Efficacy of Corticosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Participants
2.3. Study Design
2.4. Trial Intervention
2.5. Randomization and Blinding
2.6. Disease Evaluation
2.7. Outcomes
2.8. Statistical Analysis
2.9. Sample Size Calculation
3. Results
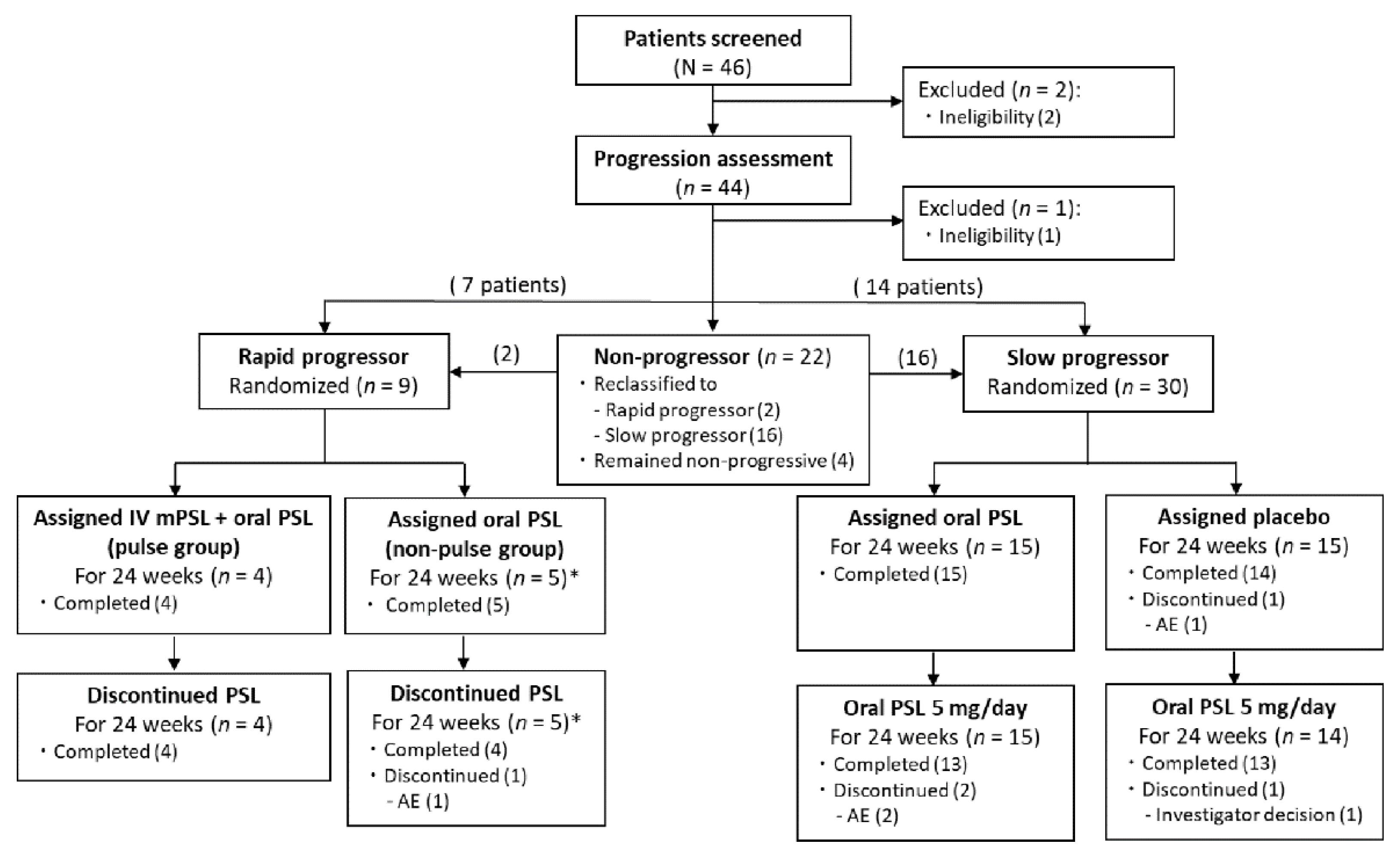
3.1. Study Population
3.2. Efficacy Analysis of Rapid Progressors
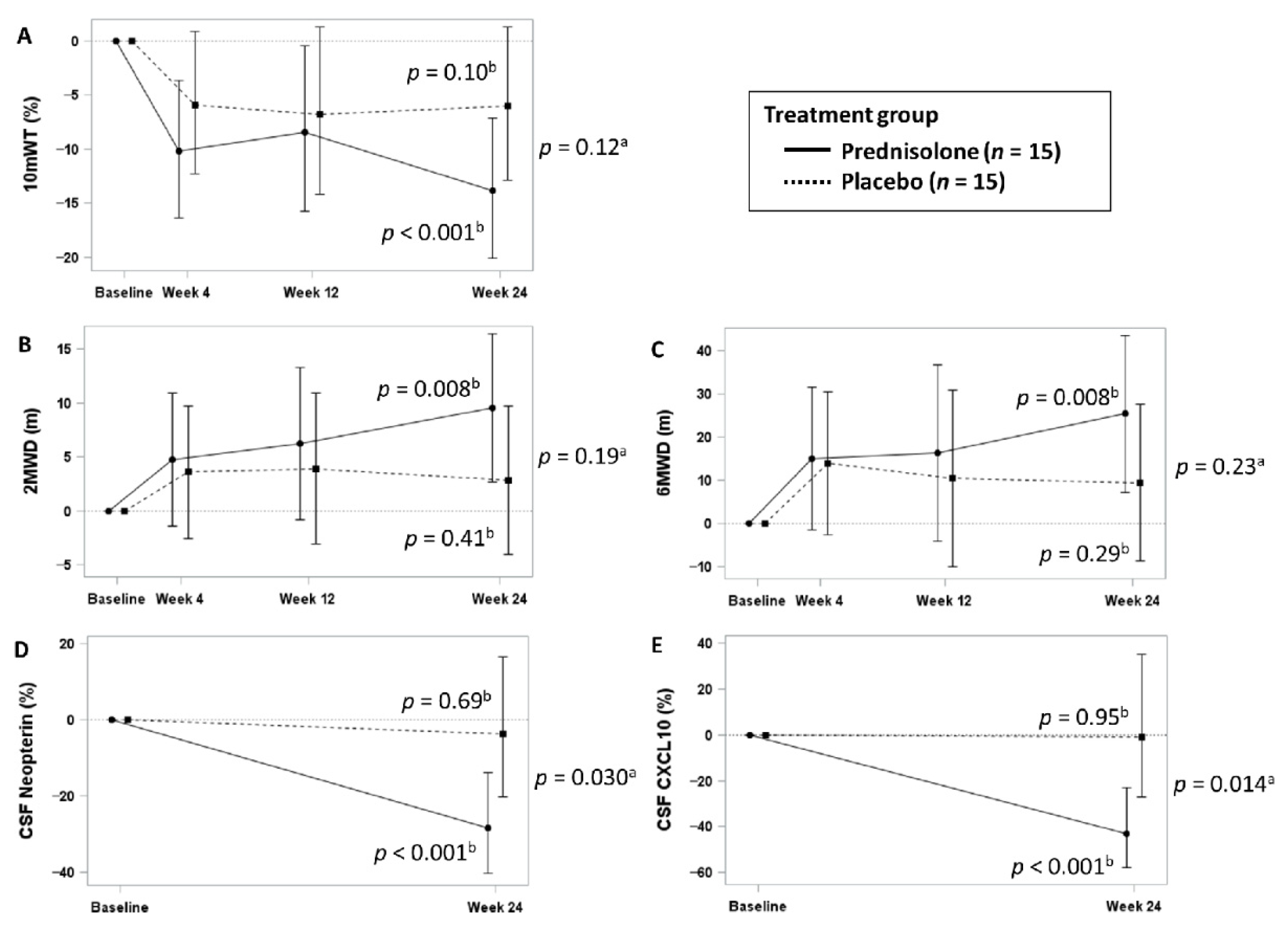
3.3. Efficacy Analysis of Slow Progressors
3.4. Safety Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [Green Version]
- Gessain, A.; Vernant, J.C.; Maurs, L.; Barin, F.; Gout, O.; Calender, A.; De Thé, G. Antibodies to human T-lymphotropic virus type-I in patients with tropical spastic paraparesis. Lancet 1985, 326, 407–410. [Google Scholar] [CrossRef]
- Osame, M.; Usuku, K.; Izumo, S.; Ijichi, N.; Amitani, H.; Igata, A.; Matsumoto, M.; Tara, M. HTLV-I associated myelopathy, a new clinical entity. Lancet 1986, 327, 1031–1032. [Google Scholar] [CrossRef]
- Bangham, C.R.M.; Araujo, A.; Yamano, Y.; Taylor, G.P. HTLV-1-associated myelopathy/tropical spastic paraparesis. Nat. Rev. Dis. Prim. 2015, 1, 15012. [Google Scholar] [CrossRef]
- Izumo, S.; Goto, I.; Itoyama, Y.; Okajima, T.; Watanabe, S.; Kuroda, Y.; Araki, S.; Mori, M.; Nagataki, S.; Matsukura, S.; et al. Interferon-alpha is effective in HTLV-I-associated myelopathy: A multicenter, randomized, double-blind, controlled trial. Neurology 1996, 46, 1016–1021. [Google Scholar] [CrossRef]
- Tsutsumi, S.; Sato, T.; Yagishita, N.; Yamauchi, J.; Araya, N.; Hasegawa, D.; Nagasaka, M.; Coler-Reilly, A.L.G.; Inoue, E.; Takata, A.; et al. Real-world clinical course of HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) in Japan. Orphanet J. Rare Dis. 2019, 14, 227. [Google Scholar] [CrossRef] [PubMed]
- Buell, K.G.; Puri, A.; Demontis, M.A.; Short, C.L.; Adonis, A.; Haddow, J.; Martin, F.; Dhasmana, D.; Taylor, G.P. Effect of pulsed methylprednisolone on pain, in patients with HTLV-1-associated myelopathy. PLoS ONE 2016, 11, e0152557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuroda, Y.; Yukitake, M.; Kurohara, K.; Takashima, H.; Matsui, M. A follow-up study on spastic paraparesis in Japanese HAM/TSP. J. Neurol. Sci. 1995, 132, 174–176. [Google Scholar] [CrossRef]
- Gotuzzo, E.; Cabrera, J.; Deza, L.; Verdonck, K.; Vandamme, A.-M.; Cairampoma, R.; Vizcarra, D.; Cabada, M.; Narvarte, G.; Casas, C.D. las Clinical Characteristics of Patients in Peru with Human T Cell Lymphotropic Virus Type 1–Associated Tropical Spastic Paraparesis. Clin. Infect. Dis. 2004, 39, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.A.; Harab, R.C.; Schor, D.; Andrada-Serpa, M.J.; Araújo, A.Q. Subacute progression of human T-lymphotropic virus type I-associated myelopathy/tropical spastic paraparesis. J. Neurovirol. 2007, 13, 468–473. [Google Scholar] [CrossRef]
