The Role of Mosquito Hemocytes in Viral Infections
Abstract
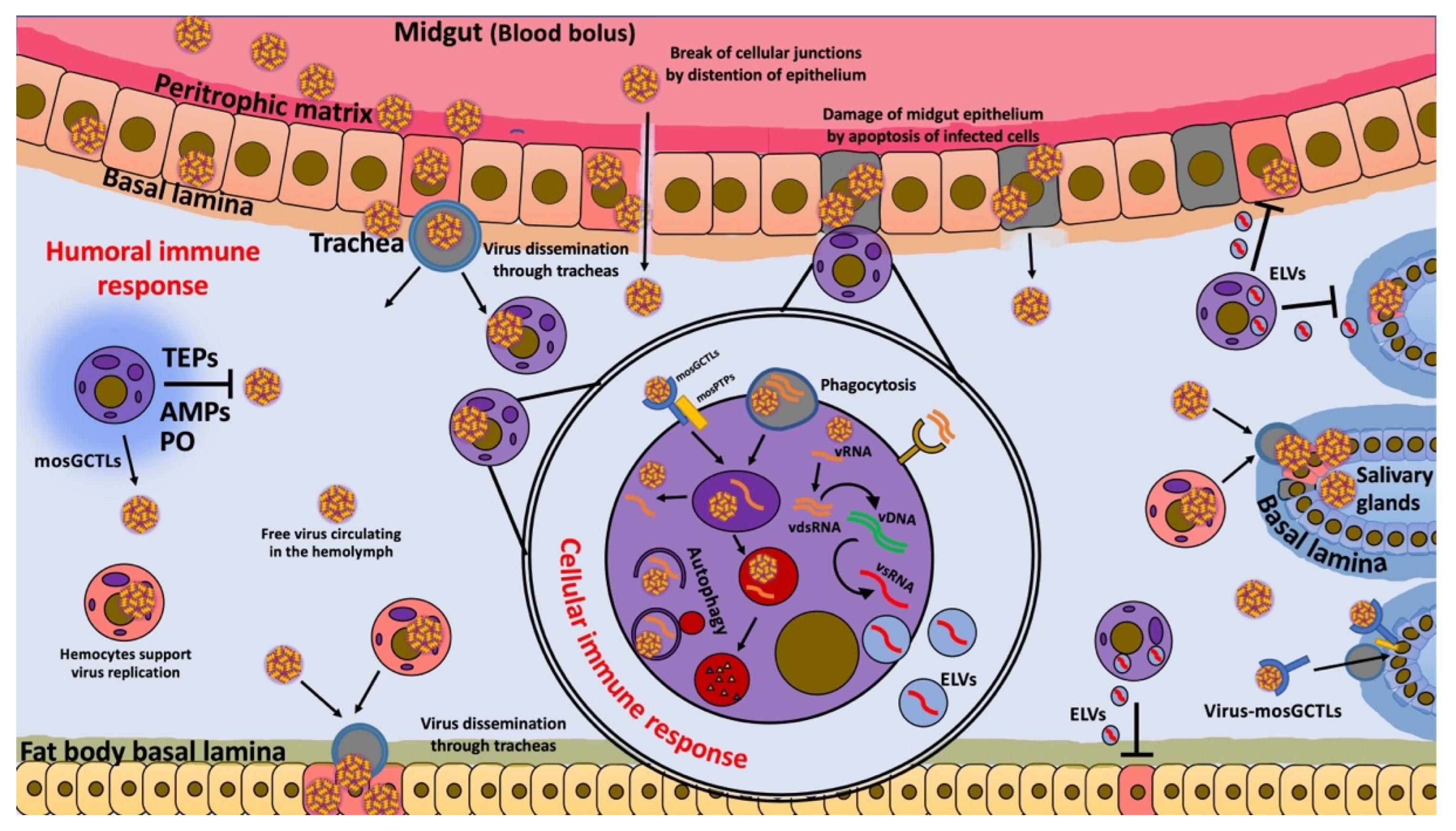
1. Introduction
2. The Role of Hemocytes in Antiviral Immunity
3. Antiviral Immune Pathway in Hemocytes
3.1. The siRNA Pathway
3.2. The Toll Pathway
3.3. The JAK-STAT Pathway
3.4. The IMD Pathway
3.5. The JNK Pathway
4. Humoral Factors Produced by Hemocytes Regulate Viral Infections
4.1. Prophenoloxidase
4.2. Antimicrobial Peptides (AMPs)
4.3. Pattern Recognition Receptors (Opsonins)
5. Cellular Antiviral Immunity in Hemocytes
5.1. Apoptosis
5.2. Autophagy and Phagocytosis
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, Y.-J.S.; Higgs, S.; Vanlandingham, D.L. Arbovirus-Mosquito Vector-Host Interactions and the Impact on Transmission and Disease Pathogenesis of Arboviruses. Front. Microbiol. 2019, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Gubler, D.J.; Weaver, S.C.; Monath, T.P.; Heymann, D.L.; Scott, T.W. Epidemic Arboviral Diseases: Priorities for Research and Public Health. Lancet Infect. Dis. 2017, 17, e101–e106. [Google Scholar] [CrossRef]
- Girard, M.; Nelson, C.B.; Picot, V.; Gubler, D.J. Arboviruses: A Global Public Health Threat. Vaccine 2020, 38, 3989–3994. [Google Scholar] [CrossRef]
- Tran, B.-L.; Tseng, W.-C.; Chen, C.-C.; Liao, S.-Y. Estimating the Threshold Effects of Climate on Dengue: A Case Study of Taiwan. Int. J. Environ. Res. Public Health 2020, 17, 1392. [Google Scholar] [CrossRef] [PubMed]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-Trotting Aedes Aegypti and Aedes Albopictus: Risk Factors for Arbovirus Pandemics. Vector-Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef]
- Kraemer, M.U.G.; Reiner, R.C.; Brady, O.J.; Messina, J.P.; Gilbert, M.; Pigott, D.M.; Yi, D.; Johnson, K.; Earl, L.; Marczak, L.B.; et al. Past and Future Spread of the Arbovirus Vectors Aedes Aegypti and Aedes Albopictus. Nat. Microbiol. 2019, 4, 854–863. [Google Scholar] [CrossRef]
- Braack, L.; Gouveia de Almeida, A.P.; Cornel, A.J.; Swanepoel, R.; de Jager, C. Mosquito-Borne Arboviruses of African Origin: Review of Key Viruses and Vectors. Parasites Vectors 2018, 11, 29. [Google Scholar] [CrossRef]
- Kraemer, M.U.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The Global Distribution of the Arbovirus Vectors Aedes Aegypti and Ae. Albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Ebi, K.L.; Nealon, J. Dengue in a Changing Climate. Environ. Res. 2016, 151, 115–123. [Google Scholar] [CrossRef]
- World Health Organization. Guidance Framework for Testing Genetically Modified Mosquitoes, 2nd ed.; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-002523-3.
