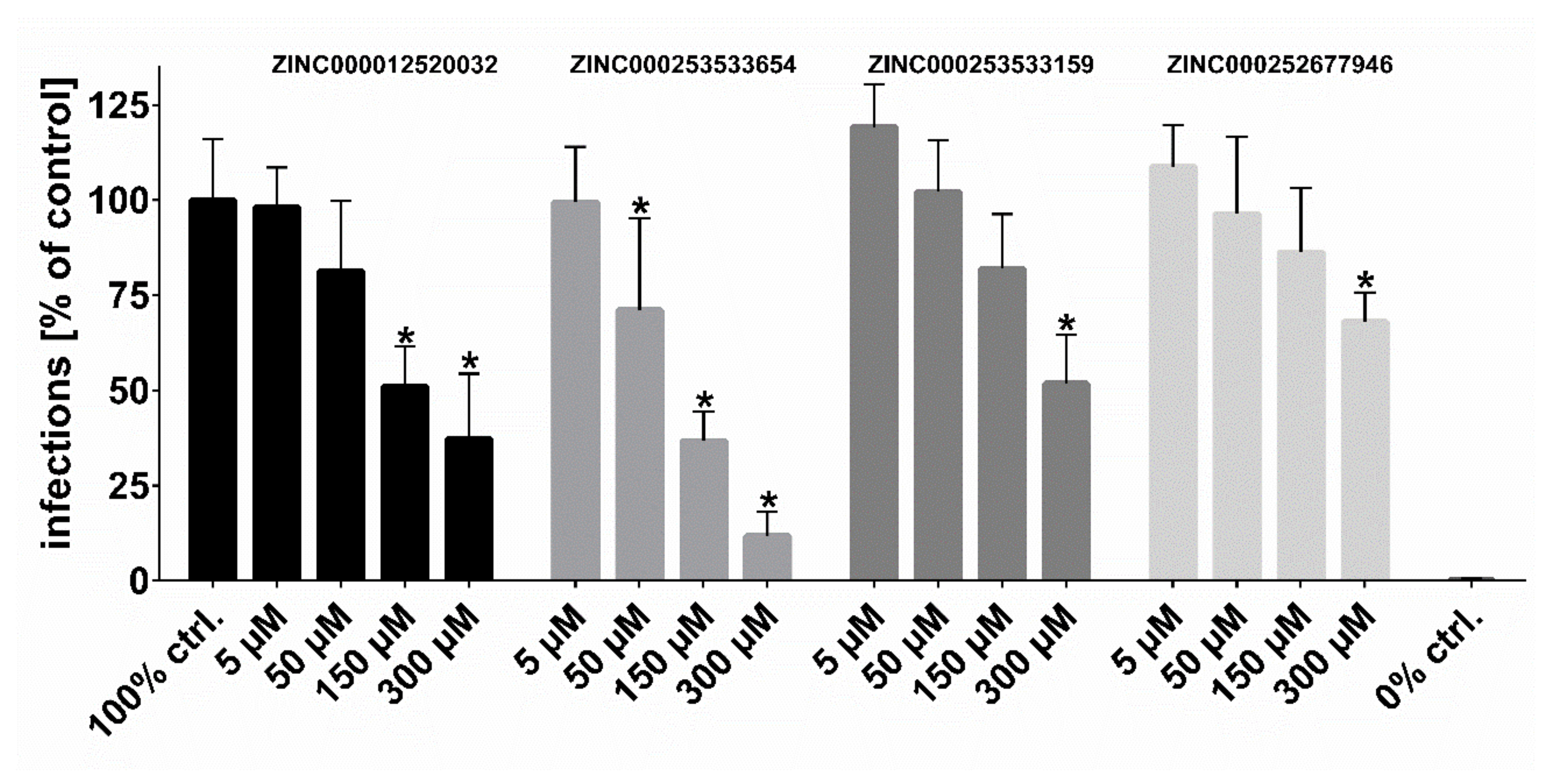
2.3. HDV Infection Experiments
2.4. Cytotoxicity Assay
2.5. Tested Compounds
2.6. Data Preparation
2.7. Pharmacophore Generation
2.8. Atom-Based QSAR-Model Generation
2.9. Virtual Screening
2.10. Statistics
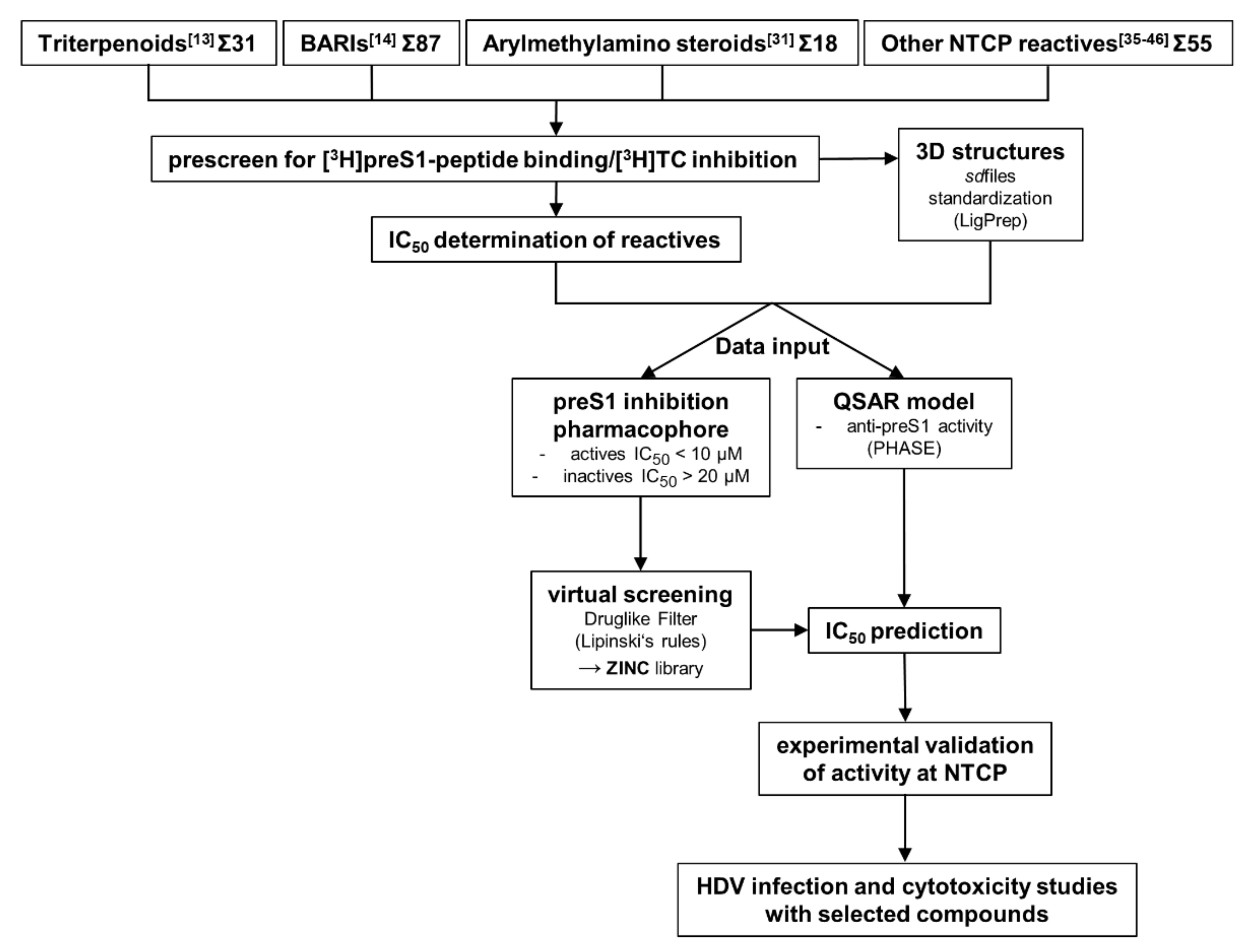
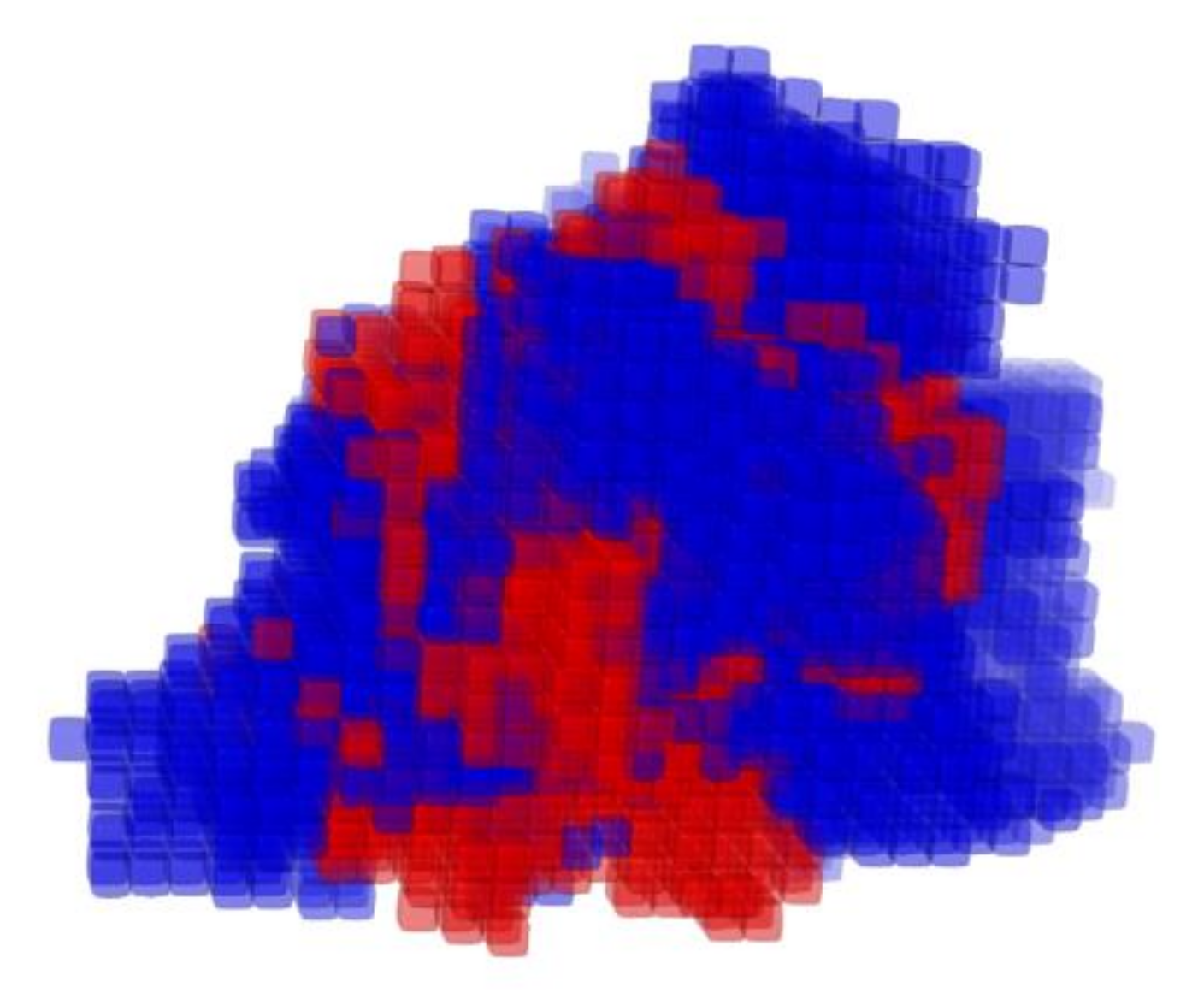
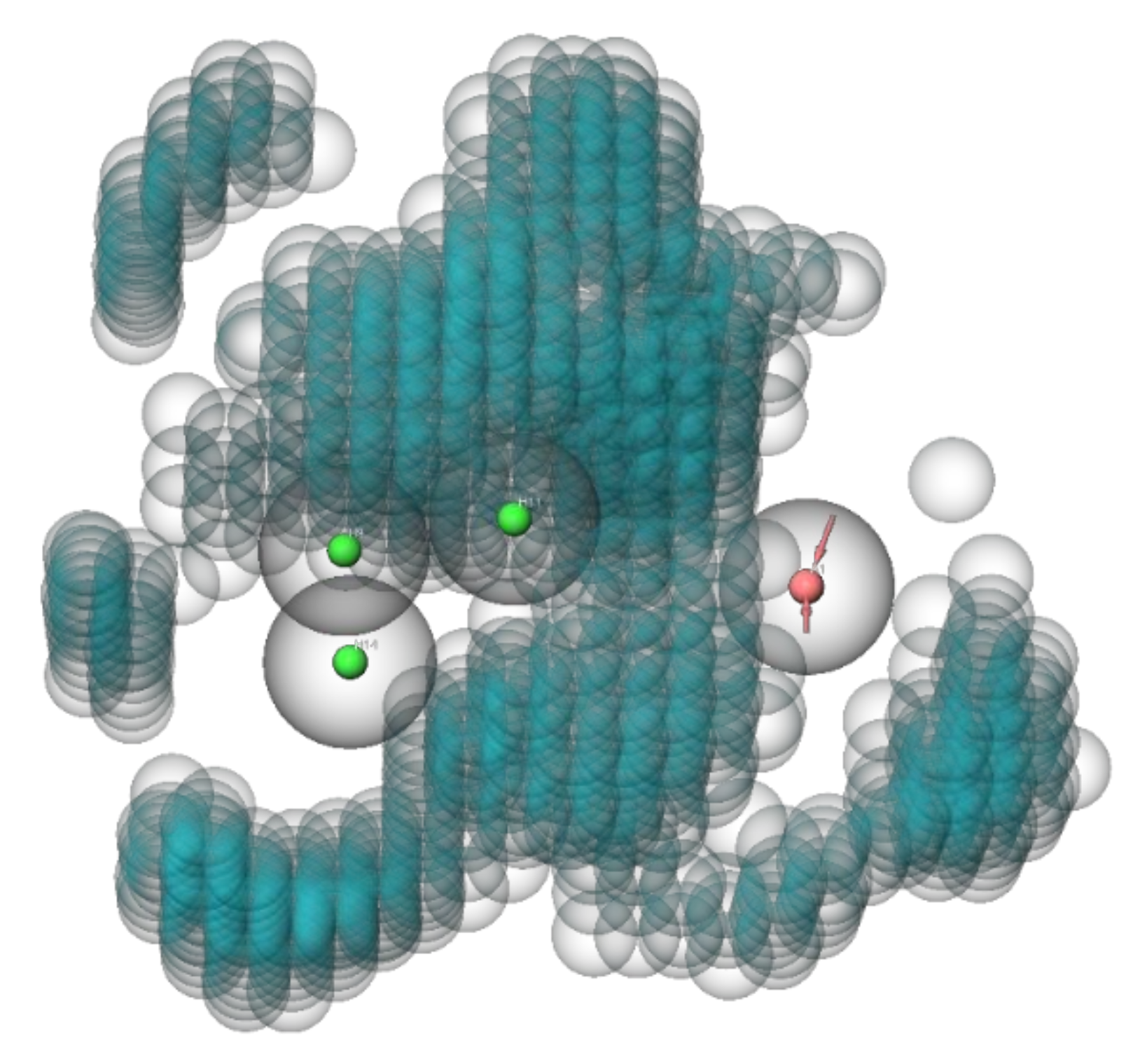
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report 2017; Geneva 2017. Licence:CC BY-NC-SA 3.0 IGO; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Glebe, D.; Bremer, C.M. The molecular virology of hepatitis B virus. Semin. Liver. Dis. 2013, 33, 103–112. [Google Scholar] [CrossRef]