- Martin, F.; Fedina, A.; Youshya, S.; Taylor, G.P. A 15-year prospective longitudinal study of disease progression in patients with HTLV-1 associated myelopathy in the UK. J. Neurol. Neurosurg. Psychiatry 2010, 81, 1336–1340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo, A.; Bangham, C.R.M.; Casseb, J.; Gotuzzo, E.; Jacobson, S.; Martin, F.; Penalva de Oliveira, A.; Puccioni-Sohler, M.; Taylor, G.P.; Yamano, Y. Management of HAM/TSP. Neurol. Clin. Pract. 2021, 11, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, M.; Nakahara, K.; Maruyama, Y.; Kawabata, M.; Higuchi, I.; Kubota, H.; Izumo, S.; Arimura, K.; Osame, M. Therapeutic trials in 200 patients with HTLV-I-associated myelopathy/ tropical spastic paraparesis. J. Neurovirol. 1996, 2, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Croda, M.G.; de Oliveira, A.C.P.; Vergara, M.P.P.; Bonasser, F.; Smid, J.; Duarte, A.J.S.; Casseb, J. Corticosteroid therapy in TSP/HAM patients: The results from a 10 years open cohort. J. Neurol. Sci. 2008, 269, 133–137. [Google Scholar] [CrossRef]
- Coler-Reilly, A.L.G.; Sato, T.; Matsuzaki, T.; Nakagawa, M.; Niino, M.; Nagai, M.; Nakamura, T.; Takenouchi, N.; Araya, N.; Yagishita, N.; et al. Effectiveness of Daily Prednisolone to Slow Progression of Human T-Lymphotropic Virus Type 1-Associated Myelopathy/Tropical Spastic Paraparesis: A Multicenter Retrospective Cohort Study. Neurotherapeutics 2017, 14, 1084–1094. [Google Scholar] [CrossRef] [Green Version]
- De Castro-Costa, C.M.; Araújo, A.Q.C.; Barreto, M.M.; Takayanagui, O.M.; Sohler, M.P.; Da Silva, E.L.M.; De Paula, S.M.B.; Ishak, R.; Ribas, J.G.R.; Rovirosa, L.C.; et al. Proposal for diagnostic criteria of tropical spastic paraparesis/HTLV-I- associated myelopathy (TSP/HAM). AIDS Res. Hum. Retrovir. 2006, 22, 931–935. [Google Scholar] [CrossRef]
- Osame, M. Review of WHO kagoshima meeting and diagnostic guidelines for HAM/TSP. In Human Retrovirology; Blattner, W.A., Ed.; Raven Press: New York, NY, USA, 1990; pp. 191–197. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef]
- Barry, M.J.; Fowler, F.J.; O’Leary, M.P.; Bruskewitz, R.C.; Holtgrewe, H.L.; Mebust, W.K.; Cockett, A.T.K. The American Urological Association Symptom Index for Benign Prostatic Hyperplasia. J. Urol. 1992, 148, 1549–1557. [Google Scholar] [CrossRef]
