Neuroinflammatory Profiling in SIV-Infected Chinese-Origin Rhesus Macaques on Antiretroviral Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and SIV Infection
2.2. Antiretroviral Therapy
2.3. Animal Euthanasia and Brain Tissue Collection
2.4. Cytokine/Chemokine Measurements in Plasma and CSF
2.5. PCR Array for Evaluation of Inflammatory Cytokine and Chemokine Gene Expression
2.6. Gene Expression and Pathway Analysis
2.7. Statistical Analysis
3. Results
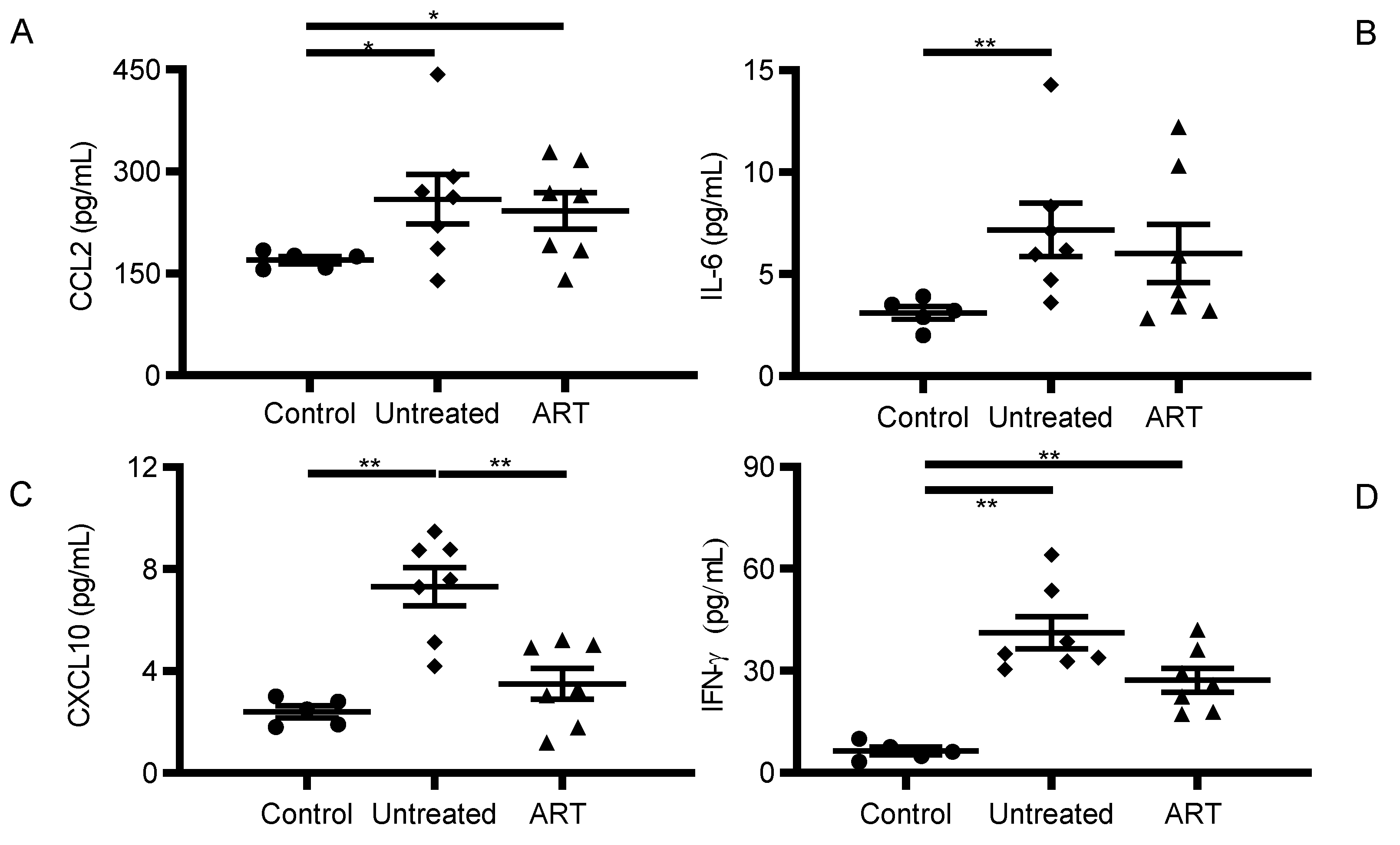
3.1. CCL2 and IFN-γ Levels Were Significantly Elevated in Peripheral Blood of SIV-Infected chRMs Receiving Suppressive ART
3.2. ART Reduced Inflammatory Cytokine and Chemokine Levels in CSF of SIV-Infected chRMs
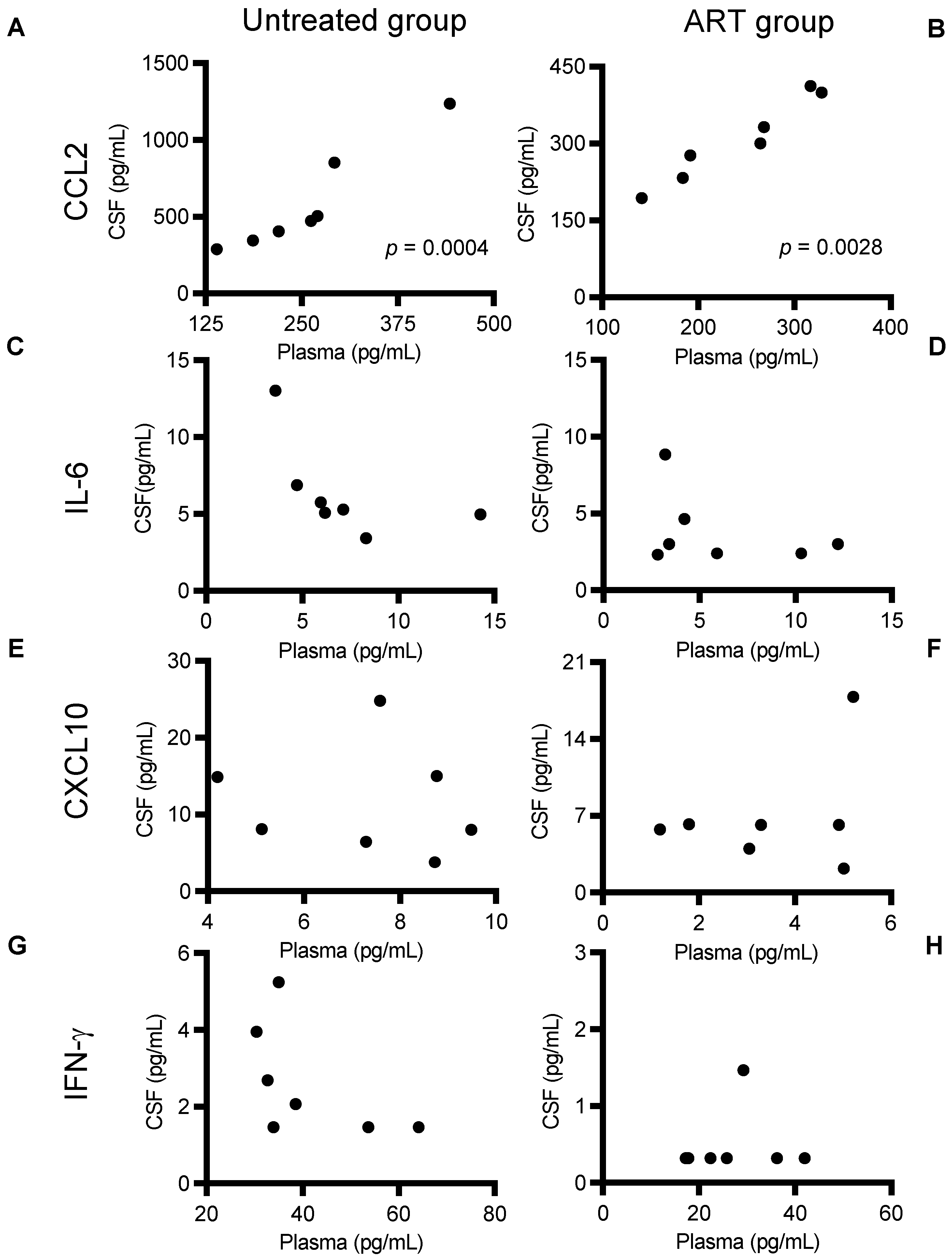
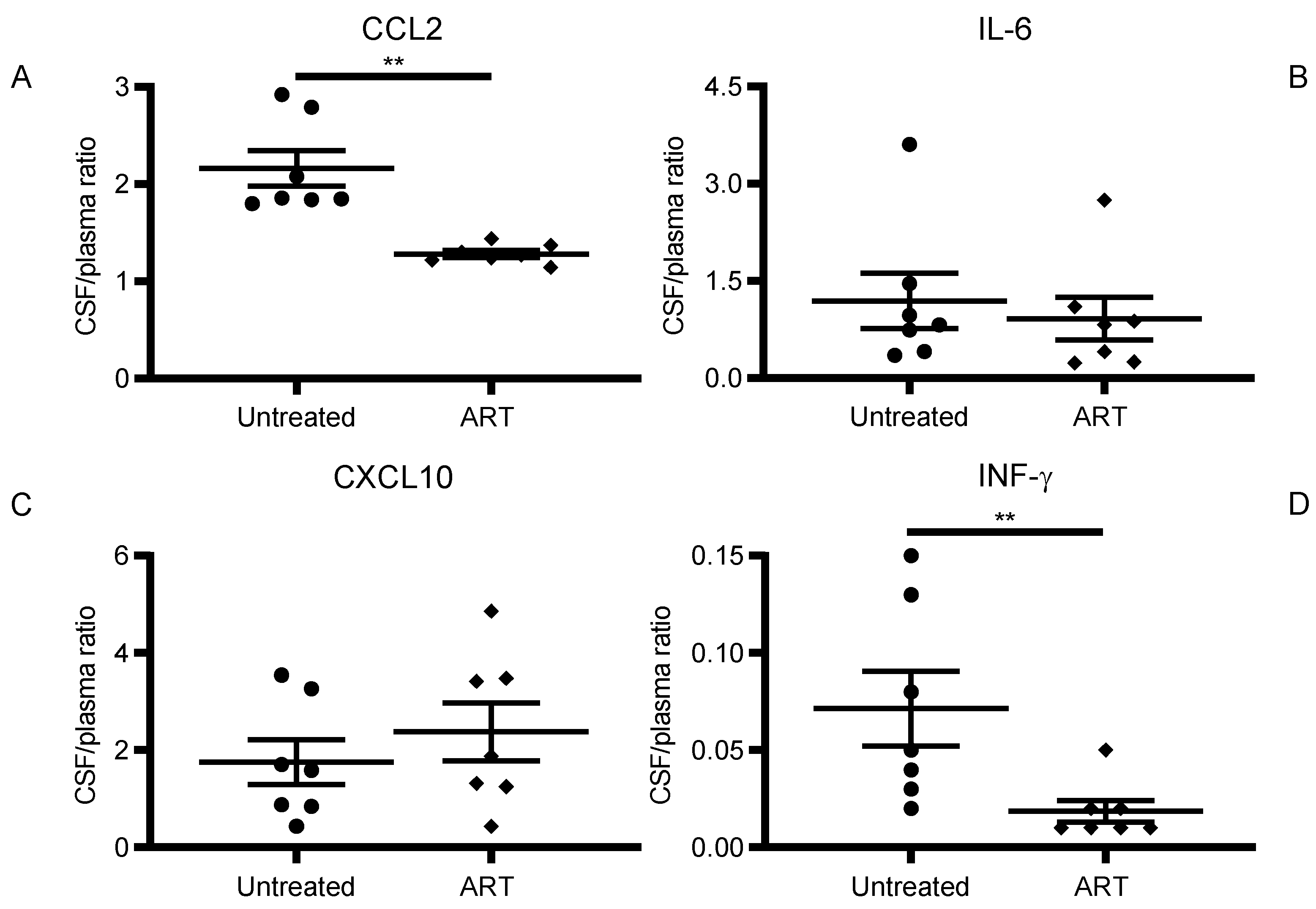
3.3. CCL2 Linear Correlation between CSF and Plasma Levels
3.4. Expression of Proinflammatory Cytokine and Chemokine Genes Remained Elevated in Basal Ganglia despite Long-Term Suppressive ART
3.5. Expression of Proinflammatory IL-11RA, IL-16, IL-6R, and IL-9 Remained Elevated in the Basal Ganglia despite Receiving Long-Term Suppressive ART
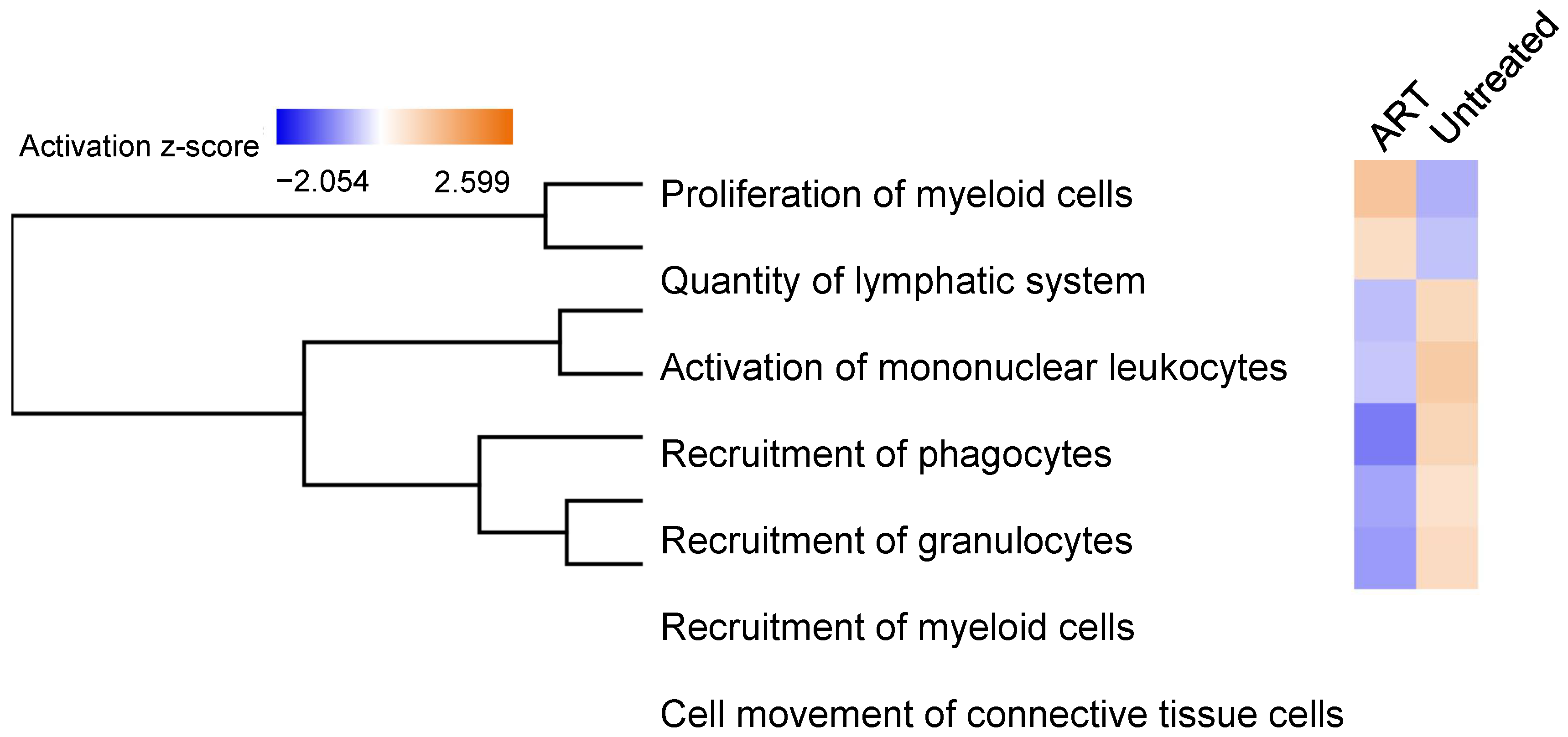
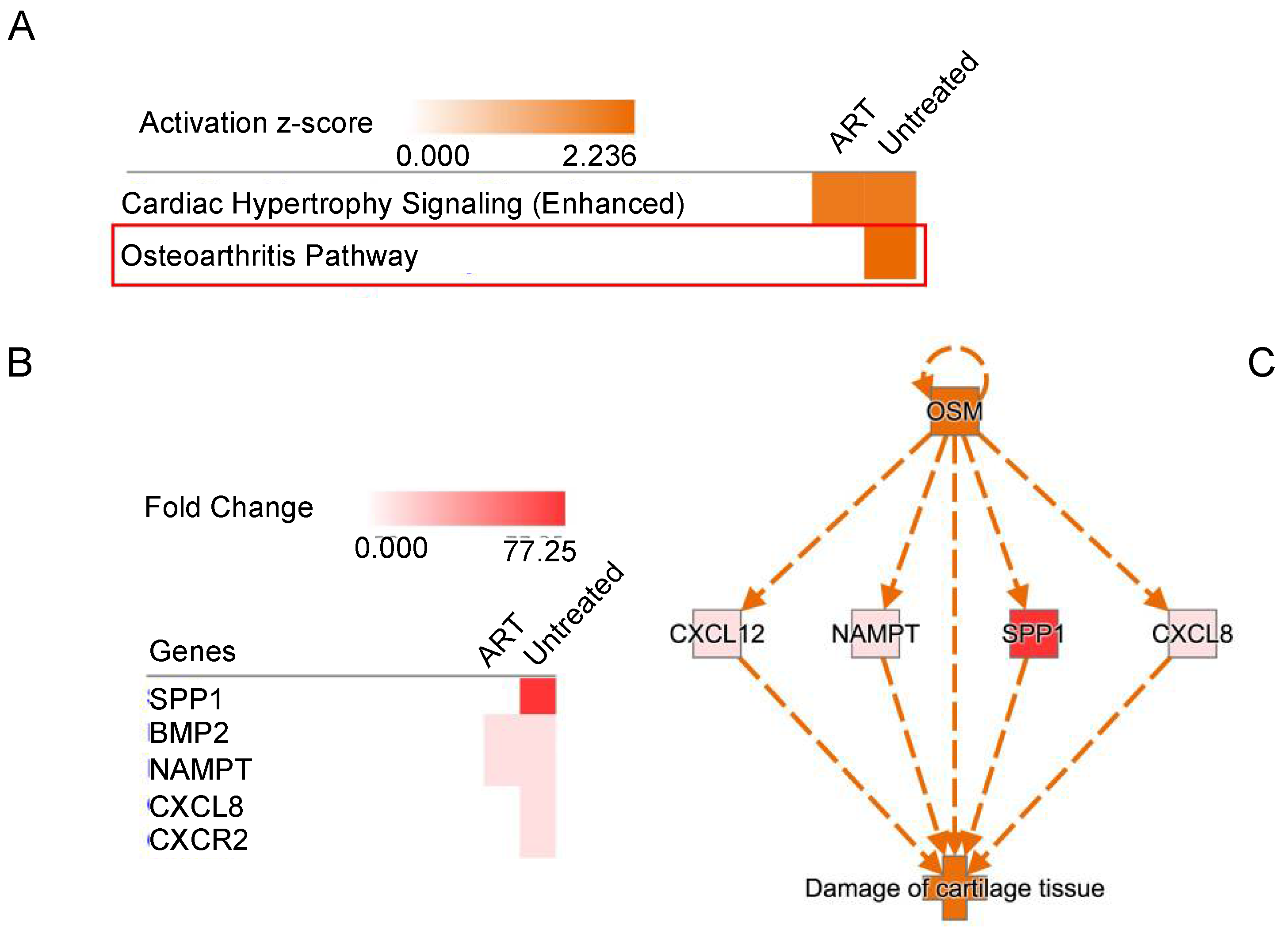
3.6. Functional Analysis of Differentially Expressed Genes in SIV+ Untreated and ART Animals
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Clifford, D.B.; Ances, B.M. HIV-associated neurocognitive disorder. Lancet Infect. Dis. 2013, 13, 976–986. [Google Scholar] [CrossRef] [Green Version]
- Marban, C.; Forouzanfar, F.; Ait-Ammar, A.; Fahmi, F.; El Mekdad, H.; Daouad, F.; Rohr, O.; Schwartz, C. Targeting the Brain Reservoirs: Toward an HIV Cure. Front. Immunol. 2016, 7, 397. [Google Scholar] [CrossRef] [Green Version]
- Hatano, H. Immune activation and HIV persistence: Considerations for novel therapeutic interventions. Curr. Opin. HIV AIDS 2013, 8, 211–216. [Google Scholar] [CrossRef] [Green Version]
- Wiley, C.A.; Soontornniyomkij, V.; Radhakrishnan, L.; Masliah, E.; Mellors, J.; Hermann, S.A.; Dailey, P.; Achim, C.L. Distribution of brain HIV load in AIDS. Brain Pathol. 1998, 8, 277–284. [Google Scholar] [CrossRef]
- Nath, A. Eradication of human immunodeficiency virus from brain reservoirs. J. Neurovirol. 2015, 21, 227–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez, S.; Johnson, A.M.; Xiang, S.H.; Li, J.; Foley, B.T.; Doyle-Meyers, L.; Panganiban, A.; Kaur, A.; Veazey, R.S.; Wu, Y.; et al. Persistence of SIV in the brain of SIV-infected Chinese rhesus macaques with or without antiretroviral therapy. J. Neurovirol. 