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- World Health Organization. World Malaria Report 2021; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-004049-6.
- Liu, N. Insecticide Resistance in Mosquitoes: Impact, Mechanisms, and Research Directions. Annu. Rev. Entomol. 2015, 60, 537–559. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Dong, Y.; Simões, M.L.; Dimopoulos, G. Mosquito Transgenesis for Malaria Control. Trends Parasitol. 2021, 38, 54–66. [Google Scholar] [CrossRef]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes Aegypti Vector Competence Studies: A Review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Palomares, L.A.; Moreno-García, M.; Lanz-Mendoza, H.; Salazar, M.I. Molecular Basis for Arbovirus Transmission by Aedes Aegypti Mosquitoes. Intervirology 2018, 61, 255–264. [Google Scholar] [CrossRef] [PubMed]
- King, J.G. Developmental and Comparative Perspectives on Mosquito Immunity. Dev. Comp. Immunol. 2020, 103, 103458. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Strand, M.R. Mosquito Hemocyte-Mediated Immune Responses. Curr. Opin. Insect Sci. 2014, 3, 14–21. [Google Scholar] [CrossRef]
- Sim, S.; Jupatanakul, N.; Dimopoulos, G. Mosquito Immunity against Arboviruses. Viruses 2014, 6, 4479–4504. [Google Scholar] [CrossRef]
- Marques, J.T.; Imler, J.-L. The Diversity of Insect Antiviral Immunity: Insights from Viruses. Curr. Opin. Microbiol. 2016, 32, 71–76. [Google Scholar] [CrossRef]
- Tikhe, C.V.; Dimopoulos, G. Mosquito Antiviral Immune Pathways. Dev. Comp. Immunol. 2021, 116, 103964. [Google Scholar] [CrossRef]
- Dong, Y.; Simões, M.L.; Dimopoulos, G. Versatile Transgenic Multistage Effector-Gene Combinations for Plasmodium Falciparum Suppression in Anopheles. Sci. Adv. 2020, 6, eaay5898. [Google Scholar] [CrossRef]
- Smith, R.C.; Vega-Rodriguez, J.; Jacobs-Lorena, M. The Plasmodium Bottleneck: Malaria Parasite Losses in the Mosquito Vector. Mem. Do Inst. Oswaldo Cruz 2014, 109, 644–661. [Google Scholar] [CrossRef]
- Levashina, E.A.; Moita, L.F.; Blandin, S.; Vriend, G.; Lagueux, M.; Kafatos, F.C. Conserved Role of a Complement-like Protein in Phagocytosis Revealed by DsRNA Knockout in Cultured Cells of the Mosquito, Anopheles Gambiae. Cell 2001, 104, 709–718. [Google Scholar] [CrossRef]
- Danielli, A.; Loukeris, T.G.; Lagueux, M.; Muller, H.-M.; Richman, A.; Kafatos, F.C. A Modular Chitin-Binding Protease Associated with Hemocytes and Hemolymph in the Mosquito Anopheles Gambiae. Proc. Natl. Acad. Sci. USA 2000, 97, 7136–7141. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Lanz, H.; Rodríguez, M.H.; Torres, J.A.; Adolfo, M.-P.; Tsutsumi, V. Morphological and Cytochemical Characterization of Female Anopheles Albimanus (Diptera: Culicidae) Hemocytes. J. Med. Entomol. 1999, 36, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Schmidt, S.L.; Christensen, B.M. Rapid Phagocytosis and Melanization of Bacteria and Plasmodium Sporozoites by Hemocytes of the Mosquito Aedes Aegypi. J. Parasitol. 2003, 89, 62–69. [Google Scholar] [CrossRef]
- Gaudet, R.G.; Bradfield, C.J.; MacMicking, J.D. Evolution of Cell-Autonomous Effector Mechanisms in Macrophages versus Non-Immune Cells. In Myeloid Cells in Health and Disease; Gordon, S., Ed.; ASM Press: Washington, DC, USA, 2017; pp. 615–635. ISBN 978-1-68367-066-7. [Google Scholar]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Castillo, J.C.; Robertson, A.E.; Strand, M.R. Characterization of Hemocytes from the Mosquitoes Anopheles Gambiae and Aedes Aegypti. Insect Biochem. Mol. Biol. 2006, 36, 891–903. [Google Scholar] [CrossRef]
- King, J.G.; Hillyer, J.F. Spatial and Temporal in Vivo Analysis of Circulating and Sessile Immune Cells in Mosquitoes: Hemocyte Mitosis Following Infection. BMC Biol. 2013, 11, 55. [Google Scholar] [CrossRef]
- King, J.G.; Hillyer, J.F. Infection-Induced Interaction between the Mosquito Circulatory and Immune Systems. PLoS Pathog. 2012, 8, e1003058. [Google Scholar] [CrossRef]
- Yan, Y.; Hillyer, J.F. The Immune and Circulatory Systems Are Functionally Integrated across Insect Evolution. Sci. Adv. 2020, 6, eabb3164. [Google Scholar] [CrossRef]
- Sigle, L.T.; Hillyer, J.F. Mosquito Hemocytes Preferentially Aggregate and Phagocytose Pathogens in the Periostial Regions of the Heart That Experience the Most Hemolymph Flow. Dev. Comp. Immunol. 2016, 55, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Castillo, J.C.; Ferreira, A.B.B.; Trisnadi, N.; Barillas-Mury, C. Activation of Mosquito Complement Antiplasmodial Response Requires Cellular Immunity. Sci. Immunol. 2017, 2, eaal1505. [Google Scholar] [CrossRef] [PubMed]
- Hillyer, J.F.; Pass, G. The Insect Circulatory System: Structure, Function, and Evolution. Annu. Rev. Entomol. 2020, 65, 121–143. [Google Scholar] [CrossRef]