- Homma, Y.; Yoshida, M.; Seki, N.; Yokoyama, O.; Kakizaki, H.; Gotoh, M.; Yamanishi, T.; Yamaguchi, O.; Takeda, M.; Nishizawa, O. Symptom assessment tool for overactive bladder syndrome—overactive bladder symptom score. Urology 2006, 68, 318–323. [Google Scholar] [CrossRef]
- Avery, K.; Donovan, J.; Peters, T.J.; Shaw, C.; Gotoh, M.; Abrams, P. ICIQ: A brief and robust measure for evaluating the symptoms and impact of urinary incontinence. Neurourol. Urodyn. 2004, 23, 322–330. [Google Scholar] [CrossRef]
- Abraham, L.; Hareendran, A.; Mills, I.W.; Martin, M.L.; Abrams, P.; Drake, M.J.; MacDonagh, R.P.; Noble, J.G. Development and validation of a quality-of-life measure for men with nocturia. Urology 2004, 63, 481–486. [Google Scholar] [CrossRef]
- Martin, F.; Castro, H.; Gabriel, C.; Adonis, A.; Fedina, A.; Harrison, L.; Brodnicki, L.; Demontis, M.A.; Babiker, A.G.; Weber, J.N.; et al. Ciclosporin A proof of concept study in patients with active, progressive HTLV-1 associated myelopathy/tropical spastic paraparesis. PLoS Negl. Trop. Dis. 2012, 6, e1675. [Google Scholar] [CrossRef] [Green Version]
- Yamano, Y.; Nagai, M.; Brennan, M.; Mora, C.A.; Soldan, S.S.; Tomaru, U.; Takenouchi, N.; Izumo, S.; Osame, M.; Jacobson, S. Correlation of human T-cell lymphotropic virus type 1 (HTLV-1) mRNA with proviral DNA load, virus-specific CD8+ T cells, and disease severity in HTLV-1-associated myelopathy (HAM/TSP). Blood 2002, 99, 88–94. [Google Scholar] [CrossRef]
- Kira, J.; Fujihara, K.; Itoyama, Y.; Goto, I.; Hasuo, K. Leukoencephalopathy in HTLV-I-associated myelopathy/tropical spastic paraparesis: MRI analysis and a two year follow-up study after corticosteroid therapy. J. Neurol. Sci. 1991, 106, 41–49. [Google Scholar] [CrossRef]
- Araújo, A.Q.; Afonso, C.R.; Leite, A.C.; Dultra, S. V Intravenous methylprednisolone in HTLV-I associated myelopathy/tropical spastic paraparesis (HAM/TSP). Arq. Neuropsiquiatr. 1993, 51, 325–328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, K.; Sato, T.; Tsugawa, J.; Fujioka, S.; Yagishita, N.; Araya, N.; Yamauchi, J.; Coler-Reilly, A.L.G.; Nagasaka, M.; Hasegawa, Y.; et al. Cerebrospinal Fluid CXCL10 as a Candidate Surrogate Marker for HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. Front. Microbiol. 2019, 10, 2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, F.; Inoue, E.; Cortese, I.C.M.; de Almeida Kruschewsky, R.; Adonis, A.; Grassi, M.F.R.; Galvão-Castro, B.; Jacobson, S.; Yamano, Y.; Taylor, G.P.; et al. Timed walk as primary outcome measure of treatment response in clinical trials for HTLV-1-associated myelopathy: A feasibility study. Pilot Feasibility Stud. 2015, 1, 35. [Google Scholar] [CrossRef] [Green Version]