2018, 24, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Steinbrink, F.; Evers, S.; Buerke, B.; Young, P.; Arendt, G.; Koutsilieri, E.; Reichelt, D.; Lohmann, H.; Husstedt, I.W.; German Competence Network, H.A. Cognitive impairment in HIV infection is associated with MRI and CSF pattern of neurodegeneration. Eur. J. Neurol. 2013, 20, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Nosik, M.; Lavrov, V.; Svitich, O. HIV Infection and Related Mental Disorders. Brain Sci. 2021, 11, 248. [Google Scholar] [CrossRef] [PubMed]
- Maki, P.M.; Cohen, M.H.; Weber, K.; Little, D.M.; Fornelli, D.; Rubin, L.H.; Perschler, P.; Gould, F.; Martin, E. Impairments in memory and hippocampal function in HIV-positive vs HIV-negative women: A preliminary study. Neurology 2009, 72, 1661–1668. [Google Scholar] [CrossRef] [Green Version]
- Abreu, C.; Shirk, E.N.; Queen, S.E.; Beck, S.E.; Mangus, L.M.; Pate, K.A.M.; Mankowski, J.L.; Gama, L.; Clements, J.E. Brain macrophages harbor latent, infectious simian immunodeficiency virus. AIDS 2019, 33 (Suppl. 2), S181–S188. [Google Scholar] [CrossRef]
- Wallet, C.; De Rovere, M.; Van Assche, J.; Daouad, F.; De Wit, S.; Gautier, V.; Mallon, P.W.G.; Marcello, A.; Van Lint, C.; Rohr, O.; et al. Microglial Cells: The Main HIV-1 Reservoir in the Brain. Front. Cell Infect. Microbiol. 2019, 9, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertrand, L.; Cho, H.J.; Toborek, M. Blood-brain barrier pericytes as a target for HIV-1 infection. Brain 2019, 142, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Henderson, L.; Nath, A. Astrocytes as an HIV Reservoir: Mechanism of HIV Infection. Curr. HIV Res. 2016, 14, 373–381. [Google Scholar] [CrossRef]
- Valdebenito, S.; Castellano, P.; Ajasin, D.; Eugenin, E.A. Astrocytes are HIV reservoirs in the brain: A cell type with poor HIV infectivity and replication but efficient cell-to-cell viral transfer. J. Neurochem. 2021, 158, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Dahl, V.; Peterson, J.; Fuchs, D.; Gisslen, M.; Palmer, S.; Price, R.W. Low levels of HIV-1 RNA detected in the cerebrospinal fluid after up to 10 years of suppressive therapy are associated with local immune activation. AIDS 2014, 28, 2251–2258. [Google Scholar] [CrossRef] [Green Version]
- Eden, A.; Price, R.W.; Spudich, S.; Fuchs, D.; Hagberg, L.; Gisslen, M. Immune activation of the central nervous system is still present after >4 years of effective highly active antiretroviral therapy. J. Infect. Dis. 2007, 196, 1779–1783. [Google Scholar] [CrossRef] [Green Version]