- Glebe, D.; Urban, S.; Knoop, E.V.; Cag, N.; Krass, P.; Grun, S.; Bulavaite, A.; Sasnauskas, K.; Gerlich, W.H. Mapping of the hepatitis B virus attachment site by use of infection-inhibiting preS1 lipopeptides and tupaia hepatocytes. Gastroenterology 2005, 129, 234–245. [Google Scholar] [CrossRef]
- Gripon, P.; Cannie, I.; Urban, S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J. Virol. 2005, 79, 1613–1622. [Google Scholar] [CrossRef]
- Hughes, S.A.; Wedemeyer, H.; Harrison, P.M. Hepatitis delta virus. Lancet 2011, 378, 73–85. [Google Scholar] [CrossRef]
- Martinez, M.G.; Villeret, F.; Testoni, B.; Zoulim, F. Can we cure hepatitis B virus with novel direct-acting antivirals? Liver Int. 2020, 40 (Suppl. S1), 27–34. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Prevention, Care and Treatment of Persons with Chronic Hepatitis b Infection; WHO: Geneva, Switzerland, 2015. [Google Scholar]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1. [Google Scholar] [CrossRef] [PubMed]
- König, A.; Döring, B.; Mohr, C.; Geipel, A.; Geyer, J.; Glebe, D. Kinetics of the bile acid transporter and hepatitis B virus receptor Na+/taurocholate cotransporting polypeptide (NTCP) in hepatocytes. J. Hepatol. 2014, 61, 867–875. [Google Scholar] [CrossRef] [PubMed]
- MYR Pharmaceuticals. Available online: http://myr-pharma.com/ (accessed on 15 May 2021).