- Ando, H.; Sato, T.; Tomaru, U.; Yoshida, M.; Utsunomiya, A.; Yamauchi, J.; Araya, N.; Yagishita, N.; Coler-Reilly, A.; Shimizu, Y.; et al. Positive feedback loop via astrocytes causes chronic inflammation in virus-associated myelopathy. Brain 2013, 136, 2876–2887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, T.; Coler-Reilly, A.; Utsunomiya, A.; Araya, N.; Yagishita, N.; Ando, H.; Yamauchi, J.; Inoue, E.; Ueno, T.; Hasegawa, Y.; et al. CSF CXCL10, CXCL9, and Neopterin as Candidate Prognostic Biomarkers for HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. PLoS Negl. Trop. Dis. 2013, 7, e2479. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Yagishita, N.; Tamaki, K.; Inoue, E.; Hasegawa, D.; Nagasaka, M.; Suzuki, H.; Araya, N.; Coler-Reilly, A.; Hasegawa, Y.; et al. Proposal of Classification Criteria for HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis Disease Activity. Front. Microbiol. 2018, 9, 1651. [Google Scholar] [CrossRef]
- Yamauchi, J.; Sato, T.; Yagishita, N.; Araya, N.; Hasegawa, D.; Tsutsumi, S.; Nagasaka, M.; Coler-Reilly, A.; Inoue, E.; Takata, A.; et al. Use of cerebrospinal fluid CXCL10 and neopterin as biomarkers in HTLV-1-associated myelopathy/tropical spastic paraparesis treated with steroids. J. Neurol. Neurosurg. Psychiatry 2020, 91, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Harrington, W.J.; Ucar, A.; Gill, P.; Snodgrass, S.; Sheremata, W.; Cabral, L.; Rabin, M.; Byrne, G.E.; Berger, J.; Voight, W.; et al. Clinical spectrum of HTLV-I in South Florida. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 1995, 8, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef] [PubMed]
- Firouzi, S.; Farmanbar, A.; Nakai, K.; Iwanaga, M.; Uchimaru, K.; Utsunomiya, A.; Suzuki, Y.; Watanabe, T. Clonality of HTLV-1–infected T cells as a risk indicator for development and progression of adult T-cell leukemia. Blood Adv. 2017, 1, 1195–1205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kataoka, K.; Koya, J. Clinical application of genomic aberrations in adult T-cell leukemia/lymphoma. J. Clin. Exp. Hematop. 2020, 60, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, M.; Yamagishi, M.; Yagishita, N.; Araya, N.; Kobayashi, S.; Makiyama, J.; Kubokawa, M.; Yamauchi, J.; Hasegawa, D.; Coler-Reilly, A.L.G.; et al. Mortality and risk of progression to adult T cell leukemia/lymphoma in HTLV-1-associated myelopathy/tropical spastic paraparesis. Proc. Natl. Acad. Sci. USA 2020, 117, 11685–11691. [Google Scholar] [CrossRef] [PubMed]