- Spudich, S.S. Immune activation in the central nervous system throughout the course of HIV infection. Curr. Opin. HIV AIDS 2016, 11, 226–233. [Google Scholar] [CrossRef] [Green Version]
- Kuehne, L.K.; Reiber, H.; Bechter, K.; Hagberg, L.; Fuchs, D. Cerebrospinal fluid neopterin is brain-derived and not associated with blood-CSF barrier dysfunction in non-inflammatory affective and schizophrenic spectrum disorders. J. Psychiatr. Res. 2013, 47, 1417–1422. [Google Scholar] [CrossRef]
- Rao, K.S.; Ghorpade, A.; Labhasetwar, V. Targeting anti-HIV drugs to the CNS. Expert Opin. Drug Deliv. 2009, 6, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Ene, L.; Duiculescu, D.; Ruta, S.M. How much do antiretroviral drugs penetrate into the central nervous system? J. Med. Life 2011, 4, 432–439. [Google Scholar]
- Bertrand, L.; Velichkovska, M.; Toborek, M. Cerebral Vascular Toxicity of Antiretroviral Therapy. J. Neuroimmune Pharmacol. 2021, 16, 74–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clements, J.E.; Gama, L.; Graham, D.R.; Mankowski, J.L.; Zink, M.C. A simian immunodeficiency virus macaque model of highly active antiretroviral treatment: Viral latency in the periphery and the central nervous system. Curr. Opin. HIV AIDS 2011, 6, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, L.; Bhaskar, V.; Pauley, D.R.; Knight, H.; Williams, K.; Lentz, M.; Ratai, E.; Westmoreland, S.V.; Gonzalez, R.G.; O’Neil, S.P. Impact of short-term combined antiretroviral therapy on brain virus burden in simian immunodeficiency virus-infected and CD8+ lymphocyte-depleted rhesus macaques. Am. J. Pathol. 2010, 177, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Spudich, S.; Gisslen, M.; Hagberg, L.; Lee, E.; Liegler, T.; Brew, B.; Fuchs, D.; Tambussi, G.; Cinque, P.; Hecht, F.M.; et al. Central nervous system immune activation characterizes primary human immunodeficiency virus 1 infection even in participants with minimal cerebrospinal fluid viral burden. J. Infect. Dis. 2011, 204, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Conductier, G.; Blondeau, N.; Guyon, A.; Nahon, J.L.; Rovere, C. The role of monocyte chemoattractant protein MCP1/CCL2 in neuroinflammatory diseases. J. Neuroimmunol. 2010, 224, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Zink, M.C.; Coleman, G.D.; Mankowski, J.L.; Adams, R.J.; Tarwater, P.M.; Fox, K.; Clements, J.E. Increased macrophage chemoattractant protein-1 in cerebrospinal fluid precedes and predicts simian immunodeficiency virus encephalitis. J. Infect. Dis. 2001, 184, 1015–1021. [Google Scholar] [CrossRef] [Green Version]