- Keddie, B.A.; Aponte, G.W.; Volkman, L.E. The Pathway of Infection of Autographa Californica Nuclear Polyhedrosis Virus in an Insect Host. Science 1989, 243, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Engelhard, E.K.; Kam-Morgan, L.N.; Washburn, J.O.; Volkman, L.E. The Insect Tracheal System: A Conduit for the Systemic Spread of Autographa Californica M Nuclear Polyhedrosis Virus. Proc. Natl. Acad. Sci. USA 1994, 91, 3224–3227. [Google Scholar] [CrossRef]
- Trudeau, D.; Washburn, J.O.; Volkman, L.E. Central Role of Hemocytes in Autographa Californica M Nucleopolyhedrovirus Pathogenesis in Heliothis Virescens and Helicoverpa Zea. J. Virol. 2001, 75, 996–1003. [Google Scholar] [CrossRef]
- Salazar, M.I.; Richardson, J.H.; Sánchez-Vargas, I.; Olson, K.E.; Beaty, B.J. Dengue Virus Type 2: Replication and Tropisms in Orally Infected Aedes Aegypti Mosquitoes. BMC Microbiol. 2007, 7, 9. [Google Scholar] [CrossRef]
- Cheng, G.; Cox, J.; Wang, P.; Krishnan, M.N.; Dai, J.; Qian, F.; Anderson, J.F.; Fikrig, E. A C-Type Lectin Collaborates with a CD45 Phosphatase Homolog to Facilitate West Nile Virus Infection of Mosquitoes. Cell 2010, 142, 714–725. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, F.; Liu, J.; Xiao, X.; Zhang, S.; Qin, C.; Xiang, Y.; Wang, P.; Cheng, G. Transmission-Blocking Antibodies against Mosquito C-Type Lectins for Dengue Prevention. PLoS Pathog. 2014, 10, e1003931. [Google Scholar] [CrossRef]
- Parikh, G.R.; Oliver, J.D.; Bartholomay, L.C. A Haemocyte Tropism for an Arbovirus. J. Gen. Virol. 2009, 90, 292–296. [Google Scholar] [CrossRef]
- Pondeville, E.; Puchot, N.; Parvy, J.-P.; Carissimo, G.; Poidevin, M.; Waterhouse, R.M.; Marois, E.; Bourgouin, C. Hemocyte-Targeted Gene Expression in the Female Malaria Mosquito Using the Hemolectin Promoter from Drosophila. Insect Biochem. Mol. Biol. 2020, 120, 103339. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Balaraman, V.; Kantor, A.M.; Lin, J.; Grant, D.G.; Held, N.L.; Franz, A.W.E. Chikungunya Virus Dissemination from the Midgut of Aedes Aegypti Is Associated with Temporal Basal Lamina Degradation during Bloodmeal Digestion. PLoS Negl. Trop. Dis. 2017, 11, e0005976. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.M.; Ehrlich, H.Y.; Magalhaes, T.; Miller, M.R.; Conway, P.J.; Bransfield, A.; Misencik, M.J.; Gloria-Soria, A.; Warren, J.L.; Andreadis, T.G.; et al. Successive Blood Meals Enhance Virus Dissemination within Mosquitoes and Increase Transmission Potential. Nat. Microbiol. 2020, 5, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Romoser, W.S.; Wasieloski, L.P.; Pushko, P.; Kondig, J.P.; Lerdthusnee, K.; Neira, M.; Ludwig, G.V. Evidence for Arbovirus Dissemination Conduits from the Mosquito (Diptera: Culicidae) Midgut. J. Med. Entomol. 2004, 41, 9. [Google Scholar] [CrossRef]
- Raquin, V.; Lambrechts, L. Dengue Virus Replicates and Accumulates in Aedes Aegypti Salivary Glands. Virology 2017, 507, 75–81. [Google Scholar] [CrossRef]
- Franz, A.; Kantor, A.; Passarelli, A.; Clem, R. Tissue Barriers to Arbovirus Infection in Mosquitoes. Viruses 2015, 7, 3741–3767. [Google Scholar] [CrossRef]
- Wang, H.; Gort, T.; Boyle, D.L.; Clem, R.J. Effects of Manipulating Apoptosis on Sindbis Virus Infection of Aedes Aegypti Mosquitoes. J. Virol. 2012, 86, 6546–6554. [Google Scholar] [CrossRef]
- Dong, S.; Kantor, A.M.; Lin, J.; Passarelli, A.L.; Clem, R.J.; Franz, A.W.E. Infection Pattern and Transmission Potential of Chikungunya Virus in Two New World Laboratory-Adapted Aedes Aegypti Strains. Sci. Rep. 2016, 6, 24729. [Google Scholar] [CrossRef]
- Cui, Y.; Grant, D.G.; Lin, J.; Yu, X.; Franz, A.W.E. Zika Virus Dissemination from the Midgut of Aedes Aegypti Is Facilitated by Bloodmeal-Mediated Structural Modification of the Midgut Basal Lamina. Viruses 2019, 11, 1056. [Google Scholar] [CrossRef]
- Merwaiss, F.; Filomatori, C.V.; Susuki, Y.; Bardossy, E.S.; Alvarez, D.E.; Saleh, M.-C. Chikungunya Virus Replication Rate Determines the Capacity of Crossing Tissue Barriers in Mosquitoes. J. Virol. 2021, 95, e01956-20. [Google Scholar] [CrossRef]
- Hillyer, J.F.; Barreau, C.; Vernick, K.D. Hemocoel, Efficiency of Salivary Gland Invasion by Malaria Sporozoites Is Controlled by Rapid Sporozoite Destruction in the Mosquito. Int. J. Parasitol. 2007, 37, 673–681. [Google Scholar] [CrossRef] [PubMed]
- Fink, K.; Ng, C.; Nkenfou, C.; Vasudevan, S.G.; van Rooijen, N.; Schul, W. Depletion of Macrophages in Mice Results in Higher Dengue Virus Titers and Highlights the Role of Macrophages for Virus Control. Eur. J. Immunol. 2009, 39, 2809–2821. [Google Scholar] [CrossRef] [PubMed]
- Tassetto, M.; Kunitomi, M.; Andino, R. Circulating Immune Cells Mediate a Systemic RNAi-Based Adaptive Antiviral Response in Drosophila. Cell 2017, 169, 314–325.e13. [Google Scholar] [CrossRef] [PubMed]