- Goh, B.; Choi, J.; Kang, J.A.; Park, S.G.; Seo, J.; Kim, T.Y. Development of a mass spectrometric screening assay for hepatitis B virus entry inhibitors. J. Pharm. Biomed. Anal. 2020, 178, 112959. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Watashi, K.; Tsukuda, S.; Aly, H.H.; Fukasawa, M.; Fujimoto, A.; Suzuki, R.; Aizaki, H.; Ito, T.; Koiwai, O.; et al. Evaluation and identification of hepatitis B virus entry inhibitors using HepG2 cells overexpressing a membrane transporter NTCP. Biochem. Biophys. Res. Commun. 2014, 443, 808–813. [Google Scholar] [CrossRef]
- Kirstgen, M.; Lowjaga, K.; Müller, S.F.; Goldmann, N.; Lehmann, F.; Alakurtti, S.; Yli-Kauhaluoma, J.; Glebe, D.; Geyer, J. Selective hepatitis B and D virus entry inhibitors from the group of pentacyclic lupane-type betulin-derived triterpenoids. Sci. Rep. 2020, 10, 21772. [Google Scholar] [CrossRef]
- Kirstgen, M.; Lowjaga, K.A.A.T.; Müller, S.F.; Goldmann, N.; Lehmann, F.; Glebe, D.; Baringhaus, K.-H.; Geyer, J. Hepatitis D Virus Entry Inhibitors Based on Repurposing Intestinal Bile Acid Reabsorption Inhibitors. Viruses 2021, 13, 666. [Google Scholar] [CrossRef] [PubMed]
- Mueller, R.; Dawson, E.S.; Meiler, J.; Rodriguez, A.L.; Chauder, B.A.; Bates, B.S.; Felts, A.S.; Lamb, J.P.; Menon, U.N.; Jadhav, S.B.; et al. Discovery of 2-(2-benzoxazoyl amino)-4-aryl-5-cyanopyrimidine as negative allosteric modulators (NAMs) of metabotropic glutamate receptor 5 (mGlu(5)): From an artificial neural network virtual screen to an in vivo tool compound. ChemMedChem 2012, 7, 406–414. [Google Scholar] [CrossRef]
- Thorne, N.; Auld, D.S.; Inglese, J. Apparent activity in high-throughput screening: Origins of compound-dependent assay interference. Curr. Opin. Chem. Biol. 2010, 14, 315–324. [Google Scholar] [CrossRef]
- Butkiewicz, M.; Lowe, E.W., Jr.; Mueller, R.; Mendenhall, J.L.; Teixeira, P.L.; Weaver, C.D.; Meiler, J. Benchmarking ligand-based virtual High-Throughput Screening with the PubChem database. Molecules 2013, 18, 735–756. [Google Scholar] [CrossRef]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Cherkasov, A.; Muratov, E.N.; Fourches, D.; Varnek, A.; Baskin, I.I.; Cronin, M.; Dearden, J.; Gramatica, P.; Martin, Y.C.; Todeschini, R.; et al. QSAR modeling: Where have you been? Where are you going to? J. Med. Chem. 2014, 57, 4977–5010. [Google Scholar] [CrossRef]
- Sterling, T.; Irwin, J.J. ZINC 15-Ligand Discovery for Everyone. J. Chem. Inf. Model. 2015, 55, 2324–2337. [Google Scholar] [CrossRef]
- Kar, S.; Roy, K. How far can virtual screening take us in drug discovery? Expert Opin. Drug Discov. 2013, 8, 245–261. [Google Scholar] [CrossRef]
- Neves, B.J.; Braga, R.C.; Melo-Filho, C.C.; Moreira-Filho, J.T.; Muratov, E.N.; Andrade, C.H. QSAR-Based Virtual Screening: Advances and Applications in Drug Discovery. Front Pharmacol. 2018, 9, 1275. [Google Scholar] [CrossRef]
- Geyer, J.; Döring, B.; Meerkamp, K.; Ugele, B.; Bakhiya, N.; Fernandes, C.F.; Godoy, J.R.; Glatt, H.; Petzinger, E. Cloning and functional characterization of human sodium-dependent organic anion transporter (SLC10A6). J. Biol. Chem. 2007, 282, 19728–19741. [Google Scholar] [CrossRef] [PubMed]
- Grosser, G.; Müller, S.F.; Kirstgen, M.; Döring, B.; Geyer, J. Substrate Specificities and Inhibition Pattern of the Solute Carrier Family 10 Members NTCP, ASBT and SOAT. Front. Mol. Biosci. 2021, 8. [Google Scholar] [CrossRef]
- Rasche, A.; Lehmann, F.; König, A.; Goldmann, N.; Corman, V.M.; Moreira-Soto, A.; Geipel, A.; van Riel, D.; Vakulenko, Y.A.; Sander, A.L.; et al. Highly diversified shrew hepatitis B viruses corroborate ancient origins and divergent infection patterns of mammalian hepadnaviruses. Proc. Natl. Acad. Sci. USA 2019, 116, 17007–17012. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho Dominguez Souza, B.F.; Konig, A.; Rasche, A.; de Oliveira Carneiro, I.; Stephan, N.; Corman, V.M.; Roppert, P.L.; Goldmann, N.; Kepper, R.; Muller, S.F.; et al. A novel hepatitis B virus species discovered in capuchin monkeys sheds new light on the evolution of primate hepadnaviruses. J. Hepatol. 2018, 68, 1114–1122. [Google Scholar] [CrossRef]
