Flavivirus–Host Interaction Landscape Visualized through Genome-Wide CRISPR Screens
Abstract
:1. Introduction
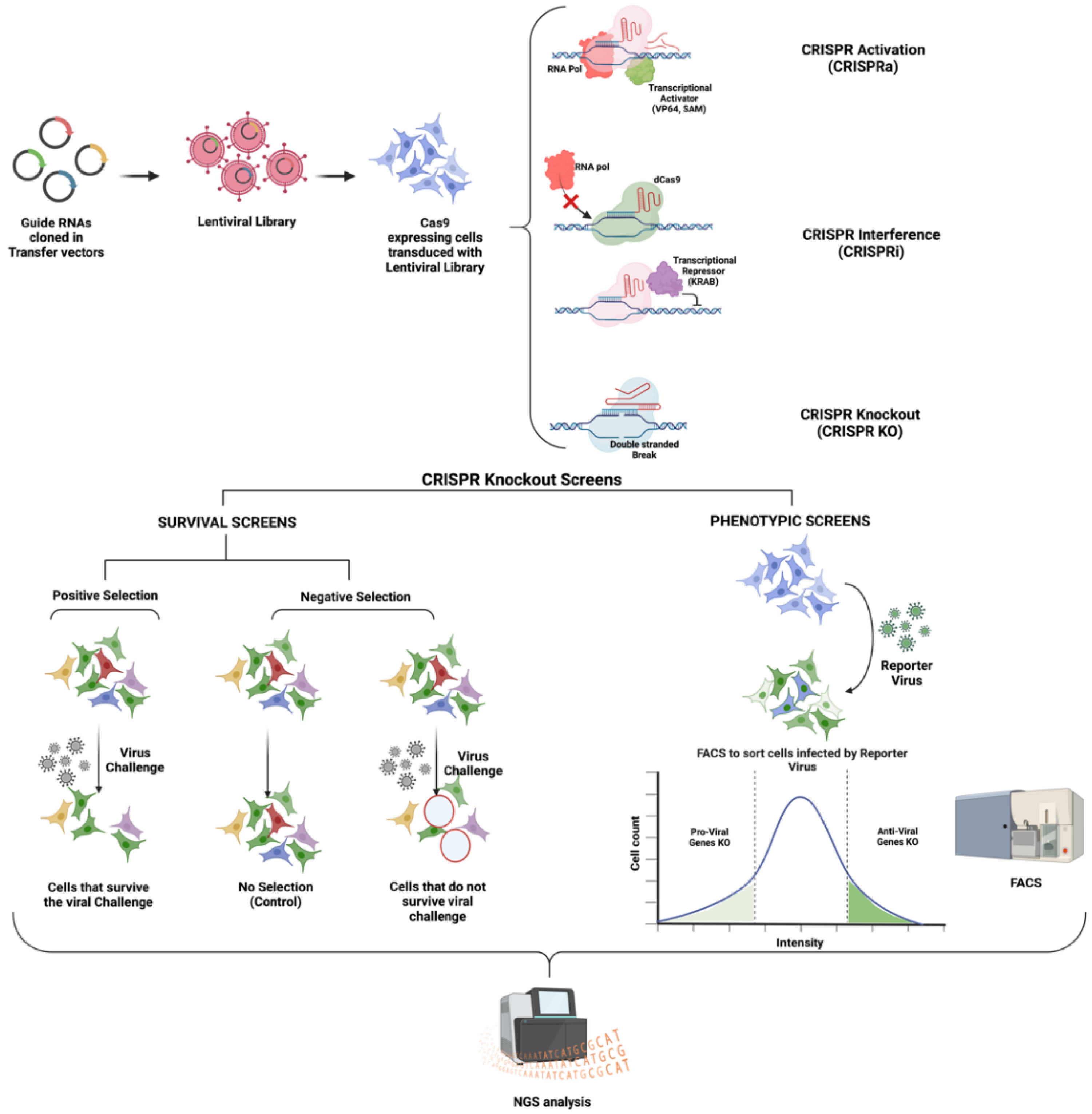
2. CRISPR-Cas Biology: An Overview
- (1)
- A catalytically dead Cas9 enzyme (dCas9) for CRISPR interference (CRISPRi) studies: This dCas9 binds to the target DNA sequence guided by the gRNA. Instead of cleaving the bound DNA, the dCas9 enzyme remains bound to the target DNA sequence, disrupting RNA polymerase or transcription factor binding to the promoter. Other than steric hindrance, CRISPRi can also repress transcription via a repressor domain, such as the Krüppel associated box (KRAB), fused to dCas9 [35,36] (Figure 1).