- Nainu, F.; Tanaka, Y.; Shiratsuchi, A.; Nakanishi, Y. Protection of Insects against Viral Infection by Apoptosis-Dependent Phagocytosis. J. Immunol. 2015, 195, 5696–5706. [Google Scholar] [CrossRef]
- Lamiable, O.; Arnold, J.; de Faria, I.J.D.S.; Olmo, R.P.; Bergami, F.; Meignin, C.; Hoffmann, J.A.; Marques, J.T.; Imler, J.-L. Analysis of the Contribution of Hemocytes and Autophagy to Drosophila Antiviral Immunity. J. Virol. 2016, 90, 5415–5426. [Google Scholar] [CrossRef]
- Leite, T.H.J.F.; Ferreira, Á.G.A.; Imler, J.-L.; Marques, J.T. Distinct Roles of Hemocytes at Different Stages of Infection by Dengue and Zika Viruses in Aedes Aegypti Mosquitoes. Front. Immunol. 2021, 12, 660873. [Google Scholar] [CrossRef]
- Li, T.; Xia, Y.; Xu, X.; Wei, G.; Wang, L. Functional Analysis of Dicer-2 Gene in Bombyx Mori Resistance to BmNPV Virus. Arch. Insect Biochem. Physiol. 2020, 105, e21724. [Google Scholar] [CrossRef]
- Feng, M.; Xia, J.; Fei, S.; Peng, R.; Wang, X.; Zhou, Y.; Wang, P.; Swevers, L.; Sun, J. Identification of Silkworm Hemocyte Subsets and Analysis of Their Response to Baculovirus Infection Based on Single-Cell RNA Sequencing. Front. Immunol. 2021, 12, 1521. [Google Scholar] [CrossRef]
- Jiang, L. Insights Into the Antiviral Pathways of the Silkworm Bombyx Mori. Front. Immunol. 2021, 12, 325. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, S.; Dizaji, N.B.; Rutkowski, N.; Pohlenz, T.; Myles, K.; Dimopoulos, G. The Aedes Aegypti SiRNA Pathway Mediates Broad-Spectrum Defense against Human Pathogenic Viruses and Modulates Antibacterial and Antifungal Defenses. PLoS Biol. 2022, 20, e3001668. [Google Scholar] [CrossRef]
- Hess, A.M.; Prasad, A.N.; Ptitsyn, A.; Ebel, G.D.; Olson, K.E.; Barbacioru, C.; Monighetti, C.; Campbell, C.L. Small RNA Profiling of Dengue Virus-Mosquito Interactions Implicates the PIWI RNA Pathway in Anti-Viral Defense. BMC Microbiol. 2011, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- McFarlane, M.; Almire, F.; Kean, J.; Donald, C.L.; McDonald, A.; Wee, B.; Lauréti, M.; Varjak, M.; Terry, S.; Vazeille, M.; et al. The Aedes Aegypti Domino Ortholog P400 Regulates Antiviral Exogenous Small Interfering RNA Pathway Activity and Ago-2 Expression. mSphere 2020, 5, e00081-20. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, O.; Niu, J.; Nji Tizi Taning, C. RNAi in Insects: A Revolution in Fundamental Research and Pest Control Applications. Insects 2020, 11, 415. [Google Scholar] [CrossRef] [PubMed]
- Tomoyasu, Y.; Miller, S.C.; Tomita, S.; Schoppmeier, M.; Grossmann, D.; Bucher, G. Exploring Systemic RNA Interference in Insects: A Genome-Wide Survey for RNAi Genes in Tribolium. Genome Biol. 2008, 9, 1–22. [Google Scholar] [CrossRef]
- Joga, M.R.; Zotti, M.J.; Smagghe, G.; Christiaens, O. RNAi Efficiency, Systemic Properties, and Novel Delivery Methods for Pest Insect Control: What We Know So Far. Front. Physiol. 2016, 7, 553. [Google Scholar] [CrossRef] [PubMed]
- Angleró-Rodríguez, Y.I.; Tikhe, C.V.; Kang, S.; Dimopoulos, G. Aedes Aegypti Toll Pathway Is Induced through DsRNA Sensing in Endosomes. Dev. Comp. Immunol. 2021, 122, 104138. [Google Scholar] [CrossRef] [PubMed]
- Qiu, P.; Pan, P.C.; Govind, S. A Role for the Drosophila Toll/Cactus Pathway in Larval Hematopoiesis. Development 1998, 125, 1909–1920. [Google Scholar] [CrossRef]
- Minakhina, S.; Tan, W.; Steward, R. JAK/STAT and the GATA Factor Pannier Control Hemocyte Maturation and Differentiation in Drosophila. Dev. Biol. 2011, 352, 308–316. [Google Scholar] [CrossRef]
- Minakhina, S.; Steward, R. Melanotic Mutants in Drosophila: Pathways and Phenotypes. Genetics 2006, 174, 253–263. [Google Scholar] [CrossRef]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral Defense Mechanisms in Honey Bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef]
- Ferreira, Á.G.; Naylor, H.; Esteves, S.S.; Pais, I.S.; Martins, N.E.; Teixeira, L. The Toll-Dorsal Pathway Is Required for Resistance to Viral Oral Infection in Drosophila. PLoS Pathog. 2014, 10, e1004507. [Google Scholar] [CrossRef] [PubMed]
- He, Y.J.; Lu, G.; Qi, Y.H.; Zhang, Y.; Zhang, X.D.; Huang, H.J.; Zhuo, J.C.; Sun, Z.T.; Yan, F.; Chen, J.P.; et al. Activation of Toll Immune Pathway in an Insect Vector Induced by a Plant Virus. Front. Immunol. 2021, 11, 3494. [Google Scholar] [CrossRef] [PubMed]
- Zambon, R.A.; Nandakumar, M.; Vakharia, V.W.; Wu, L.P. The Toll Pathway Is Important for an Antiviral Response in Drosophila. Proc. Natl. Acad. Sci. USA 2005, 102, 7257–7262. [Google Scholar] [CrossRef] [PubMed]
- The Aedes Aegypti Toll Pathway Controls Dengue Virus Infection PLOS Pathogens. Available online: https://journals.plos.org/plospathogens/article?id=10.1371/journal.ppat.1000098 (accessed on 1 August 2022).