| Characteristic | All Patients (n = 8) | Pulse (n = 4) | Non-Pulse (n = 4) | |
|---|---|---|---|---|
| Age (years) | 62.0 (55.5–65.5) | 61.0 (55.5–65.5) | 62.0 (56.0–69.5) | |
| Sex | Female | 8 (100.0%) | 4 (100.0%) | 4 (100.0%) |
| Race | Asian | 8 (100.0%) | 4 (100.0%) | 4 (100.0%) |
| Disease duration of HAM (months) | 7.0 (2.5–62.0) | 7.0 (3.5–36.0) | 32.0 (2.5–63.5) | |
| Prior corticosteroid treatment (yes) | 2 (25.0%) | 1 (25.0%) | 1 (25.0%) | |
| OMDS | 4 (needs handrail to climb stairs) | 2 (25.0%) | 1 (25.0%) | 1 (25.0%) |
| 5 (needs unilateral support to walk) | 5 (62.5%) | 2 (50.0%) | 3 (75.0%) | |
| 6 (needs bilateral support to walk) | 1 (12.5%) | 1 (25.0%) | 0 (0.0%) | |
| 10 mWT (seconds) | 14.5 (10.5–30.4) | 14.3 (10.5–18.5) | 26.4 (10.0–44.0) | |
| 2 MWD (m) | 66.9 (37.3–84.1) | 66.9 (48.4–83.2) | 56.6 (26.5–101.6) | |
| 6 MWD (m) | 187.1 (67.9–241.1) | 188.8 (125.1–241.2) | 129.9 (31.1–288.8) | |
| Timed up-and-go test (seconds) | 12.7 (10.0–18.6) | 12.7 (11.4–15.0) | 14.9 (8.9–30.9) | |
| MAS | 1 | 3 (37.5%) | 1 (25.0%) | 2 (50.0%) |
| 1+ | 2 (25.0%) | 0 (0.0%) | 2 (50.0%) | |
| 2 | 3 (37.5%) | 3 (75.0%) | 0 (0.0%) | |
| IPEC1 score | 13.0 (10.5–15.5) | 13.0 (10.5–18.0) | 13.5 (9.5–15.5) | |
| CSF neopterin concentration (pmol/mL) | 37.5 (21.0–49.0) | 32.0 (21.0–41.5) | 45.0 (24.0–57.5) | |
| CSF CXCL10 concentration (pg/mL) | 5099.2 (3608.8–5671.8) | 4979.4 (3608.8–5344.9) | 5426.1 (3297.2–6319.3) | |
| HTLV-1 proviral load in PBMCs (copies/100 PBMCs) | 6.6 (3.5–13.1) | 5.0 (3.5–10.0) | 9.78 (4.0–17.0) | |
| HTLV-1 proviral load in CSF cells (copies/100 CSF cells) | 9.7 (4.2–12.9) | 7.1 (4.2–11.6) | 10.7 (5.6–16.7) | |
| HTLV-1 proviral load in CSF (copies/mL CSF) | 538.2 (90.2–1002.7) | 288.8 (90.2–1214.1) | 754.4 (317.2–1002.7) | |
| OABSS score | 6.5 (3.5–7.5) | 5.5 (2.0–7.5) | 6.5 (4.5–8.0) | |
| IPSS score | 13.5 (5.0–24.0) | 13.5 (5.0–24.0) | 13.5 (9.0–23.5) | |
| ICIQ-SF score | 7.0 (3.5–12.0) | 8.5 (1.5–15.0) | 7.0 (5.0–9.0) | |
| N-QOL score | 77.1 (54.2–92.7) | 92.7 (74.0–99.0) | 62.5 (34.2–77.1) | |
| VAS score for global condition of HAM (mm) | 15.0 (2.5 –25.0) | 10.5 (1.0–25.0) | 15.0 (7.0–25.5) | |
| VAS score for walking (mm) | 11.0 (1.5–28.0) | 6.5 (0.0–25.5) | 17.0 (6.0–28.0) | |
| VAS score for pain (mm) | 80.0 (26.0–100.0) | 98.5 (70.0–100.0) | 66.5 (32.0–92.0) | |
| Characteristic | All Patients (n = 30) | Prednisolone (n = 15) | Placebo (n = 15) | |
|---|---|---|---|---|
| Age | 64.0 (58.0–68.0) | 65.0 (63.0–67.0) | 63.0 (55.0–69.0) | |