- (2)
- Cas9 tethered with a transcriptional activator such as SunTAG [33], Synergistic Activation Mediator (SAM) [37], VP64 [38], etc., for CRISPR activation (CRISPRa) studies: Such Cas9 leads to the recruitment of transcriptional machinery to the targeted promoter. CRISPRa studies are employed to perform gain-of-function studies [35,39].
3. CRISPR Screens for Studying Flavivirus Infections
3.1. Virus Receptors and Attachment Factors
3.2. Viral Translation and Insertion into ER Membrane
- Signal Peptidase Complex (SPCS): After being translated, the flavivirus polyprotein is inserted into the ER membrane as a single multipass protein and cleaved by viral and host proteases, including the host signal peptidase complex (SPCS). Knocking out SPCS1, a significant component of the SPCS, ablated the replication of all flaviviruses but not that of the unrelated RNA viruses, suggesting that it is needed for flavivirus replication specifically. Mechanistic studies revealed that the SPCS1 is involved in the cleavage of the polyprotein’s structural proteins prM and E [60].
- Translocon-associated protein complex (TRAP): The SRP ribonucleotide complex recognizes and binds to a hydrophobic transmembrane region of the nascent polypeptide, arrests translation, and brings the ribosome to a translocon where translation continues. Since the translated polyprotein contains several transmembrane domains that need to be appropriately integrated into the ER membrane, the host SRP-translocon pathway proteins such as SEC61A1 and SEC63 also showed up in several CRISPR Screens [53,60,64,68] and in an RNAi Screen [66]. Additionally, several protein-protein interaction studies have revealed interactions between ZIKV/DENV NS4A and SEC62, SEC61γ, and SRPR; NS4A/2B and SEC61β; and NS4B with SEC61α [69,70]. Interestingly, pharmacological modulation of this complex has been shown to inhibit DENV and ZIKV replication [70,71].
- Endoplasmic-reticulum-associated protein degradation (ERAD) Pathway: ERAD is a protein quality control mechanism that recognizes incorrectly folded proteins in the ER lumen. These proteins are then retro-translocated through the ER membrane to the cytosol to be targeted for proteasomal degradation. Certain components of the classical ERAD machinery, especially the ones that form the retro-translocation complex, were shown to be essential for infectious virus particle formation and virus-induced cell death for DENV, ZIKV, JEV, and WNV. These include proteins such as SEL1L, derlin 2 (DERL2), and ubiquitin-conjugating enzyme E2 J1 (UBE2J1). Knocking out these genes conferred robust protection against WNV-induced cell death. Remarkably, WNV replication was unaffected. Thus, these factors have been speculated to be the chief drivers of WNV-induced cell death [59].
- The Endoplasmic reticulum membrane protein complex (EMC): EMC is an evolutionarily conserved complex responsible for stabilizing and helping in the insertion of multipass membrane proteins in the ER. Several genetic screens have independently shown the EMC proteins to be essential for correct viral protein insertion into the ER membrane [53,58,59,68]. A 2019 study suggested that biogenesis and co-translational stabilization of DENV and ZIKV multipass proteins NS4A and NS4B rely on the interaction with EMC components [72]. The authors used a dual-fluorescence reporter system to map the hydrophobic transmembrane regions of NS4B utilized for the interaction with the EMC complex. An independent study showed a very prominent loss of replication of DENV, ZIKV, and YFV upon knocking out protein complex EMC4. Interestingly, there was no effect on the replication of WNV. The authors speculated that this difference could be because Culex mosquitoes, rather than Aedes, primarily transmit WNV. To support this vector-specific hypothesis, they also interrogated the DENV titer in Aedes mosquito midguts, which was found to be depleted post-siRNA-mediated targeting of EMC2/3/4. All in all, the study suggested that the EMC is a critical host factor utilized by Aedes-transmitted flaviviruses [73].
- Additionally, two subunits of the endoplasmic reticulum (ER) resident dolichol-phosphate mannose synthase (DPMS) complex were identified as host dependency factors for DENV and ZIKV. The DPMS complex catalyzes the synthesis of dolichol-phosphate mannose (DPM), which serves as a mannosyl donor in pathways leading to N-glycosylation, glycosylphosphatidylinositol (GPI) anchor biosynthesis, and C- or O-mannosylation of proteins in the ER lumen. This DPMS complex was shown to be required for optimal viral RNA amplification and proper glycosylation and folding of viral structural proteins prM and E [55].