- Barletta, A.B.F.; Saha, B.; Trisnadi, N.; Talyuli, O.; Raddi, G.; Barillas-Mury, C. Hemocyte Differentiation to the Megacyte Lineage Enhances Mosquito Immunity against Plasmodium. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Visweswariah, S.S. Intramacrophage ROS Primes the Innate Immune System via JAK/STAT and Toll Activation. Cell Rep. 2020, 33, 108368. [Google Scholar] [CrossRef]
- Pan, X.; Zhou, G.; Wu, J.; Bian, G.; Lu, P.; Raikhel, A.S.; Xi, Z. Wolbachia Induces Reactive Oxygen Species (ROS)-Dependent Activation of the Toll Pathway to Control Dengue Virus in the Mosquito Aedes Aegypti. Proc. Natl. Acad. Sci. USA 2012, 109, E23–E31. [Google Scholar] [CrossRef]
- Dudzic, J.P.; Hanson, M.A.; Iatsenko, I.; Kondo, S.; Lemaitre, B. More Than Black or White: Melanization and Toll Share Regulatory Serine Proteases in Drosophila. Cell Rep. 2019, 27, 1050–1061.e3. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, L.; Wang, X.; Yang, H.; Zhang, X.; Pan, G.; Li, C.; Ji, H.; Abbas, M.N.; Li, C.; et al. Scavenger Receptor C Regulates Antimicrobial Peptide Expression by Activating Toll Signaling in Silkworm, Bombyx Mori. Int. J. Biol. Macromol. 2021, 191, 396–404. [Google Scholar] [CrossRef]
- Engineered Aedes Aegypti JAK/STAT Pathway-Mediated Immunity to Dengue Virus PLOS Neglected Tropical Diseases. Available online: https://journals.plos.org/plosntds/article?id=10.1371/journal.pntd.0005187 (accessed on 1 August 2022).
- Bang, I.S. JAK/STAT Signaling in Insect Innate Immunity. Entomol. Res. 2019, 49, 339–353. [Google Scholar] [CrossRef]
- Barillas-Mury, C.; Han, Y.-S.; Seeley, D.; Kafatos, F.C. Anopheles Gambiae Ag-STAT, a New Insect Member of the STAT Family, Is Activated in Response to Bacterial Infection. EMBO J. 1999, 18, 959–967. [Google Scholar] [CrossRef]
- Yan, Y.; Sigle, L.T.; Rinker, D.C.; Estévez-Lao, T.Y.; Capra, J.A.; Hillyer, J.F. The IMD and JNK Pathways Drive the Functional Integration of the Immune and Circulatory Systems of Mosquitoes. bioRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Costa, A.; Jan, E.; Sarnow, P.; Schneider, D. The Imd Pathway Is Involved in Antiviral Immune Responses in Drosophila. PLoS ONE 2009, 4, e7436. [Google Scholar] [CrossRef] [PubMed]
- Avadhanula, V.; Weasner, B.P.; Hardy, G.G.; Kumar, J.P.; Hardy, R.W. A Novel System for the Launch of Alphavirus RNA Synthesis Reveals a Role for the Imd Pathway in Arthropod Antiviral Response. PLoS Pathog. 2009, 5, e1000582. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.; Freisinger, T.; Ishii, K.; Okado, K.; Shinzawa, N.; Fukumoto, S.; Kanuka, H. Activation of Imd Pathway in Hemocyte Confers Infection Resistance through Humoral Response in Drosophila. Biochem. Biophys. Res. Commun. 2013, 430, 1120–1125. [Google Scholar] [CrossRef]
- Sherri, N.; Salloum, N.; Mouawad, C.; Haidar-Ahmad, N.; Shirinian, M.; Rahal, E.A. Epstein-Barr Virus DNA Enhances Diptericin Expression and Increases Hemocyte Numbers in Drosophila Melanogaster via the Immune Deficiency Pathway. Front. Microbiol. 2018, 9, 1268. [Google Scholar] [CrossRef]
- Tafesh-Edwards, G.; Eleftherianos, I. JNK Signaling in Drosophila Immunity and Homeostasis. Immunol. Lett. 2020, 226, 7–11. [Google Scholar] [CrossRef]
- Chowdhury, A.; Modahl, C.M.; Tan, S.T.; Xiang, B.W.W.; Missé, D.; Vial, T.; Kini, R.M.; Pompon, J.F. JNK Pathway Restricts DENV2, ZIKV and CHIKV Infection by Activating Complement and Apoptosis in Mosquito Salivary Glands. PLoS Pathog. 2020, 16, e1008754. [Google Scholar] [CrossRef]
- Garver, L.S.; de Almeida Oliveira, G.; Barillas-Mury, C. The JNK Pathway Is a Key Mediator of Anopheles Gambiae Antiplasmodial Immunity. PLoS Pathog. 2013, 9, e1003622. [Google Scholar] [CrossRef]
- Hernández-Martínez, S.; Lanz-Mendoza, H.; Martínez-Barnetche, J.; Rodríguez, M.H. Antimicrobial Properties of Anopheles Albimanus Pericardial Cells. Cell Tissue Res. 2013, 351, 127–137. [Google Scholar] [CrossRef]
- Cardoso-Jaime, V.; Maya-Maldonado, K.; Celestino-Montes, A.; Tsutsumi, V.; Hernández-Martínez, S. Lysozyme C-1 Gene Is Overexpressed in Anopheles Albimanus Pericardial Cells after an Immune Challenge. Dev. Comp. Immunol. 2021, 114, 103830. [Google Scholar] [CrossRef]
- Cevik, D.; Acker, M.; Michalski, C.; Jacobs, J.R. Pericardin, a Drosophila Collagen, Facilitates Accumulation of Hemocytes at the Heart. Dev. Biol. 2019, 454, 52–65. [Google Scholar] [CrossRef] [PubMed]