| Sex | Female | 23 (76.7%) | 11 (73.3%) | 12 (80.0%) |
| Race | Asian | 30 (100.0%) | 15 (100.0%) | 15 (100.0%) |
| Disease duration of HAM (months) | 31.0 (6.0–85.0) | 24.0 (4.0–81.0) | 37.0 (14.0–89.0) | |
| Prior corticosteroid treatment (yes) | 11 (36.7%) | 6 (40.0%) | 5 (33.3%) | |
| OMDS | 2 (abnormal gait, stumbling, stiffness) | 2 (6.7%) | 1 (6.7%) | 1 (6.7%) |
| 3 (unable to run) | 5 (16.7%) | 0 (0.0%) | 5 (33.3%) | |
| 4 (needs handrail to climb stairs) | 9 (30.0%) | 4 (26.7%) | 5 (33.3%) | |
| 5 (needs unilateral support to walk) | 12 (40.0%) | 8 (53.3%) | 4 (26.7%) | |
| 6 (needs bilateral support to walk) | 2 (6.7%) | 2 (13.3%) | 0 (0.0%) | |
| 10 mWT (seconds) | 9.4 (8.0–13.7) | 12.5 (8.8–19.4) | 8.1 (7.3–10.2) | |
| 2 MWD (m) | 98.8 (75.8–124.7) | 81.0 (50.0–103.5) | 120.0 (81.8–131.9) | |
| 6 MWD (m) | 278.0 (201.2–370.0) | 240.0 (130.0–298.5) | 340.0 (234.6–381.7) | |
| Timed up-and-go test (seconds) | 9.6 (7.5–12.5) | 10.6 (8.4–17.7) | 8.1 (7.1–10.7) | |
| MAS | 0 | 2 (6.7%) | 1 (6.7%) | 1 (6.7%) |
| 1 | 15 (50.0%) | 9 (60.0%) | 6 (40.0%) | |
| 1+ | 8 (26.7%) | 5 (33.3%) | 3 (20.0%) | |
| 2 | 5 (16.7%) | 0 (0.0%) | 5 (33.3%) | |
| IPEC1 (points) | 13.0 (10.0–15.0) | 15.0 (12.0–17.0) | 13.0 (10.0–13.0) | |
| CSF neopterin concentration (pmol/mL) | 12.0 (7.0–19.0) | 12.0 (6.0–24.0) | 13.0 (7.0–18.0) | |
| CSF CXCL10 concentration (pg/mL) | 2275.8 (1269.3–3536.9) | 1854.0 (1030.0–4539.5) | 2306.4 (1350.2–3192.8) | |
| HTLV-1 proviral load in PBMCs (copies/100 PBMCs) | 4.9 (3.4–7.2) | 5.3 (3.8–7.2) | 4.7 (3.0–7.2) | |
| HTLV-1 proviral load in CSF cells (copies/100 CSF cells) | 8.9 (5.9–11.3) | 8.9 (4.9–10.6) | 8.9 (6.5–11.4) | |
| HTLV-1 proviral load in CSF (copies/mL CSF) | 312.2 (178.6–518.5) | 297.0 (96.6–527.0) | 323.0 (226.6–518.5) | |
| OABSS score | 5.5 (4.0–9.0) | 5.0 (3.0–9.0) | 6.0 (4.0–11.0) | |
| IPSS score | 15.0 (9.0–27.0) | 13.0 (9.0–25.0) | 16.0 (9.0–28.0) | |
| ICIQ-SF score | 6.0 (0.0–10.0) | 6.0 (0.0–10.0) | 6.0 (0.0–12.0) | |
| N-QOL score | 67.4 (58.3–81.3) | 64.6 (45.8–83.3) | 68.8 (65.9–81.3) | |
| VAS score for global condition of HAM (mm) | 31.5 (18.0–47.0) | 23.0 (10.0–46.0) | 32.0 (20.0–48.0) | |
| VAS score for walking (mm) | 38.0 (13.0–52.0) | 27.0 (9.0–53.0) | 39.0 (27.0–52.0) | |
| VAS score for pain (mm) | 51.0 (41.0–97.0) | 45.0 (21.0–97.0) | 64.0 (50.0–100.0) | |
| Measurement | Pulse Group (n = 4) | Non-Pulse Group (n = 4) | p Value | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Improvement in OMDS (≥1 grade) or 10 mWT (≥30%) at week 2 | 4 (100.0%; 95% CI: 39.8 to 100.0) | 1 (25.0%; 95% CI: 0.6 to 80.6) | 0.14 | |