- Müller, S.F.; König, A.; Döring, B.; Glebe, D.; Geyer, J. Characterisation of the hepatitis B virus cross-species transmission pattern via Na+/taurocholate co-transporting polypeptides from 11 New World and Old World primate species. PLoS ONE 2018, 13, e0199200. [Google Scholar] [CrossRef] [PubMed]
- Alakurtti, S.; Heiska, T.; Kiriazis, A.; Sacerdoti-Sierra, N.; Jaffe, C.L.; Yli-Kauhaluoma, J. Synthesis and anti-leishmanial activity of heterocyclic betulin derivatives. Bioorg. Med. Chem. 2010, 18, 1573–1582. [Google Scholar] [CrossRef] [PubMed]
- Pohjala, L.; Alakurtti, S.; Ahola, T.; Yli-Kauhaluoma, J.; Tammela, P. Betulin-derived compounds as inhibitors of alphavirus replication. J. Nat. Prod. 2009, 72, 1917–1926. [Google Scholar] [CrossRef] [PubMed]
- Salin, O.; Alakurtti, S.; Pohjala, L.; Siiskonen, A.; Maass, V.; Maass, M.; Yli-Kauhaluoma, J.; Vuorela, P. Inhibitory effect of the natural product betulin and its derivatives against the intracellular bacterium Chlamydia pneumoniae. Biochem. Pharmacol. 2010, 80, 1141–1151. [Google Scholar] [CrossRef]
- Krieg, R.; Jortzik, E.; Goetz, A.A.; Blandin, S.; Wittlin, S.; Elhabiri, M.; Rahbari, M.; Nuryyeva, S.; Voigt, K.; Dahse, H.M.; et al. Arylmethylamino steroids as antiparasitic agents. Nat. Commun. 2017, 8, 14478. [Google Scholar] [CrossRef]
- Chen, I.J.; Foloppe, N. Drug-like bioactive structures and conformational coverage with the LigPrep/ConfGen suite: Comparison to programs MOE and catalyst. J. Chem. Inf. Model. 2010, 50, 822–839. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.L.; Smondyrev, A.M.; Knoll, E.H.; Rao, S.N.; Shaw, D.E.; Friesner, R.A. PHASE: A new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary results. J. Comput. Aided Mol. Des. 2006, 20, 647–671. [Google Scholar] [CrossRef]
- Williams, A.J.; Ekins, S. A quality alert and call for improved curation of public chemistry databases. Drug Discov. Today 2011, 16, 747–750. [Google Scholar] [CrossRef]
- Fukano, K.; Tsukuda, S.; Oshima, M.; Suzuki, R.; Aizaki, H.; Ohki, M.; Park, S.Y.; Muramatsu, M.; Wakita, T.; Sureau, C.; et al. Troglitazone Impedes the Oligomerization of Sodium Taurocholate Cotransporting Polypeptide and Entry of Hepatitis B Virus Into Hepatocytes. Front. Microbiol. 2018, 9, 3257. [Google Scholar] [CrossRef] [PubMed]
- McRae, M.P.; Lowe, C.M.; Tian, X.; Bourdet, D.L.; Ho, R.H.; Leake, B.F.; Kim, R.B.; Brouwer, K.L.; Kashuba, A.D. Ritonavir, saquinavir, and efavirenz, but not nevirapine, inhibit bile acid transport in human and rat hepatocytes. J. Pharmacol. Exp. Ther. 2006, 318, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ekins, S.; Polli, J.E. Structure-activity relationship for FDA approved drugs as inhibitors of the human sodium taurocholate cotransporting polypeptide (NTCP). Mol. Pharm. 2013, 10, 1008–1019. [Google Scholar] [CrossRef]
- Liu, Y.; Ruan, H.; Li, Y.; Sun, G.; Liu, X.; He, W.; Mao, F.; He, M.; Yan, L.; Zhong, G.; et al. Potent and Specific Inhibition of NTCP-Mediated HBV/HDV Infection and Substrate Transporting by a Novel, Oral-Available Cyclosporine A Analogue. J. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Shen, Z.-W.; Luo, M.-Y.; Hu, H.-H.; Zhou, H.; Jiang, H.-D.; Yu, L.-S.; Zeng, S. Screening and verifying potential NTCP inhibitors from herbal medicinal ingredients using the LLC-PK1 cell model stably expressing human NTCP. Chin. J. Nat. Med. 2016, 14, 549–560. [Google Scholar] [CrossRef]