- Bissel, S.J.; Gurnsey, K.; Jedema, H.P.; Smith, N.F.; Wang, G.; Bradberry, C.W.; Wiley, C.A. Aged Chinese-origin rhesus macaques infected with SIV develop marked viremia in absence of clinical disease, inflammation or cognitive impairment. Retrovirology 2018, 15, 17. [Google Scholar] [CrossRef] [Green Version]
- Crowe, S. SIVmac pathogenesis in rhesus macaques of Chinese and Indian origin compared with primary HIV infections in humans, by Stellbrink et al. AIDS 2003, 17 (Suppl. 4), S107–S108. [Google Scholar]
- Lahiri, C.D.; Reed-Walker, K.; Sheth, A.N.; Acosta, E.P.; Vunnava, A.; Ofotokun, I. Cerebrospinal fluid concentrations of tenofovir and emtricitabine in the setting of HIV-1 protease inhibitor-based regimens. J. Clin. Pharmacol. 2016, 56, 492–496. [Google Scholar] [CrossRef] [Green Version]
- Kramer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef]
- Kamat, A.; Misra, V.; Cassol, E.; Ancuta, P.; Yan, Z.; Li, C.; Morgello, S.; Gabuzda, D. A plasma biomarker signature of immune activation in HIV patients on antiretroviral therapy. PLoS ONE 2012, 7, e30881. [Google Scholar] [CrossRef] [Green Version]
- Reinhart, T.A.; Qin, S.; Sui, Y. Multiple roles for chemokines in the pathogenesis of SIV infection. Curr. HIV Res. 2009, 7, 73–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, P.P.; Fu, Y.; Masliah, E.; Lefebvre, C.; Repunte-Canonigo, V. Central nervous system (CNS) transcriptomic correlates of human immunodeficiency virus (HIV) brain RNA load in HIV-infected individuals. Sci. Rep. 2021, 11, 12176. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.H.; Cho, J.G.; Yoon, K.J.; Lim, D.S.; Kim, C.H.; Lee, S.W.; Park, S.G. The antibody atliximab attenuates collagen-induced arthritis by neutralizing AIMP1, an inflammatory cytokine that enhances osteoclastogenesis. Biomaterials 2015, 44, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.P.; Sheikh, F.; Kotenko, S.V.; Dickensheets, H. The expanded family of class II cytokines that share the IL-10 receptor-2 (IL-10R2) chain. J. Leukoc. Biol. 2004, 76, 314–321. [Google Scholar] [CrossRef]
- Acuner-Ozbabacan, E.S.; Engin, B.H.; Guven-Maiorov, E.; Kuzu, G.; Muratcioglu, S.; Baspinar, A.; Chen, Z.; Van Waes, C.; Gursoy, A.; Keskin, O.; et al. The structural network of Interleukin-10 and its implications in inflammation and cancer. BMC Genom. 2014, 15 (Suppl. 4), S2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starkey, M.R.; Nguyen, D.H.; Essilfie, A.T.; Kim, R.Y.; Hatchwell, L.M.; Collison, A.M.; Yagita, H.; Foster, P.S.; Horvat, J.C.; Mattes, J.; et al. Tumor necrosis factor-related apoptosis-inducing ligand translates neonatal respiratory infection into chronic lung disease. Mucosal Immunol. 2014, 7, 478–488. [Google Scholar] [CrossRef]