| Secondary endpoints | ||||
| Improvement in OMDS (≥1 grade) at week 2 | 4 (100.0%; 95% CI: 39.8 to 100.0) | 0 (0.0%; 95% CI: 0.0 to 60.2) | 0.029 | |
| Improvement in 10 mWT (≥30%) at week 2 | 1 (25.0%; 95% CI: 0.6–80.6) | 1 (25.0%; 95% CI: 0.6 to 80.6) | 1.00 | |
| Changes in 10 mWT (%) | Week 2 | −21.6 (−50.1to2.8) | −16.8 (−36.7 to −11.4) | 0.56 |
| Week 4 | −17.0 (−19.8 to 2.3) | −18.7 (−35.9 to −15.7) | 0.56 | |
| Week 12 | −25.8 (−32.3 to 21.5) | −14.3 (−44.5 to 8.6) | 1.00 | |
| Week 24 | −20.4 (−40.1 to 5.2) | −20.5 (−47.7 to 30.7) | 1.00 | |
| Changes in 2 MWD (%) | Week 2 | 23.3 (2.7 to 84.7) | 11.1 (−15.7 to 38.0) | 0.39 |
| Week 4 | 21.8 (9.8 to 35.3) | 18.5 (−17.4 to 38.7) | 0.77 | |
| Week 12 | 41.8 (0.4 to 59.5) | 16.4 (−36.1 to 43.3) | 0.25 | |
| Week 24 | 37.5 (6.0 to 85.0) | 20.0 (−27.0 to 63.3) | 0.39 | |
| Changes in 6 MWD (%) | Week 2 | 32.8 (−4.1 to 94.2) | 30.5 (5.7 to 58.1) | 1.00 |
| Week 4 | 26.4 (7.4 to 60.3) | 30.0 (11.4 to 38.7) | 1.00 | |
| Week 12 | 49.4 (−8.5 to 64.4) | 20.6 (17.4 to 43.3) | 0.39 | |
| Week 24 | 51.8 (3.7 to 91.9) | 21.4 (−33.5 to 63.3) | 0.39 | |
| Changes in CSF neopterin concentrations (%) | Week 2 | −60.3 (−65.1 to −50.0) | −58.2 (−62.9 to −30.8) | 0.56 |
| Week 24 | −30.4 (−41.9 to −22.2) | −49.5 (−68.6 to 0.0) | 0.56 | |
| Changes in CSF CXCL10 concentrations (%) | Week 2 | −74.2 (−77.9 to −52.0) | −73.4 (−88.7 to −59.9) | 0.56 |
| Week 24 | –32.2 (−37.8 to −7.0) | −30.4 (−83.7 to −20.6) | 0.56 | |
| Patients who received intravenous methylprednisolone therapy between week 4 and 24 | 0 (0.0%; 95% CI: 0.0 to 60.2) | 1 (25.0%; 95% CI: 0.6 to 80.6) | 1.00 | |
| Patients in whom the 10 mWT worsened by ≥ 100% compared with week 4 | 0 (0.0%; 95% CI: 0.0 to 60.2) | 0 (0.0%; 95% CI: 0.0 to 60.2) | 1.00 | |
| Patients who could not stop treatment with prednisolone at week 26 | 0 (0.0%; 95% CI: 0.0 to 60.2) | 2 (50.0%; 95% CI: 6.8 to 93.2) | 0.43 | |
| Patients who resumed prednisolone treatment after week 26 | 3/4 (75.0%; 95% CI: 19.4 to 99.4) | 2/2 (100.0%; 95% CI: 15.8 to 100.0) | 1.00 | |
| Measurement | Prednisolone Group (n = 15) | Placebo Group (n = 15) | p Value a | |
|---|---|---|---|---|
| Primary endpoint | ||||
| Changes in 10 mWT (%) | Week 4 | −10.2 (−16.3 to −3.6) | −6.0 (−12.3 to 0.8) | |
| Week 12 | −8.4 (−15.8 to −0.5) | −6.8 (−14.2 to 1.3) | ||
| Week 24 | −13.8 (−20.1 to −7.1) | −6.0 (−12.8 to 1.3) | 0.12 | |
| p value b | <0.001 | 0.10 | ||
| Secondary endpoints | ||||
| Changes in 2 MWD (m) | Week 4 | 4.8 (−1.4 to 11.0) | 3.6 (−2.5 to 9.8) | |
| Week 12 | 6.2 (−0.8 to 13.3) | 3.9 (−3.1 to 10.9) | ||
