Challenges in the Application of Glyco-Technology to Hepatitis B Virus Therapy and Diagnosis
Abstract
:1. Introduction
2. What Is Glycosylation?
3. Biological Significance of the Glycosylation of HBV Surface Proteins
4. Is HBV Glycosylation a Useful Target for HBV Therapy and/or Monitoring?
5. Glyco-Biomarkers for Hepatitis B-Related Liver Diseases
6. NTCP Glycosylation
7. HBV Infection and Cell Surface Glycosylation
8. Closing Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017; ISBN 9789241565455. [Google Scholar]
- Rybicka, M.; Bielawski, K.P. Recent advances in understanding, diagnosing, and treating hepatitis B virus infection. Microorganisms 2020, 8, 1416. [Google Scholar] [CrossRef]
- Burns, G.S.; Thompson, A.J. Viral hepatitis B: Clinical and epidemiological characteristics. Cold Spring Harb. Perspect. Med. 2014, 4, a024935. [Google Scholar] [CrossRef] [Green Version]
- Smolders, E.J.; Burger, D.M.; Feld, J.J.; Kiser, J.J. Review article: Clinical pharmacology of current and investigational hepatitis B virus therapies. Aliment. Pharmacol. Ther. 2020, 51, 231–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H.; et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. eLife 2012, 1, e00049. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Z.; Hu, F.; Su, L. Cell culture models and animal models for HBV study. Adv. Exp. Med. Biol. 2020, 1179, 109–135. [Google Scholar]
- Julithe, R.; Abou-Jaoudé, G.; Sureau, C. Modification of the hepatitis B virus envelope protein glycosylation pattern interferes with secretion of viral particles, infectivity, and susceptibility to neutralizing antibodies. J. Virol. 2014, 88, 9049–9059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomme, B.; Steenkiste, C.V.; Callewaert, N.; Vlierberghe, H.V. Alteration of protein glycosylation in liver diseases. J. Hepatol. 2009, 50, 592–603. [Google Scholar] [CrossRef]
- Li, Y.; Liu, D.; Wang, Y.; Su, W.; Liu, G.; Dong, W. The importance of glycans of viral and host proteins in enveloped virus infection. Front. Immunol. 2021, 12, 1544. [Google Scholar]
- Varki, A.; Cummings, R.D.; Aebi, M.; Packer, N.H.; Seeberger, P.H.; Esko, J.D.; Stanley, P.; Hart, G.; Darvill, A.; Kinoshita, T.; et al. Symbol nomenclature for graphical representations of glycans. Glycobiology 2015, 25, 1323–1324. [Google Scholar] [CrossRef] [Green Version]
- Pinho, S.S.; Reis, C.A. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Miyoshi, E.; Moriwaki, K.; Nakagawa, T. Biological function of fucosylation in cancer biology. J. Biochem. 2008, 143, 725–729. [Google Scholar] [CrossRef]
- Lau, K.S.; Dennis, J.W. N-Glycans in cancer progression. Glycobiology 2008, 18, 750–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyoshi, E.; Kamada, Y.; Suzuki, T. Functional glycomics: Application to medical science and hepatology. Hepatol. Res. 2020, 50, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Miyake, T.; Kuno, A.; Imai, Y.; Sawai, Y.; Hino, K.; Hara, Y.; Hige, S.; Sakamoto, M.; Yamada, G.; et al. Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J. Gastroenterol. 2015, 50, 776–784. [Google Scholar] [CrossRef] [PubMed]
- Marques-da-Silva, D.; Ferreira, V.d.R.; Monticelli, M.; Janeiro, P.; Videira, P.A.; Witters, P.; Jaeken, J.; Cassiman, D. Liver involvement in congenital disorders of glycosylation (CDG). A systematic review of the literature. J. Inherit. Metab. Dis. 2017, 40, 195–207. [Google Scholar] [CrossRef]