- Dong, Z.; Ekins, S.; Polli, J.E. A substrate pharmacophore for the human sodium taurocholate co-transporting polypeptide. Int. J. Pharm. 2015, 478, 88–95. [Google Scholar] [CrossRef]
- Saso, W.; Tsukuda, S.; Ohashi, H.; Fukano, K.; Morishita, R.; Matsunaga, S.; Ohki, M.; Ryo, A.; Park, S.Y.; Suzuki, R.; et al. A new strategy to identify hepatitis B virus entry inhibitors by AlphaScreen technology targeting the envelope-receptor interaction. Biochem. Biophys. Res. Commun. 2018. [Google Scholar] [CrossRef]
- Hata, S.; Wang, P.; Eftychiou, N.; Ananthanarayanan, M.; Batta, A.; Salen, G.; Pang, K.S.; Wolkoff, A.W. Substrate specificities of rat oatp1 and ntcp: Implications for hepatic organic anion uptake. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, 829–839. [Google Scholar] [CrossRef] [PubMed]
- Mita, S.; Suzuki, H.; Akita, H.; Hayashi, H.; Onuki, R.; Hofmann, A.F.; Sugiyama, Y. Inhibition of bile acid transport across Na+/taurocholate cotransporting polypeptide (SLC10A1) and bile salt export pump (ABCB 11)-coexpressing LLC-PK1 cells by cholestasis-inducing drugs. Drug Metab. Dispos. 2006, 34, 1575–1581. [Google Scholar] [CrossRef]
- Nkongolo, S.; Ni, Y.; Lempp, F.A.; Kaufman, C.; Lindner, T.; Esser-Nobis, K.; Lohmann, V.; Mier, W.; Mehrle, S.; Urban, S. Cyclosporin A inhibits hepatitis B and hepatitis D virus entry by cyclophilin-independent interference with the NTCP receptor. J. Hepatol. 2014, 60, 723–731. [Google Scholar] [CrossRef] [PubMed]
- Shimura, S.; Watashi, K.; Fukano, K.; Peel, M.; Sluder, A.; Kawai, F.; Iwamoto, M.; Tsukuda, S.; Takeuchi, J.S.; Miyake, T.; et al. Cyclosporin derivatives inhibit hepatitis B virus entry without interfering with NTCP transporter activity. J. Hepatol. 2017, 66, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Watashi, K.; Sluder, A.; Daito, T.; Matsunaga, S.; Ryo, A.; Nagamori, S.; Iwamoto, M.; Nakajima, S.; Tsukuda, S.; Borroto-Esoda, K.; et al. Cyclosporin A and its analogs inhibit hepatitis B virus entry into cultured hepatocytes through targeting a membrane transporter, sodium taurocholate cotransporting polypeptide (NTCP). Hepatology 2014, 59, 1726–1737. [Google Scholar] [CrossRef]
- Dong, Z.; Ekins, S.; Polli, J.E. Quantitative NTCP pharmacophore and lack of association between DILI and NTCP Inhibition. Eur. J. Pharm. Sci. 2015, 66, 1–9. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beeley, N.R.A.; Sage, C. GPCRs: An update on structural approaches to drug discovery. Targets 2003, 2, 19–25. [Google Scholar] [CrossRef]
- Klebe, G. Virtual ligand screening: Strategies, perspectives and limitations. Drug Discov. Today 2006, 11, 580–594. [Google Scholar] [CrossRef]
- DePristo, M.A.; de Bakker, P.I.; Blundell, T.L. Heterogeneity and inaccuracy in protein structures solved by X-ray crystallography. Structure 2004, 12, 831–838. [Google Scholar] [CrossRef]
- Srivastava, A.; Nagai, T.; Srivastava, A.; Miyashita, O.; Tama, F. Role of Computational Methods in Going beyond X-ray Crystallography to Explore Protein Structure and Dynamics. Int. J. Mol. Sci. 2018, 19, 3401. [Google Scholar] [CrossRef]
- Dixon, S.L.; Duan, J.; Smith, E.; Von Bargen, C.D.; Sherman, W.; Repasky, M.P. AutoQSAR: An automated machine learning tool for best-practice QSAR modeling. Future Med. Chem. 2016, 8, 1825–1839. [Google Scholar] [CrossRef]
- Appelman, M.D.; Wettengel, J.M.; Protzer, U.; Oude Elferink, R.P.J.; van de Graaf, S.F.J. Molecular regulation of the hepatic bile acid uptake transporter and HBV entry receptor NTCP. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2021, 1866, 158960. [Google Scholar] [CrossRef]



| Ligand Name | Binding Affinity | Prediction | Error |
|---|---|---|---|
| Rosiglitazone [35] | 5.699 | 5.836 | 0.137 |
| 28-O-Succinoylbetulin [13] | 5.523 | 5.278 | −0.245 |
| 3-O-Acetylbetulinic acid [13] | 5.523 | 5.214 | −0.309 |