- Marcondes, M.C.; Ojakian, R.; Bortell, N.; Flynn, C.; Conti, B.; Fox, H.S. Osteopontin expression in the brain triggers localized inflammation and cell death when immune cells are activated by pertussis toxin. Mediat. Inflamm. 2014, 2014, 358218. [Google Scholar] [CrossRef] [Green Version]
- Marcondes, M.C.; Lanigan, C.M.; Burdo, T.H.; Watry, D.D.; Fox, H.S. Increased expression of monocyte CD44v6 correlates with the deveopment of encephalitis in rhesus macaques infected with simian immunodeficiency virus. J. Infect. Dis. 2008, 197, 1567–1576. [Google Scholar] [CrossRef] [Green Version]
- Vera, J.H.; Guo, Q.; Cole, J.H.; Boasso, A.; Greathead, L.; Kelleher, P.; Rabiner, E.A.; Kalk, N.; Bishop, C.; Gunn, R.N.; et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology 2016, 86, 1425–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Ma, S.S.; Zhang, W.Y.; Duan, J.P. Changes in peripheral blood inflammatory factors (TNF-alpha and IL-6) and intestinal flora in AIDS and HIV-positive individuals. J. Zhejiang Univ. Sci. B 2019, 20, 793–802. [Google Scholar] [CrossRef]
- Covino, D.A.; Sabbatucci, M.; Fantuzzi, L. The CCL2/CCR2 Axis in the Pathogenesis of HIV-1 Infection: A New Cellular Target for Therapy? Curr. Drug Targets 2016, 17, 76–110. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.H.; O’Connor, J.L.; Phillips, A.N.; Ronsholt, F.F.; Pett, S.; Vjecha, M.J.; French, M.A.; Lundgren, J.D.; Insight, S.; Groups, E.S.; et al. Factors Associated With Plasma IL-6 Levels During HIV Infection. J. Infect. Dis. 2015, 212, 585–595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef]
- Valverde-Villegas, J.M.; de Medeiros, R.M.; Ellwanger, J.H.; Santos, B.R.; Melo, M.G.; Almeida, S.E.M.; Chies, J.A.B. High CXCL10/IP-10 levels are a hallmark in the clinical evolution of the HIV infection. Infect. Genet. Evol. 2018, 57, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Popko, B.; Corbin, J.G.; Baerwald, K.D.; Dupree, J.; Garcia, A.M. The effects of interferon-gamma on the central nervous system. Mol. Neurobiol. 1997, 14, 19–35. [Google Scholar] [CrossRef]
- Allers, K.; Fehr, M.; Conrad, K.; Epple, H.J.; Schurmann, D.; Geelhaar-Karsch, A.; Schinnerling, K.; Moos, V.; Schneider, T. Macrophages accumulate in the gut mucosa of untreated HIV-infected patients. J. Infect. Dis. 2014, 209, 739–748. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.C.; Dann, S.M.; Okhuysen, P.C.; Lewis, D.E.; Chappell, C.L.; Adler, D.G.; White, A.C., Jr. High levels of CXCL10 are produced by intestinal epithelial cells in AIDS patients with active cryptosporidiosis but not after reconstitution of immunity. Infect. Immun. 2007, 75, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.; Meyers, C.A.; Yan, N.; Agarwal, S.; Levi, B.; James, A.W. BMP-2-induced bone formation and neural inflammation. J. Orthop. 2017, 14, 252–256. [Google Scholar] [CrossRef]