- Gera, J.; Budakoti, P.; Suhag, M.; Mandal, L.; Mandal, S. Physiological ROS Controls Upd3-Dependent Modeling of ECM to Support Cardiac Function in Drosophila. Sci. Adv. 2022, 8, eabj4991. [Google Scholar] [CrossRef] [PubMed]
- Cerenius, L.; Söderhäll, K. Immune Properties of Invertebrate Phenoloxidases. Dev. Comp. Immunol. 2021, 122, 104098. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Revenis, C. Role and Importance of Phenoloxidase in Insect Hemostasis. J. Innate. Immun. 2011, 3, 28–33. [Google Scholar] [CrossRef] [PubMed]
- González-Santoyo, I.; Córdoba-Aguilar, A. Phenoloxidase: A Key Component of the Insect Immune System: Biochemical and Evolutionary Ecology of PO. Entomol. Exp. Appl. 2012, 142, 1–16. [Google Scholar] [CrossRef]
- McNeil, J.; Cox-Foster, D.; Slavicek, J.; Hoover, K. Contributions of Immune Responses to Developmental Resistance in Lymantria Dispar Challenged with Baculovirus. J. Insect Physiol. 2010, 56, 1167–1177. [Google Scholar] [CrossRef]
- Rodriguez-Andres, J.; Rani, S.; Varjak, M.; Chase-Topping, M.E.; Beck, M.H.; Ferguson, M.C.; Schnettler, E.; Fragkoudis, R.; Barry, G.; Merits, A.; et al. Phenoloxidase Activity Acts as a Mosquito Innate Immune Response against Infection with Semliki Forest Virus. PLoS Pathog. 2012, 8, e1002977. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect Antimicrobial Peptides and Their Applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Wang, G. (Ed.) Antimicrobial Peptides: Discovery, Design and Novel Therapeutic Strategies; Advances in molecular and cellular microbiology; CABI: Wallingford, UK; Cambridge, MA, USA, 2010; ISBN 978-1-84593-657-0. [Google Scholar]
- Hillyer, J.F. Mosquito Immunity. In Invertebrate Immunity; Söderhäll, K., Ed.; Advances in Experimental Medicine and Biology; Springer US: Boston, MA, USA, 2010; Volume 708, pp. 218–238. ISBN 978-1-4419-8058-8. [Google Scholar]
- Feng, M.; Fei, S.; Xia, J.; Labropoulou, V.; Swevers, L.; Sun, J. Antimicrobial Peptides as Potential Antiviral Factors in Insect Antiviral Immune Response. Front. Immunol. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Xiao, X.; Liu, Y.; Zhang, X.; Wang, J.; Li, Z.; Pang, X.; Wang, P.; Cheng, G. Complement-Related Proteins Control the Flavivirus Infection of Aedes Aegypti by Inducing Antimicrobial Peptides. PLoS Pathog. 2014, 10, e1004027. [Google Scholar] [CrossRef]
- Liu, W.-Q.; Chen, S.-Q.; Bai, H.-Q.; Wei, Q.-M.; Zhang, S.-N.; Chen, C.; Zhu, Y.-H.; Yi, T.-W.; Guo, X.-P.; Chen, S.-Y.; et al. The Ras/ERK Signaling Pathway Couples Antimicrobial Peptides to Mediate Resistance to Dengue Virus in Aedes Mosquitoes. PLoS Negl. Trop. Dis. 2020, 14, e0008660. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Mohammed, M.; Franzén, O.; Ankarklev, J.; Smith, R.C. Single-Cell Analysis of Mosquito Hemocytes Identifies Signatures of Immune Cell Subtypes and Cell Differentiation. eLife 2021, 10, e66192. [Google Scholar] [CrossRef] [PubMed]
- Raddi, G.; Barletta, A.B.F.; Efremova, M.; Ramirez, J.L.; Cantera, R.; Teichmann, S.A.; Barillas-Mury, C.; Billker, O. Mosquito Cellular Immunity at Single-Cell Resolution. Science 2020, 369, 1128–1132. [Google Scholar] [CrossRef] [PubMed]
- Severo, M.S.; Landry, J.J.M.; Lindquist, R.L.; Goosmann, C.; Brinkmann, V.; Collier, P.; Hauser, A.E.; Benes, V.; Henriksson, J.; Teichmann, S.A.; et al. Unbiased Classification of Mosquito Blood Cells by Single-Cell Genomics and High-Content Imaging. Proc Natl Acad Sci USA 2018, 115, E7568–E7577. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-J.; Fuchs, J.F.; Mayhew, G.F.; Yu, H.E.; Christensen, B.M. Tissue-Enriched Expression Profiles in Aedes Aegypti Identify Hemocyte-Specific Transcriptome Responses to Infection. Insect Biochem. Mol. Biol. 2012, 42, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, Y.; Zhang, R.; Zhang, J. The Diversity of Pattern Recognition Receptors (PRRs) Involved with Insect Defense against Pathogens. Curr. Opin. Insect Sci. 2019, 33, 105–110. [Google Scholar] [CrossRef]
- Dubovskiy, I.; Kryukova, N.; Glupov, V.V.; Ratcliffe, N. Encapsulation and Nodulation in Insects. Invertebr. Surviv. J. 2016, 13, 229–246. [Google Scholar] [CrossRef]
- Shokal, U.; Eleftherianos, I. Evolution and Function of Thioester-Containing Proteins and the Complement System in the Innate Immune Response. Front. Immunol. 2017, 8, 759. [Google Scholar] [CrossRef]