- Reily, C.; Stewart, T.J.; Renfrow, M.B.; Novak, J. Glycosylation in health and disease. Nat. Rev. Nephrol. 2019, 15, 346–366. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Garner, R.; Salehi, S.; La Rocca, M.; Duncan, D. Association between ABO blood types and coronavirus disease 2019 (COVID-19), genetic associations, and underlying molecular mechanisms: A literature review of 23 studies. Ann. Hematol. 2021, 100, 1123–1132. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Uchiyama, N.; Koseki-Kuno, S.; Ebe, Y.; Takashima, S.; Yamada, M.; Hirabayashi, J. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat. Methods 2005, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Narimatsu, H.; Kaji, H.; Vakhrushev, S.Y.; Clausen, H.; Zhang, H.; Noro, E.; Togayachi, A.; Nagai-Okatani, C.; Kuno, A.; Zou, X.; et al. Current technologies for complex glycoproteomics and their applications to biology/disease-driven glycoproteomics. J. Proteome Res. 2018, 17, 4097–4112. [Google Scholar] [CrossRef]
- Miyoshi, E.; Nakano, M. Fucosylated haptoglobin is a novel marker for pancreatic cancer: Detailed analyses of oligosaccharide structures. Proteomics 2008, 8, 3257–3262. [Google Scholar] [CrossRef]
- Heermann, K.H.; Goldmann, U.; Schwartz, W.; Seyffarth, T.; Baumgarten, H.; Gerlich, W.H. Large surface proteins of hepatitis B virus containing the pre-s sequence. J. Virol. 1984, 52, 396–402. [Google Scholar] [CrossRef] [Green Version]
- Heermann, K.-H.; Kruse, F.; Seifer, M.; Gerlich, W.H. Immunogenicity of the gene S and pre-S domains in hepatitis B virions and HBsAg filaments. Intervirology 1987, 28, 14–25. [Google Scholar] [CrossRef]
- Bruss, V. Hepatitis B virus morphogenesis. World J. Gastroenterol. WJG 2007, 13, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, S.; Glebe, D.; Tolle, T.K.; Lochnit, G.; Linder, D.; Geyer, R.; Gerlich, W.H.Y. Structure of pre-S2 N- and O-linked glycans in surface proteins from different genotypes of hepatitis B virus. J. Gen. Virol. 2004, 85, 2045–2053. [Google Scholar] [CrossRef]
- Sureau, C.; Fournier-Wirth, C.; Maurel, P. Role of N glycosylation of hepatitis B virus envelope proteins in morphogenesis and infectivity of hepatitis delta virus. J. Virol. 2003, 77, 5519–5523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, D.-M.; Li, X.-H.; Mom, V.; Lu, Z.-H.; Liao, X.-W.; Han, Y.; Pichoud, C.; Gong, Q.-M.; Zhang, D.; Zhang, Y.; et al. N-glycosylation mutations within hepatitis B virus surface major hydrophilic region contribute mostly to immune escape. J. Hepatol. 2014, 60, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Mehta, A.; Dadmarz, M.; Dwek, R.; Blumberg, B.S.; Block, T.M. Aberrant trafficking of hepatitis B virus glycoproteins in cells in which N-glycan processing is inhibited. Proc. Natl. Acad. Sci. USA 1997, 94, 2380–2385. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, H.; Zhang, J.; Yang, J.; Bai, L.; Zheng, B.; Zheng, T.; Wang, Y.; Li, J.; Zhang, W. SERINC5 Inhibits the Secretion of complete and genome-free hepatitis B virions through interfering with the glycosylation of the HBV envelope. Front. Microbiol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Qiao, Y.; Lu, S.; Xu, Z.; Li, X.; Zhang, K.; Liu, Y.; Zhao, L.; Chen, R.; Si, L.; Lin, S.; et al. Additional N-glycosylation mutation in the major hydrophilic region of hepatitis B virus S gene is a risk indicator for hepatocellular carcinoma occurrence in patients with coexistence of HBsAg/anti-HBs. Oncotarget 2017, 8, 61719–61730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Qin, Y.; Liu, Y.; Li, F.; Liao, H.; Lu, S.; Qiao, Y.; Xu, D.; Li, J. PreS deletion profiles of hepatitis B virus (HBV) are associated with clinical presentations of chronic HBV infection. J. Clin. Virol. 2016, 82, 27–32. [Google Scholar] [CrossRef]