- Wu, D.H.; Hatzopoulos, A.K. Bone morphogenetic protein signaling in inflammation. Exp. Biol. Med. 2019, 244, 147–156. [Google Scholar] [CrossRef]
- Li, M.; Ransohoff, R.M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol. 2008, 84, 116–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, S.C.; Nair, A.; Marpadga, R.; Chubinskaya, S.; Doherty, M.; Valdes, A.M.; Scanzello, C.R. IL-15 and IL15RA in Osteoarthritis: Association With Symptoms and Protease Production, but Not Structural Severity. Front. Immunol. 2020, 11, 1385. [Google Scholar] [CrossRef]
- Audrito, V.; Messana, V.G.; Deaglio, S. NAMPT and NAPRT: Two Metabolic Enzymes With Key Roles in Inflammation. Front. Oncol. 2020, 10, 358. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.; Xu, D.; Austin, C.D.; Caplazi, P.; Senger, K.; Sun, Y.; Jeet, S.; Young, J.; Delarosa, D.; Suto, E.; et al. Function of CSF1 and IL34 in Macrophage Homeostasis, Inflammation, and Cancer. Front. Immunol. 2019, 10, 2019. [Google Scholar] [CrossRef]
- Nakamichi, Y.; Udagawa, N.; Takahashi, N. IL-34 and CSF-1: Similarities and differences. J. Bone Miner. Metab. 2013, 31, 486–495. [Google Scholar] [CrossRef]
- Knight, A.C.; Brill, S.A.; Queen, S.E.; Tarwater, P.M.; Mankowski, J.L. Increased Microglial CSF1R Expression in the SIV/Macaque Model of HIV CNS Disease. J. Neuropathol. Exp. Neurol. 2018, 77, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Imai, T.; Yasuda, N. Therapeutic intervention of inflammatory/immune diseases by inhibition of the fractalkine (CX3CL1)-CX3CR1 pathway. Inflamm. Regen. 2016, 36, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, J.K.; Jiang, Y.; Chen, S.; Xia, Y.; Maciejewski, D.; McNamara, R.K.; Streit, W.J.; Salafranca, M.N.; Adhikari, S.; Thompson, D.A.; et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. USA 1998, 95, 10896–10901. [Google Scholar] [CrossRef] [Green Version]
- Cardona, A.E.; Pioro, E.P.; Sasse, M.E.; Kostenko, V.; Cardona, S.M.; Dijkstra, I.M.; Huang, D.; Kidd, G.; Dombrowski, S.; Dutta, R.; et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006, 9, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Duan, M.; Yao, H.; Cai, Y.; Liao, K.; Seth, P.; Buch, S. HIV-1 Tat disrupts CX3CL1-CX3CR1 axis in microglia via the NF-kappaBYY1 pathway. Curr. HIV Res. 2014, 12, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Irons, D.L.; Meinhardt, T.; Allers, C.; Kuroda, M.J.; Kim, W.K. Overexpression and activation of colony-stimulating factor 1 receptor in the SIV/macaque model of HIV infection and neuroHIV. Brain Pathol. 2019, 29, 826–836. [Google Scholar] [CrossRef]