| Ginkgolic acid 17:1 [39] | 5.523 | 5.821 | 0.298 |
| Troglitazone [35] | 5.523 | 5.448 | −0.075 |
| Ginkgolic acid 15:1 [39] | 5.523 | 5.461 | −0.062 |
| 28-O-(3,3-dimethylglutaroyl)betulin [13] | 5.398 | 5.365 | −0.033 |
| Ritonavir [36] | 5.398 | 5.243 | −0.155 |
| 20,29-Dihydrobetulonic acid [13] | 5.319 | 4.993 | −0.326 |
| 3,28-Di-O-succinoylbetulin [13] | 5.301 | 5.200 | −0.101 |
| 3-O-Caffeoylbetulin [13] | 5.222 | 5.310 | 0.088 |
| 3,28-Di-O-(3,3-dimethylglutaroyl)betulin [13] | 5.155 | 5.163 | 0.008 |
| Steroid 12c [31] | 5.097 | 5.043 | −0.054 |
| 28-O-(Bromoacetyl)betulin [13] | 5.097 | 4.878 | −0.219 |
| S973509 [14] | 5.046 | 5.027 | −0.019 |
| A000028897 [14] | 5.046 | 5.090 | 0.044 |
| A000295231 [14] | 5.000 | 5.004 | 0.004 |
| Erythrosin B [39] | 5.000 | 5.087 | 0.087 |
| Betulinaldehyde oxime [13] | 4.959 | 4.848 | −0.111 |
| 28-(2H-Tetrahydropyran-2-yl)betulin [13] | 4.854 | 4.746 | −0.108 |
| Allobetulin [13] | 4.854 | 4.843 | −0.011 |
| Efavirenz [36] | 4.796 | 4.646 | −0.149 |
| Rifampicin [47] | 4.745 | 4.849 | 0.105 |
| Steroid 9c [31] | 4.745 | 4.718 | −0.026 |
| 28-O-Cinnamoylbetulin [13] | 4.721 | 4.715 | −0.006 |
| Rapamycin [41] | 4.678 | 4.683 | 0.005 |
| Simvastatin [37] | 4.638 | 4.554 | −0.084 |
| Cyclosporine A [38,43−46] | 4.585 | 4.472 | −0.113 |
| Losartan [37] | 4.538 | 4.620 | 0.082 |
| Methyl betulinate [13] | 4.481 | 4.692 | 0.211 |
| Steroid 8c [31] | 4.469 | 4.538 | 0.070 |
| 3-O-Acetyl-28-(2H-tetrahydropyran-2-yl)betulin [13] | 4.456 | 4.593 | 0.137 |
| Nimodipine [37] | 4.444 | 4.429 | −0.015 |
| Betulonic aldehyde [13] | 4.432 | 4.426 | −0.006 |
| Bromosulfophthalein [42] | 4.420 | 4.506 | 0.085 |
| 3-O-Acetylbetulin [13] | 4.377 | 4.389 | 0.012 |
| Steroid 13c [31] | 4.367 | 4.182 | −0.184 |
| 3,28-Di-O-acetyl-20,30-epoxybetulin [13] | 4.357 | 4.321 | −0.035 |
| Steroid 1g [31] | 4.310 | 4.148 | −0.162 |
| Steroid 1o [31] | 4.260 | 4.255 | −0.005 |
| 28-O-Nicotinoylbetulin [13] | 4.201 | 4.258 | 0.057 |
| 3,28-Di-O-acetyl-18,19-dehydro-20,29-dihydrobetulin [13] | 4.201 | 4.235 | 0.034 |
| Bendroflumethiazide [37] | 4.201 | 4.263 | 0.062 |
| Tioconazole [37] | 4.125 | 4.308 | 0.183 |
| S973515 [14] | 4.119 | 3.989 | −0.130 |
| Steroid 7c [31] | 4.066 | 4.142 | 0.076 |
| Steroid 5c [31] | 4.056 | 4.066 | 0.011 |
| A000295480 [14] | 4.009 | 4.098 | 0.089 |
| Steroid 3c [31] | 3.996 | 3.994 | −0.002 |
| Betulin [13] | 3.963 | 4.454 | 0.492 |
| Steroid 4c [31] | 3.959 | 4.005 | 0.046 |
| A000295013 [14] | 3.924 | 3.865 | −0.059 |
| Steroid 1e [31] | 3.910 | 3.988 | 0.078 |
| 4′-Ethyl-1′,2′,4′-triazoline-3′,5′-dione-fused 3,28-di-O-acetylbetulin [13] | 3.848 | 3.741 | −0.107 |
| 3,28-Di-O-acetylbetulin [13] | 3.701 | 3.939 | 0.238 |
| Steroid 1c [31] | 3.652 | 3.642 | −0.010 |
| Olmesartan [37] | 3.129 | 3.057 | −0.072 |
| Irbesartan [37] | 3.032 | 3.076 | 0.045 |
| Rosuvastatin [37] | 1.000 | 0.918 | −0.082 |
| # PLS Factors | SD | R2 | Stability | F | P | Pearson-r |
|---|---|---|---|---|---|---|
| 1 | 0.4671 | 0.5998 | 0.245 | 95.9 | −14 | 0.2709 |
| 2 | 0.3093 | 0.8273 | 0.055 | 150.9 | −25 | 0.3074 |
| 3 | 0.2314 | 0.9048 | −0.216 | 196.5 | −31 | 0.358 |
| 4 | 0.153 | 0.9591 | −0.242 | 357.3 | −41 | 0.4614 |
| 5 | 0.1038 | 0.9815 | −0.298 | 635.2 | −50 | 0.408 |
| 6 | 0.079 | 0.9894 | −0.335 | 922 | −56 | 0.4073 |
| 7 | 0.053 | 0.9953 | −0.345 | 1766.9 | −65 | 0.4124 |
| # PLS Factors | H-Bond Donor | Hydrophobic/non-Polar | Negative Ionic | Positive Ionic | Electron-Withdrawing | Other |
|---|---|---|---|---|---|---|
| 1 | 0.045 | 0.635 | 0 | 0 | 0.285 | 0.035 |
| 2 | 0.048 | 0.618 | 0 | 0 | 0.297 | 0.037 |
| 3 | 0.049 | 0.613 | 0 | 0 | 0.300 | 0.038 |
| 4 | 0.053 | 0.606 | 0 | 0 | 0.301 | 0.040 |
| 5 | 0.055 | 0.601 | 0 | 0 | 0.301 | 0.041 |
| 6 | 0.056 | 0.600 | 0.001 | 0.001 | 0.302 | 0.041 |