- Cohen, D.; Ghosh, S.; Shimakawa, Y.; Ramou, N.; Garcia, P.S.; Dubois, A.; Guillot, C.; Kakwata-Nkor Deluce, N.; Tilloy, V.; Durand, G.; et al. Hepatitis B virus preS2Δ38–55 variants: A newly identified risk factor for hepatocellular carcinoma. JHEP Rep. 2020, 2, 100144. [Google Scholar] [CrossRef]
- Block, T.M.; Comunale, M.A.; Lowman, M.; Steel, L.F.; Romano, P.R.; Fimmel, C.; Tennant, B.C.; London, W.T.; Evans, A.A.; Blumberg, B.S.; et al. Use of targeted glycoproteomics to identify serum glycoproteins that correlate with liver cancer in woodchucks and humans. Proc. Natl. Acad. Sci. USA 2005, 102, 779–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marrero, J.A.; Romano, P.R.; Nikolaeva, O.; Steel, L.; Mehta, A.; Fimmel, C.J.; Comunale, M.A.; D’Amelio, A.; Lok, A.S.; Block, T.M. GP73, a resident Golgi glycoprotein, is a novel serum marker for hepatocellular carcinoma. J. Hepatol. 2005, 43, 1007–1012. [Google Scholar] [CrossRef]
- Dai, Z.; Liu, Y.-K.; Cui, J.-F.; Shen, H.-L.; Chen, J.; Sun, R.-X.; Zhang, Y.; Zhou, X.-W.; Yang, P.-Y.; Tang, Z.-Y. Identification and analysis of altered α1,6-fucosylated glycoproteins associated with hepatocellular carcinoma metastasis. Proteomics 2006, 6, 5857–5867. [Google Scholar] [CrossRef] [PubMed]
- Shirabe, K.; Bekki, Y.; Gantumur, D.; Araki, K.; Ishii, N.; Kuno, A.; Narimatsu, H.; Mizokami, M. Mac-2 binding protein glycan isomer (M2BPGi) is a new serum biomarker for assessing liver fibrosis: More than a biomarker of liver fibrosis. J. Gastroenterol. 2018, 53, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Jun, T.; Hsu, Y.-C.; Ogawa, S.; Huang, Y.-T.; Yeh, M.-L.; Tseng, C.-H.; Huang, C.-F.; Tai, C.-M.; Dai, C.-Y.; Huang, J.-F.; et al. Mac-2 binding protein glycosylation isomer as a hepatocellular carcinoma marker in patients with chronic hepatitis B or C infection. Hepatol. Commun. 2019, 3, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Baudi, I.; Inoue, T.; Tanaka, Y. Novel biomarkers of hepatitis B and hepatocellular carcinoma: Clinical significance of HBcrAg and M2BPGi. Int. J. Mol. Sci. 2020, 21, 949. [Google Scholar] [CrossRef] [Green Version]
- Ho, C.-H.; Tsai, H.-W.; Lee, C.-Y.; Huang, L.-J.; Chien, R.-N.; Wu, I.-C.; Chiu, Y.-C.; Liu, W.-C.; Cheng, P.-N.; Chang, T.-T.; et al. Favorable response to long-term nucleos(t)ide analogue therapy in HBeAg-positive patients with high serum fucosyl-agalactosyl IgG. Sci. Rep. 2017, 7, 1957. [Google Scholar] [CrossRef] [Green Version]
- Appelman, M.D.; Chakraborty, A.; Protzer, U.; McKeating, J.A.; Graaf, S.F.J. van de N-Glycosylation of the Na+-Taurocholate Cotransporting Polypeptide (NTCP) determines its trafficking and stability and is required for hepatitis B virus infection. PLoS ONE 2017, 12, e0170419. [Google Scholar] [CrossRef]
- Sargiacomo, C.; El-Kehdy, H.; Pourcher, G.; Stieger, B.; Najimi, M.; Sokal, E. Age-dependent glycosylation of the sodium taurocholate cotransporter polypeptide: From fetal to adult human livers. Hepatol. Commun. 2018, 2, 693–702. [Google Scholar] [CrossRef]