- Blandin, S. Thioester-Containing Proteins and Insect Immunity. Mol. Immunol. 2004, 40, 903–908. [Google Scholar] [CrossRef]
- Williams, M.; Baxter, R. The Structure and Function of Thioester-Containing Proteins in Arthropods. Biophys. Rev. 2014, 6, 261–272. [Google Scholar] [CrossRef]
- Blandin, S.; Shiao, S.-H.; Moita, L.F.; Janse, C.J.; Waters, A.P.; Kafatos, F.C.; Levashina, E.A. Complement-Like Protein TEP1 Is a Determinant of Vectorial Capacity in the Malaria Vector Anopheles Gambiae. Cell 2004, 116, 661–670. [Google Scholar] [CrossRef]
- Kwon, H.; Smith, R.C. Chemical Depletion of Phagocytic Immune Cells in Anopheles Gambiae Reveals Dual Roles of Mosquito Hemocytes in Anti- Plasmodium Immunity. Proc Natl Acad Sci USA 2019, 116, 14119–14128. [Google Scholar] [CrossRef] [PubMed]
- Bou Aoun, R.; Hetru, C.; Troxler, L.; Doucet, D.; Ferrandon, D.; Matt, N. Analysis of Thioester-Containing Proteins during the Innate Immune Response of Drosophila Melanogaster. J. Innate. Immun. 2011, 3, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Weng, S.-C.; Li, H.-H.; Li, J.-C.; Liu, W.-L.; Chen, C.-H.; Shiao, S.-H. A Thioester-Containing Protein Controls Dengue Virus Infection in Aedes Aegypti Through Modulating Immune Response. Front. Immunol. 2021, 12, 670122. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Liu, L.; Wang, P.; Zhang, Y.; Zhao, Y.O.; Colpitts, T.M.; Feitosa, F.; Anderson, J.F.; Fikrig, E. An In Vivo Transfection Approach Elucidates a Role for Aedes Aegypti Thioester-Containing Proteins in Flaviviral Infection. PLoS ONE 2011, 6, e22786. [Google Scholar] [CrossRef] [PubMed]
- Souvannaseng, L.; Hun, L.V.; Baker, H.; Klyver, J.M.; Wang, B.; Pakpour, N.; Bridgewater, J.M.; Napoli, E.; Giulivi, C.; Riehle, M.A.; et al. Inhibition of JNK Signaling in the Asian Malaria Vector Anopheles Stephensi Extends Mosquito Longevity and Improves Resistance to Plasmodium Falciparum Infection. PLoS Pathog. 2018, 14, e1007418. [Google Scholar] [CrossRef] [PubMed]
- Xia, X.; You, M.; Rao, X.-J.; Yu, X.-Q. Insect C-Type Lectins in Innate Immunity. Dev. Comp. Immunol. 2018, 83, 70–79. [Google Scholar] [CrossRef]
- Simões, M.L.; Mlambo, G.; Tripathi, A.; Dong, Y.; Dimopoulos, G. Immune Regulation of Plasmodium Is Anopheles Species Specific and Infection Intensity Dependent. mBio 2017, 8, e01631-17. [Google Scholar] [CrossRef]
- Simões, M.L.; Dong, Y.; Mlambo, G.; Dimopoulos, G. C-Type Lectin 4 Regulates Broad-Spectrum Melanization-Based Refractoriness to Malaria Parasites. PLoS Biol. 2022, 20, e3001515. [Google Scholar] [CrossRef]
- Adelman, Z.; Myles, K. The C-Type Lectin Domain Gene Family in Aedes Aegypti and Their Role in Arbovirus Infection. Viruses 2018, 10, 367. [Google Scholar] [CrossRef]
- Caragata, E.P.; Tikhe, C.V.; Dimopoulos, G. Curious Entanglements: Interactions between Mosquitoes, Their Microbiota, and Arboviruses. Curr. Opin. Virol. 2019, 37, 26–36. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Xiao, X.; Liu, Y.; Zhang, R.; Liu, J.; Liu, Q.; Wang, P.; Cheng, G. Mosquito C-Type Lectins Maintain Gut Microbiome Homeostasis. Nat. Microbiol. 2016, 1, 16023. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A Review of Programmed Cell Death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Orme, M.; Meier, P. Inhibitor of Apoptosis Proteins in Drosophila: Gatekeepers of Death. Apoptosis 2009, 14, 950–960. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Clem, R.J. Defining the Core Apoptosis Pathway in the Mosquito Disease Vector Aedes Aegypti: The Roles of Iap1, Ark, Dronc, and Effector Caspases. Apoptosis 2011, 16, 105–113. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, K.; Olson, B.J.S.C.; Huang, N.; Unis, D.; Clem, R.J. Rapid Selection against Arbovirus-Induced Apoptosis during Infection of a Mosquito Vector. Proc. Natl. Acad. Sci. USA 2015, 112, E1152–E1161. [Google Scholar] [CrossRef] [PubMed]
- Sim, S.; Ramirez, J.L.; Dimopoulos, G. Dengue Virus Infection of the Aedes Aegypti Salivary Gland and Chemosensory Apparatus Induces Genes That Modulate Infection and Blood-Feeding Behavior. PLoS Pathog. 2012, 8, e1002631. [Google Scholar] [CrossRef]
- Kelly, E.M.; Moon, D.C.; Bowers, D.F. Apoptosis in Mosquito Salivary Glands: Sindbis Virus-Associated and Tissue Homeostasis. J. Gen. Virol. 2012, 93, 2419–2424. [Google Scholar] [CrossRef]