- Knight, A.C.; Brill, S.A.; Solis, C.V.; Richardson, M.R.; McCarron, M.E.; Queen, S.E.; Bailey, C.C.; Mankowski, J.L. Differential regulation of TREM2 and CSF1R in CNS macrophages in an SIV/macaque model of HIV CNS disease. J. Neurovirol. 2020, 26, 511–519. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, J.; Che, Y.Q. CXCL8 gene silencing promotes neuroglial cells activation while inhibiting neuroinflammation through the PI3K/Akt/NF-kappaB-signaling pathway in mice with ischemic stroke. J. Cell Physiol. 2019, 234, 7341–7355. [Google Scholar] [CrossRef]
- Haarmann, A.; Schuhmann, M.K.; Silwedel, C.; Monoranu, C.M.; Stoll, G.; Buttmann, M. Human Brain Endothelial CXCR2 is Inflammation-Inducible and Mediates CXCL5- and CXCL8-Triggered Paraendothelial Barrier Breakdown. Int. J. Mol. Sci. 2019, 20, 602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, J.I.; Bell, J.S.; DeLuca, G.C. Vascular pathology in multiple sclerosis: Reframing pathogenesis around the blood-brain barrier. J. Neurol. Neurosurg. Psychiatry 2018, 89, 42–52. [Google Scholar] [CrossRef]
- Weiss, N.; Miller, F.; Cazaubon, S.; Couraud, P.O. The blood-brain barrier in brain homeostasis and neurological diseases. Biochim. Biophys. Acta 2009, 1788, 842–857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurfein, B.T.; Zhang, Y.; Lopez, C.B.; Argaw, A.T.; Zameer, A.; Moran, T.M.; John, G.R. IL-11 regulates autoimmune demyelination. J. Immunol. 2009, 183, 4229–4240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruocco, G.; Rossi, S.; Motta, C.; Macchiarulo, G.; Barbieri, F.; De Bardi, M.; Borsellino, G.; Finardi, A.; Grasso, M.G.; Ruggieri, S.; et al. T helper 9 cells induced by plasmacytoid dendritic cells regulate interleukin-17 in multiple sclerosis. Clin. Sci. 2015, 129, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Donninelli, G.; Saraf-Sinik, I.; Mazziotti, V.; Capone, A.; Grasso, M.G.; Battistini, L.; Reynolds, R.; Magliozzi, R.; Volpe, E. Interleukin-9 regulates macrophage activation in the progressive multiple sclerosis brain. J. Neuroinflamm. 2020, 17, 149. [Google Scholar] [CrossRef]
- Mathy, N.L.; Scheuer, W.; Lanzendorfer, M.; Honold, K.; Ambrosius, D.; Norley, S.; Kurth, R. Interleukin-16 stimulates the expression and production of pro-inflammatory cytokines by human monocytes. Immunology 2000, 100, 63–69. [Google Scholar] [CrossRef]