- Hu, Q.; Zhang, F.; Duan, L.; Wang, B.; Ye, Y.; Li, P.; Li, D.; Yang, S.; Zhou, L.; Chen, W. E-cadherin plays a role in hepatitis B virus entry through affecting glycosylated sodium-taurocholate cotransporting polypeptide distribution. Front. Cell. Infect. Microbiol. 2020, 10, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwamoto, M.; Saso, W.; Sugiyama, R.; Ishii, K.; Ohki, M.; Nagamori, S.; Suzuki, R.; Aizaki, H.; Ryo, A.; Yun, J.-H.; et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl. Acad. Sci. USA 2019, 116, 8487–8492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamatsu, S.; Shimomura, M.; Kamada, Y.; Maeda, H.; Sobajima, T.; Hikita, H.; Iijima, M.; Okamoto, Y.; Misaki, R.; Fujiyama, K.; et al. Core-fucosylation plays a pivotal role in hepatitis B pseudo virus infection: A possible implication for HBV glycotherapy. Glycobiology 2016, 26, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- Tsurimoto, T.; Fujiyama, A.; Matsubara, K. Stable expression and replication of hepatitis B virus genome in an integrated state in a human hepatoma cell line transfected with the cloned viral DNA. Proc. Natl. Acad. Sci. USA 1987, 84, 444–448. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.-S.; Lee, T.-Y.; Chou, R.-H.; Yen, C.-J.; Huang, W.-C.; Wu, C.-Y.; Yu, Y.-L. Development of a highly sensitive glycan microarray for quantifying AFP-L3 for early prediction of hepatitis B virus–related hepatocellular carcinoma. PLoS ONE 2014, 9, e99959. [Google Scholar] [CrossRef] [PubMed]
- Iijima, J.; Kobayashi, S.; Kitazume, S.; Kizuka, Y.; Fujinawa, R.; Korekane, H.; Shibata, T.; Saitoh, S.-I.; Akashi-Takamura, S.; Miyake, K.; et al. Core fucose is critical for CD14-dependent Toll-like receptor 4 signaling. Glycobiology 2017, 27, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.; Chan, K.F.; Lin, P.C.; Song, Z. The “less-is-more” in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity. mAbs 2018, 10, 693–711. [Google Scholar] [CrossRef] [PubMed]

| Glyco-Biomarkers | Glycan Changes | Relationship with HBV | Monosaccharides |
|---|---|---|---|
| M2BP-Gi |  Increase LacdiNAc | appropriate surrogative biomarkers for predicting disease progression of hepatitis B [Baudi et al., 2020] |  |
| IgG | 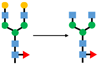 Agalactosylation | Highly associated with histological liver damage and reversed by anti-viral therapy [Ho et al., 2017] High levels of serum fucosyl-agalactosyl IgG at the treatment endpoint is favorable response to long-term nucleoside analogue therapy in patients with chronic hepatitis B. [Ho et al., 2017] | |
| Anti glycan IgG |  | Serum concentrations of anti-DSGG, anti-fucosyl GM1 and anti-Gb2 were significantly higher in patients with HCC than in chronic HBV infection individuals not in chronic HCV infection patients. [Wu et al., 2012] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ouchida, T.; Takamatsu, S.; Maeda, M.; Asuka, T.; Morita, C.; Kondo, J.; Ueda, K.; Miyoshi, E. Challenges in the Application of Glyco-Technology to Hepatitis B Virus Therapy and Diagnosis. Viruses 2021, 13, 1860. https://doi.org/10.3390/v13091860
Ouchida T, Takamatsu S, Maeda M, Asuka T, Morita C, Kondo J, Ueda K, Miyoshi E. Challenges in the Application of Glyco-Technology to Hepatitis B Virus Therapy and Diagnosis. Viruses. 2021; 13(9):1860. https://doi.org/10.3390/v13091860
Chicago/Turabian StyleOuchida, Tsunenori, Shinji Takamatsu, Megumi Maeda, Tatsuya Asuka, Chiharu Morita, Jumpei Kondo, Keiji Ueda, and Eiji Miyoshi. 2021. "Challenges in the Application of Glyco-Technology to Hepatitis B Virus Therapy and Diagnosis" Viruses 13, no. 9: 1860. https://doi.org/10.3390/v13091860