- Vijayakumar, K.; Cho, G. Autophagy: An Evolutionarily Conserved Process in the Maintenance of Stem Cells and Aging. Cell Biochem. Funct. 2019, 37, 452–458. [Google Scholar] [CrossRef]
- Aman, Y.; Schmauck-Medina, T.; Hansen, M.; Morimoto, R.I.; Simon, A.K.; Bjedov, I.; Palikaras, K.; Simonsen, A.; Johansen, T.; Tavernarakis, N.; et al. Autophagy in Healthy Aging and Disease. Nat. Aging 2021, 1, 634–650. [Google Scholar] [CrossRef]
- Kroemer, G.; Mariño, G.; Levine, B. Autophagy and the Integrated Stress Response. Mol. Cell 2010, 40, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Nagy, P.; Sándor, G.O.; Juhász, G. Autophagy Maintains Stem Cells and Intestinal Homeostasis in Drosophila. Sci. Rep. 2018, 8, 4644. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.L.; Baehrecke, E.H. Growth Arrest and Autophagy Are Required for Salivary Gland Cell Degradation in Drosophila. Cell 2007, 131, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Bryant, B.; Raikhel, A.S. Programmed Autophagy in the Fat Body of Aedes Aegypti Is Required to Maintain Egg Maturation Cycles. PLoS ONE 2011, 6, e25502. [Google Scholar] [CrossRef] [PubMed]
- Gunay, B.; Goncu, E. Role of Autophagy in Midgut Stem Cells of Silkworm Bombyx Mori, during Larval–Pupal Metamorphosis. Arch Insect Biochem. Physiol. 2021, 108, e21832. [Google Scholar] [CrossRef]
- Tian, L.; Ma, L.; Guo, E.; Deng, X.; Ma, S.; Xia, Q.; Cao, Y.; Li, S. 20-Hydroxyecdysone Upregulates Atg Genes to Induce Autophagy in the Bombyx Fat Body. Autophagy 2013, 9, 1172–1187. [Google Scholar] [CrossRef]
- Shiba, H.; Yabu, T.; Sudayama, M.; Mano, N.; Arai, N.; Nakanishi, T.; Hosono, K. Sequential Steps of Macroautophagy and Chaperone-Mediated Autophagy Are Involved in the Irreversible Process of Posterior Silk Gland Histolysis during Metamorphosis of Bombyx Mori. J. Exp. Biol. 2016, 219, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Tindwa, H.; Jo, Y.H.; Patnaik, B.B.; Lee, Y.S.; Kang, S.S.; Han, Y.S. Molecular Cloning and Characterization of Autophagy-Related Gene TmATG8 in Listeria-Invaded Hemocytes of Tenebrio Molitor. Dev. Comp. Immunol. 2015, 51, 88–98. [Google Scholar] [CrossRef]
- Barletta, A.B.F.; Silva, M.C.L.N.; Sorgine, M.H.F. Validation of Aedes Aegypti Aag-2 Cells as a Model for Insect Immune Studies. Parasites Vectors 2012, 5, 148. [Google Scholar] [CrossRef]
- Brackney, D.E.; Correa, M.A.; Cozens, D.W. The Impact of Autophagy on Arbovirus Infection of Mosquito Cells. PLoS Negl. Trop. Dis. 2020, 14, e0007754. [Google Scholar] [CrossRef]
- Chen, T.-Y.; Smartt, C.T. Activation of the Autophagy Pathway Decreases Dengue Virus Infection in Aedes Aegypti Cells. Parasites Vectors 2021, 14, 551. [Google Scholar] [CrossRef] [PubMed]
- Henderson, C.; Brustolin, M.; Hegde, S.; Dayama, G.; Lau, N.; Hughes, G.L.; Bergey, C.; Rasgon, J.L. Mayaro Virus Infection Elicits an Innate Immune Response in Anopheles Stephensi. bioRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Wang, L.-L.; Wang, X.-R.; Wei, X.-M.; Huang, H.; Wu, J.-X.; Chen, X.-X.; Liu, S.-S.; Wang, X.-W. The Autophagy Pathway Participates in Resistance to Tomato Yellow Leaf Curl Virus Infection in Whiteflies. Autophagy 2016, 12, 1560–1574. [Google Scholar] [CrossRef]
- Wang, S.; Guo, H.; Zhu-Salzman, K.; Ge, F.; Sun, Y. PEBP Balances Apoptosis and Autophagy in Whitefly upon Arbovirus Infection. Nat. Commun. 2022, 13, 846. [Google Scholar] [CrossRef] [PubMed]
- Ramesh Kumar, J.; Smith, J.P.; Kwon, H.; Smith, R.C. Use of Clodronate Liposomes to Deplete Phagocytic Immune Cells in Drosophila Melanogaster and Aedes Aegypti. Front. Cell Dev. Biol. 2021, 9, 627976. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cardoso-Jaime, V.; Tikhe, C.V.; Dong, S.; Dimopoulos, G. The Role of Mosquito Hemocytes in Viral Infections. Viruses 2022, 14, 2088. https://doi.org/10.3390/v14102088
Cardoso-Jaime V, Tikhe CV, Dong S, Dimopoulos G. The Role of Mosquito Hemocytes in Viral Infections. Viruses. 2022; 14(10):2088. https://doi.org/10.3390/v14102088
Chicago/Turabian StyleCardoso-Jaime, Victor, Chinmay Vijay Tikhe, Shengzhang Dong, and George Dimopoulos. 2022. "The Role of Mosquito Hemocytes in Viral Infections" Viruses 14, no. 10: 2088. https://doi.org/10.3390/v14102088
APA StyleCardoso-Jaime, V., Tikhe, C. V., Dong, S., & Dimopoulos, G. (2022). The Role of Mosquito Hemocytes in Viral Infections. Viruses, 14(10), 2088. https://doi.org/10.3390/v14102088