| Week 24 | 9.6 (2.7 to 16.4) | 2.8 (−4.0 to 9.7) | 0.19 | |
| p value b | 0.008 | 0.41 | ||
| Changes in 6 MWD (m) | Week 4 | 15.0 (−1.6 to 31.6) | 14.0 (−2.5 to 30.5) | |
| Week 12 | 16.3 (−4.1 to 36.7) | 10.5 (−9.8 to 30.8) | ||
| Week 24 | 25.3 (7.3 to 43.4) | 9.5 (−8.6 to 27.5) | 0.23 | |
| p value b | 0.008 | 0.29 | ||
| Changes in CSF neopterin concentrations (%) | Week 24 | −28.3 (−40.2 to −13.8) | −3.6 (−20.3 to 16.5) | 0.030 |
| p value b | <0.001 | 0.69 | ||
| Changes in CSF CXCL10 concentrations (%) | Week 24 | −43.0 (−57.7 to −23.1) | −0.9 (−27.3 to 35.0) | 0.014 |
| p value b | <0.001 | 0.95 | ||
| Rapid Progressors | Slow Progressors | ||||
|---|---|---|---|---|---|
| Event | Pulse (n = 4) | Non-Pulse (n = 5) | Prednisolone (n = 15) | Placebo (n = 15) | |
| Week 0 to 24 | Any AEs a | 4 (25) | 5 (25) | 14 (38) | 13 (19) |
| AEs related to trial regimen b | 4 (18) | 5 (13) | 11 (23) | 3 (3) | |
| Serious AEs | 0 | 0 | 0 | 0 | |
| Discontinuation due to AEs c | 0 | 0 | 0 | 1 (1) | |
| Week 25 to 48 | Any AEs d | 4 (9) | 5 (22) | 12 (36) | 9 (23) |
| AEs related to trial regimen e | 3 (4) | 2 (3) | 8 (13) | 5 (6) | |
| Serious AEs f | 0 | 1 (1) | 2 (2) | 1 (1) | |
| Discontinuation due to AEs f | 0 | 1 (1) | 1 (1) | 1 (1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamauchi, J.; Tanabe, K.; Sato, T.; Nakagawa, M.; Matsuura, E.; Tsuboi, Y.; Tamaki, K.; Sakima, H.; Ishihara, S.; Ohta, Y.; et al. Efficacy of Corticosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P). Viruses 2022, 14, 136. https://doi.org/10.3390/v14010136
Yamauchi J, Tanabe K, Sato T, Nakagawa M, Matsuura E, Tsuboi Y, Tamaki K, Sakima H, Ishihara S, Ohta Y, et al. Efficacy of Corticosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P). Viruses. 2022; 14(1):136. https://doi.org/10.3390/v14010136
Chicago/Turabian StyleYamauchi, Junji, Kenichiro Tanabe, Tomoo Sato, Masanori Nakagawa, Eiji Matsuura, Yoshio Tsuboi, Keiko Tamaki, Hirokuni Sakima, Satoshi Ishihara, Yuki Ohta, and et al. 2022. "Efficacy of Corticosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P)" Viruses 14, no. 1: 136. https://doi.org/10.3390/v14010136
APA StyleYamauchi, J., Tanabe, K., Sato, T., Nakagawa, M., Matsuura, E., Tsuboi, Y., Tamaki, K., Sakima, H., Ishihara, S., Ohta, Y., Matsumoto, N., Kono, K., Yagishita, N., Araya, N., Takahashi, K., Kunitomo, Y., Nagasaka, M., Coler-Reilly, A., Hasegawa, Y., ... Yamano, Y. (2022). Efficacy of Corticosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P). Viruses, 14(1), 136. https://doi.org/10.3390/v14010136