- Scheller, J.; Chalaris, A.; Schmidt-Arras, D.; Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 2011, 1813, 878–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Liao, W.J.; Liao, J.M.; Liao, P.; Lu, H. Ribosomal proteins: Functions beyond the ribosome. J. Mol. Cell Biol. 2015, 7, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.H.; Benveniste, E.N. Oncostatin M: A pleiotropic cytokine in the central nervous system. Cytokine Growth Factor Rev. 2004, 15, 379–391. [Google Scholar] [CrossRef] [PubMed]

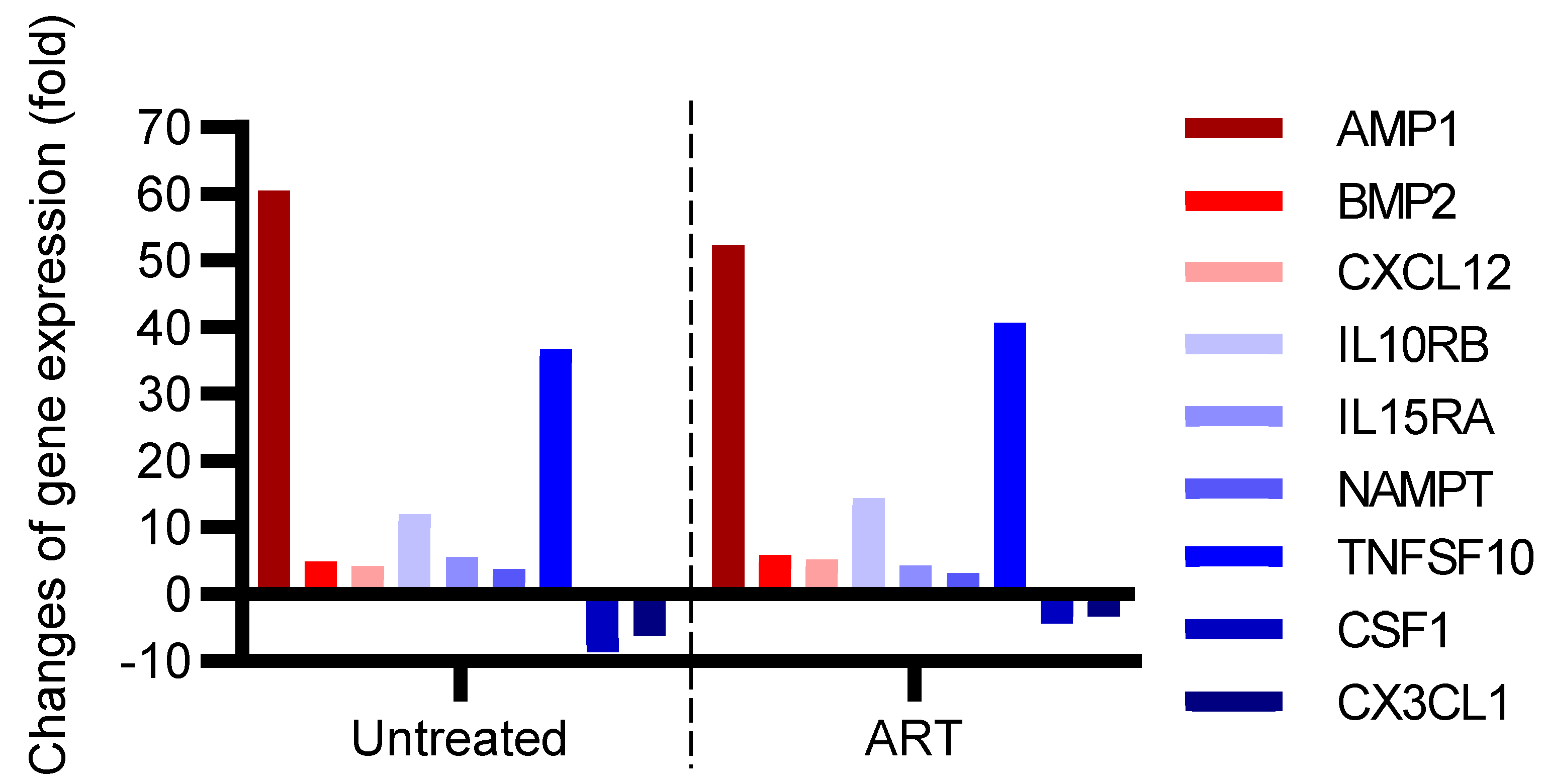

| Gene | Fold Change in Untreated Group vs. Control Group | p-Value | Gene | Fold Change in ART Group vs. Control Group | p-Value |
|---|---|---|---|---|---|
| AIMP1 | 60.62 | 0.0006 | AIMP1 | 52.35 | 0.0003 |
| BMP2 | 4.90 | 0.0138 | BMP2 | 5.85 | 0.0184 |
| CXCL12 | 4.23 | 0.0380 | CXCL12 | 5.17 | 0.0021 |
| IL10RB | 11.93 | 0.0014 | IL10RB | 14.35 | 0.0054 |
| IL15RA | 5.58 | 0.0021 | IL15RA | 4.24 | 0.0219 |
| NAMPT | 3.72 | 0.0039 | NAMPT | 3.20 | 0.0145 |
| TNFSF10 | 36.72 | 0.0003 | TNFSF10 | 40.67 | 0.0004 |
| CSF1 | −8.58 | 0.0006 | CSF1 | −4.28 | 0.0010 |
| CX3CL1 | −6.26 | 0.0093 | CX3CL1 | −3.28 | 0.0087 |
| CXCL8 | 3.93 | 0.0057 | IL11RA | 11.79 | 0.0208 |
| CXCR2 | 2.51 | 0.0426 | IL16 | 8.23 | 0.0035 |
| SPP1 | 77.25 | 0.0165 | IL6R | 5.96 | 0.0010 |
| IL9 | 9.26 | 0.0106 | |||
| RPL13A | −2.20 | 0.0389 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Solis-Leal, A.; Siddiqui, S.; Wu, F.; Mohan, M.; Hu, W.; Doyle-Meyers, L.A.; Dufour, J.P.; Ling, B. Neuroinflammatory Profiling in SIV-Infected Chinese-Origin Rhesus Macaques on Antiretroviral Therapy. Viruses 2022, 14, 139. https://doi.org/10.3390/v14010139
Solis-Leal A, Siddiqui S, Wu F, Mohan M, Hu W, Doyle-Meyers LA, Dufour JP, Ling B. Neuroinflammatory Profiling in SIV-Infected Chinese-Origin Rhesus Macaques on Antiretroviral Therapy. Viruses. 2022; 14(1):139. https://doi.org/10.3390/v14010139
Chicago/Turabian StyleSolis-Leal, Antonio, Summer Siddiqui, Fei Wu, Mahesh Mohan, Wenhui Hu, Lara A. Doyle-Meyers, Jason P. Dufour, and Binhua Ling. 2022. "Neuroinflammatory Profiling in SIV-Infected Chinese-Origin Rhesus Macaques on Antiretroviral Therapy" Viruses 14, no. 1: 139. https://doi.org/10.3390/v14010139
APA StyleSolis-Leal, A., Siddiqui, S., Wu, F., Mohan, M., Hu, W., Doyle-Meyers, L. A., Dufour, J. P., & Ling, B. (2022). Neuroinflammatory Profiling in SIV-Infected Chinese-Origin Rhesus Macaques on Antiretroviral Therapy. Viruses, 14(1), 139. https://doi.org/10.3390/v14010139