| 7 | 0.056 | 0.601 | 0 | 0 | 0.302 | 0.040 |
| Ligand Name | Binding Affinity | Prediction | Error |
|---|---|---|---|
| Ciglitazone [35] | 6.000 | 4.570 | −1.430 |
| Betulinic acid [13] | 5.699 | 4.677 | −1.022 |
| 20,29-Dihydrobetulin [13] | 5.456 | 4.815 | −0.641 |
| 3-O-(3,3-Dimethylglutaroyl)betulinic acid [13] | 5.398 | 5.073 | −0.325 |
| 3,28-Di-O-acetyl-29-hydroxybetulin [13] | 5.097 | 4.433 | −0.664 |
| Saquinavir [36] | 5.097 | 4.349 | −0.748 |
| Emodin [39] | 5.046 | 4.845 | −0.201 |
| Ginkgolic acid 13:0 [39] | 4.959 | 4.496 | −0.462 |
| S985852 [14] | 4.854 | 4.774 | −0.080 |
| Pioglitazone [35] | 4.770 | 4.888 | 0.119 |
| Nitrendipine [37] | 4.745 | 4.390 | −0.354 |
| Glyburide [37] | 4.678 | 4.161 | −0.517 |
| Lupenone [13] | 4.658 | 4.398 | −0.259 |
| Betulonoyl dimethyl-L-aspartate [13] | 4.638 | 4.300 | −0.338 |
| 3-Oxoallobetulin [13] | 4.509 | 4.228 | −0.281 |
| Steroid 6c [31] | 4.432 | 4.040 | −0.392 |
| Steroid 7s [31] | 4.420 | 4.410 | −0.010 |
| Raloxifene [37] | 4.319 | 4.376 | 0.057 |
| Lupeol [37] | 4.292 | 4.497 | 0.204 |
| Steroid 1s [31] | 4.237 | 3.881 | −0.356 |
| 3,28-Di-O-(dihydrocinnamoyl)betulin [13] | 4.125 | 4.902 | 0.777 |
| A000289041 [14] | 4.051 | 4.708 | 0.657 |
| Nifedipine [37] | 4.018 | 4.463 | 0.445 |
| Steroid 2c [31] | 3.900 | 4.002 | 0.102 |
| Steroid 2s [31] | 3.666 | 4.075 | 0.410 |
| Steroid 2o [31] | 3.600 | 4.176 | 0.576 |
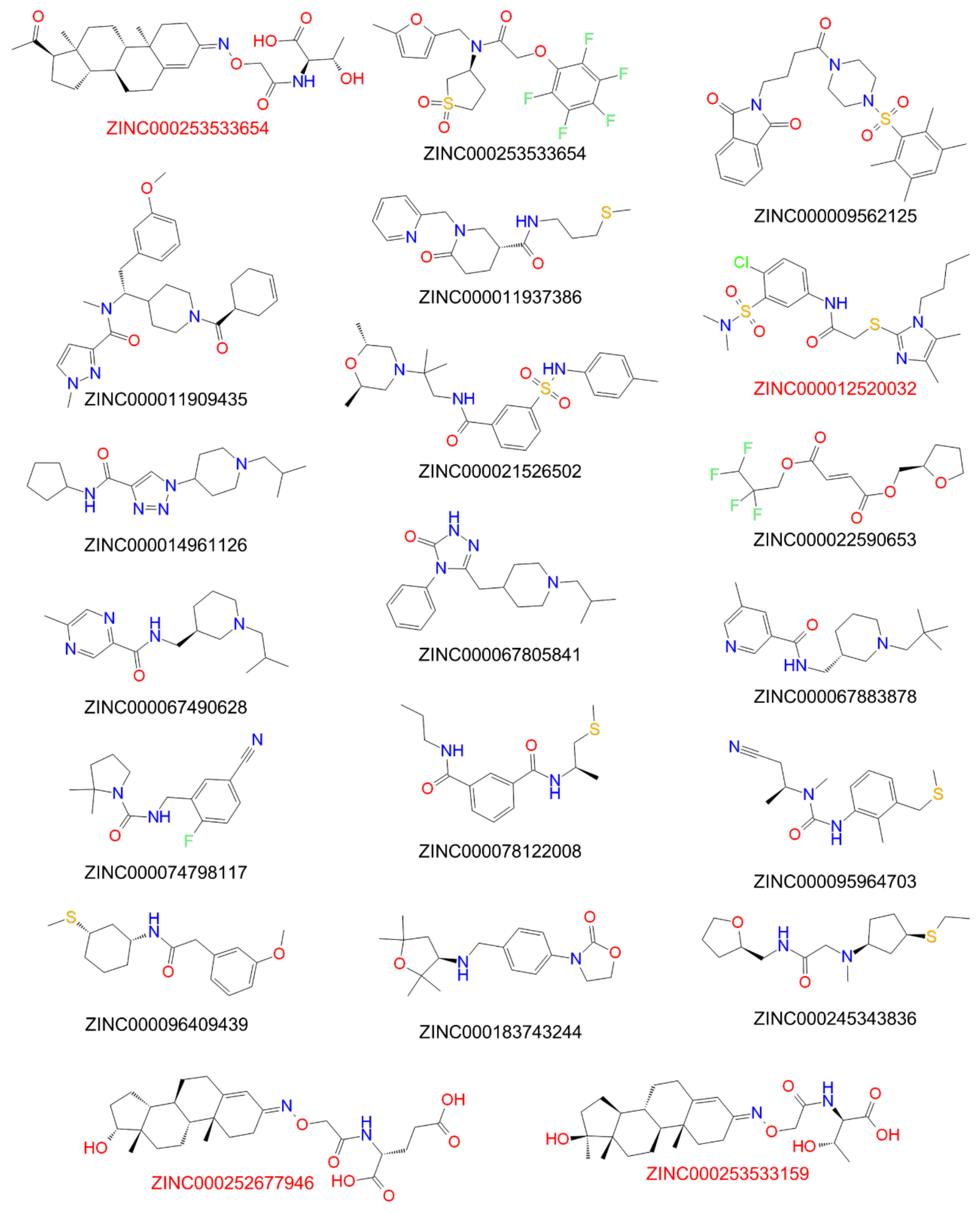
| Compound ID | Predicted Anti-PreS1 Activity (IC50 µM) | Compound ID | Predicted Anti-PreS1 Activity (IC50 µM) |
|---|---|---|---|
| ZINC000021526502 | 7 | ZINC000014961126 | 9 |
| ZINC000253533654 | 7 | ZINC000009431397 | 9 |
| ZINC000067883878 | 8 | ZINC000096409439 | 9 |
| ZINC000009562125 | 8 | ZINC000245343836 | 9 |
| ZINC000253533159 | 8 | ZINC000095964703 | 10 |
| ZINC000183743244 | 8 | ZINC000022590653 | 10 |
| ZINC000011937386 | 8 | ZINC000074798117 | 10 |
| ZINC000067805841 | 9 | ZINC000011909435 | 11 |
| ZINC000067490628 | 9 | ZINC000252677946 | 13 |
| ZINC000078122008 | 9 | ZINC000012520032 | 16 |
| Compound ID | Predicted Anti-PreS1 Activity (IC50 µM) | Experimentally Determined Anti-PreS1 Activity (IC50 µM) | Experimentally Determined Anti-TC Activity (IC50 µM) |
|---|---|---|---|
| ZINC000012520032 | 16 | 35 | 37 |
| ZINC000253533654 | 7 | 9 | 11 |
| ZINC000253533159 | 8 | 19 | 51 |
| ZINC000252677946 | 13 | 20 | 51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirstgen, M.; Müller, S.F.; Lowjaga, K.A.A.T.; Goldmann, N.; Lehmann, F.; Alakurtti, S.; Yli-Kauhaluoma, J.; Baringhaus, K.-H.; Krieg, R.; Glebe, D.; et al. Identification of Novel HBV/HDV Entry Inhibitors by Pharmacophore- and QSAR-Guided Virtual Screening. Viruses 2021, 13, 1489. https://doi.org/10.3390/v13081489
Kirstgen M, Müller SF, Lowjaga KAAT, Goldmann N, Lehmann F, Alakurtti S, Yli-Kauhaluoma J, Baringhaus K-H, Krieg R, Glebe D, et al. Identification of Novel HBV/HDV Entry Inhibitors by Pharmacophore- and QSAR-Guided Virtual Screening. Viruses. 2021; 13(8):1489. https://doi.org/10.3390/v13081489
Chicago/Turabian StyleKirstgen, Michael, Simon Franz Müller, Kira Alessandra Alicia Theresa Lowjaga, Nora Goldmann, Felix Lehmann, Sami Alakurtti, Jari Yli-Kauhaluoma, Karl-Heinz Baringhaus, Reimar Krieg, Dieter Glebe, and et al. 2021. "Identification of Novel HBV/HDV Entry Inhibitors by Pharmacophore- and QSAR-Guided Virtual Screening" Viruses 13, no. 8: 1489. https://doi.org/10.3390/v13081489
APA StyleKirstgen, M., Müller, S. F., Lowjaga, K. A. A. T., Goldmann, N., Lehmann, F., Alakurtti, S., Yli-Kauhaluoma, J., Baringhaus, K.-H., Krieg, R., Glebe, D., & Geyer, J. (2021). Identification of Novel HBV/HDV Entry Inhibitors by Pharmacophore- and QSAR-Guided Virtual Screening. Viruses, 13(8), 1489. https://doi.org/10.3390/v13081489