3.3. Formation of Replication Complexes (RCs) and Viral RNA Synthesis
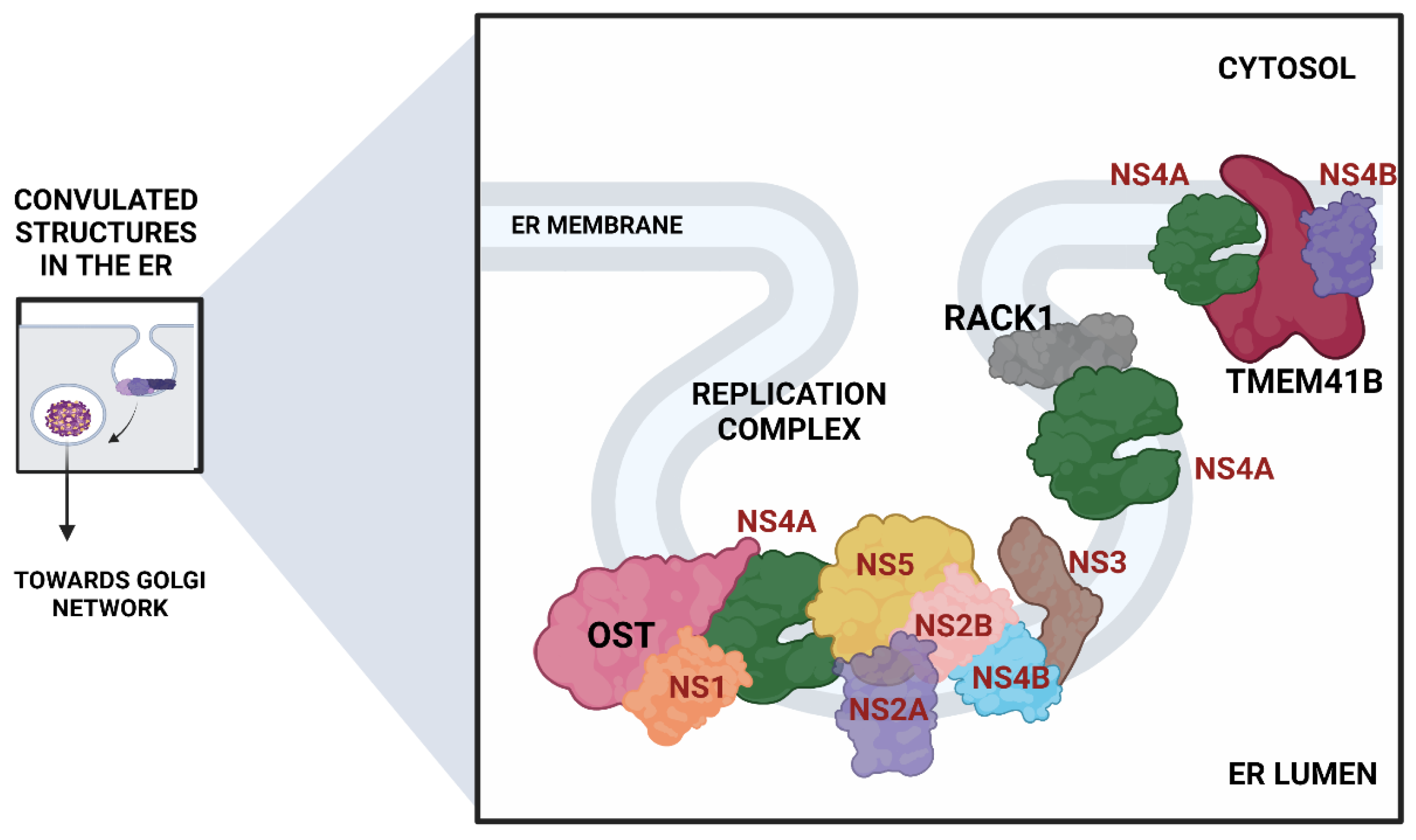
- The Oligosaccharyltransferase (OST) complex: The OST complex is associated with N-linked glycosylation of host proteins in mammalian cells. Interestingly, different flaviviruses exhibit different dependencies on the two OST complex catalytic subunits: STT3A and STT3B. While the STT3A complex is needed for the co-translational N-linked glycosylation of the majority of the glycoproteins, the STT3B complex is essential for the co-translational or post-translational glycosylation of acceptor sites that have been skipped by the STT3A complex [74]. The OST complex was shown to be necessary for the viral RNA synthesis but not for the entry and translation. Both complexes were individually required for the replication of DENV. However, ZIKV replication was shown to be exclusively dependent on the STT3A complex, pointing out divergent virus-host interactions. Knocking out OST complex component STT3A abrogated the replication of YFV, WNV, and JEV as well. However, these replication defects were rescued by the expression of catalytically dead STT3A mutants, suggesting that the ability of OST complex to glycosylate proteins is not required for flavivirus replication. Additionally, physical interactions between flavivirus replication complex members NS1, NS2B, NS3, and NS4B and OST Complex in the ER suggest that the OST complex might act as a scaffold to orchestrate the assembly of the viral replication complex [53]. Lin et al. employed the same genome-wide CRISPR KO approach and extended this work to show that the oxidoreductase activity of the OST complex subunit MAGT1 was essential for DENV propagation. They further showed that the expression of MAGT1 depends on the presence of STT3B but not on its catalytic activity. MAGT1 was also associated with DENV NS1 and NS4B proteins during viral infection [54]. Collectively, these two studies suggested that the OST complex not only interacts physically with the replication complexes but is also engaged in post-translationally modifying and stabilizing the viral non-structural proteins associated with the complex.
- In another interesting genome-wide CRISPR KO study, Transmembrane Protein 41B (TMEM41B) was shown to be required for infection and replication of several mosquito-borne and tick-borne flaviviruses, making it a pan-flavivirus host factor. Based on mechanistic studies, the authors proposed a model whereupon flavivirus entry and subsequent translation of the viral polyprotein; this protein, TMEM41B, is recruited to the ER membrane together with viral proteins NS4A and NS4B, which are involved in inducing membrane curvature so that replication complexes (RCs) can form and make a protected environment for viral genome replication. The study also showed how the absence of TMEM41B leads to the formation of poor RCs, which ultimately causes the dsRNA replication intermediates to become exposed to innate immunity pattern recognition receptors (PRRs) in the host cell. This recognition and activation of innate immune responses lead to the abortion of the infectious replication cycle [68].
- Another significant flavivirus host factor is the Receptor for Activated C Kinase 1 (RACK1) protein. This protein has functions correlated with protein shuttling, anchoring, stabilization, and mediating specific cellular pathways through protein interactions. A recent CRISPR KO screen in Huh7 cells found that silencing of RACK1 affected the replication of several flaviviruses, including ZIKV, DENV, and WNV but not YFV. They utilized a Renilla luciferase DENV replicon to proclaim that RACK1 specifically played a role in viral genome replication rather than viral entry or translation. The authors used a replication-independent expression system to delineate the mechanism that induces the formation of RCs in the ER without virus infection. RACK1 silencing was shown to limit the organization of these structures in the ER membrane [56].
- Apart from these pathways and complexes, FAD biosynthesis, catalyzed by riboflavin (vitamin B2), kinase (RFK), and FAD synthase (FLAD1), was shown to be critical for the synthesis of HCV RNA. ELAVL1, an RNA-binding protein that binds to host mRNAs and increases their stability [75], was shown to attach to the 3′ UTR of HCV RNA to aid its replication via circularization [53]. Significantly, a protein called Cyclophilin A (CYPA) that has been shown previously to interact with HCV replication protein NS5A was also enriched (3). Some host cyclophilin inhibitors have shown promising effects in curing HCV infection in both in vitro and in vivo settings and have advanced to phase II/III clinical trials [76]. This study on cyclophilin inhibitors also highlights how targeting the host factors instead of viral factors is associated with the reduced emergence of resistance [76]. This is important because HCV exhibits a brisk mutation rate as an RNA virus, and a single mutation in the viral target can render the antiviral ineffective.
4. CRISPR Screens to Identify Anti-Flavivirus Host Factors
5. Conclusions and Prospective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, J.; Liu, Y.; Yuan, Z. Critical Role of Dengue Virus NS1 Protein in Viral Replication. Virol. Sin. 2014, 29, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Wilson, J.R.; de Sessions, P.F.; Leon, M.A.; Scholle, F. West Nile Virus Nonstructural Protein 1 Inhibits TLR3 Signal Transduction. J. Virol. 2008, 82, 8262–8271. [Google Scholar] [CrossRef] [PubMed]
- Falgout, B.; Pethel, M.; Zhang, Y.M.; Lai, C.J. Both Nonstructural Proteins NS2B and NS3 Are Required for the Proteolytic Processing of Dengue Virus Nonstructural Proteins. J. Virol. 1991, 65, 2467–2475. [Google Scholar] [CrossRef] [PubMed]
- Steinkühler, C.; Tomei, L.; De Francesco, R. In Vitro Activity of Hepatitis C Virus Protease NS3 Purified from Recombinant Baculovirus-Infected Sf9 Cells. J. Biol. Chem. 1996, 271, 6367–6373. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Jordan, J.L.; Sánchez-Burgos, G.G.; Laurent-Rolle, M.; García-Sastre, A. Inhibition of Interferon Signaling by Dengue Virus. Proc. Natl. Acad. Sci. USA 2003, 100, 14333–14338. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.H.; Fu, J.; Sugrue, R.J.; Yap, E.H.; Chan, Y.C.; Tan, Y.H. Recombinant Dengue Type 1 Virus NS5 Protein Expressed in Escherichia coli Exhibits RNA-Dependent RNA Polymerase Activity. Virology 1996, 216, 317–325. [Google Scholar] [CrossRef]
- Behrens, S.E.; Tomei, L.; De Francesco, R. Identification and Properties of the RNA-Dependent RNA Polymerase of Hepatitis C Virus. EMBO J. 1996, 15, 12–22. [Google Scholar] [CrossRef]
- Alvarez, D.E.; De Lella Ezcurra, A.L.; Fucito, S.; Gamarnik, A.V. Role of RNA Structures Present at the 3′UTR of Dengue Virus on Translation, RNA Synthesis, and Viral Replication. Virology 2005, 339, 200–212. [Google Scholar] [CrossRef]
- Gillespie, L.K.; Hoenen, A.; Morgan, G.; Mackenzie, J.M. The Endoplasmic Reticulum Provides the Membrane Platform for Biogenesis of the Flavivirus Replication Complex. J. Virol. 2010, 84, 10438–10447. [Google Scholar] [CrossRef]
- Klaitong, P.; Smith, D.R. Roles of Non-Structural Protein 4A in Flavivirus Infection. Viruses 2021, 13, 2077. [Google Scholar] [CrossRef]
- Welsch, S.; Miller, S.; Romero-Brey, I.; Merz, A.; Bleck, C.K.E.; Walther, P.; Fuller, S.D.; Antony, C.; Krijnse-Locker, J.; Bartenschlager, R. Composition and Three-Dimensional Architecture of the Dengue Virus Replication and Assembly Sites. Cell Host Microbe 2009, 5, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Reed, K.E.; Gorbalenya, A.E.; Rice, C.M. The NS5A/NS5 Proteins of Viruses from Three Genera of the Family Flaviviridae Are Phosphorylated by Associated Serine/Threonine Kinases. J. Virol. 1998, 72, 6199–6206. [Google Scholar] [CrossRef] [PubMed]
- Dengue and Severe Dengue. Available online: https://www.who.int/news-room/fact-sheets/detail/dengue-and-severe-dengue (accessed on 22 August 2022).
- Fitzgerald, B.; Boyle, C.; Honein, M.A. Birth Defects Potentially Related to Zika Virus Infection During Pregnancy in the United States. JAMA 2018, 319, 1195–1196. [Google Scholar] [CrossRef] [PubMed]
- Factsheet about Japanese Encephalitis. Available online: https://www.ecdc.europa.eu/en/japanese-encephalitis/facts (accessed on 10 September 2022).
- Reed, K.D.; Meece, J.K.; Henkel, J.S.; Shukla, S.K. Birds, Migration and Emerging Zoonoses: West Nile Virus, Lyme Disease, Influenza A and Enteropathogens. Clin. Med. Res. 2003, 1, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Valentine, M.J.; Murdock, C.C.; Kelly, P.J. Sylvatic Cycles of Arboviruses in Non-Human Primates. Parasit. Vectors 2019, 12, 463. [Google Scholar] [CrossRef] [PubMed]
- Germain, M.; Francy, D.B.; Monath, T.P.; Ferrara, L.; Bryan, J.; Salaun, J.J.; Heme, G.; Renaudet, J.; Adam, C.; Digoutte, J.P. Yellow Fever in the Gambia, 1978–1979: Entomological Aspects and Epidemiological Correlations. Am. J. Trop. Med. Hyg. 1980, 29, 929–940. [Google Scholar] [CrossRef]
- Hanley, K.A.; Monath, T.P.; Weaver, S.C.; Rossi, S.L.; Richman, R.L.; Vasilakis, N. Fever versus Fever: The Role of Host and Vector Susceptibility and Interspecific Competition in Shaping the Current and Future Distributions of the Sylvatic Cycles of Dengue Virus and Yellow Fever Virus. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 2013, 19, 292–311. [Google Scholar] [CrossRef]
- Puschnik, A.S.; Majzoub, K.; Ooi, Y.S.; Carette, J.E. A CRISPR Toolbox to Study Virus-Host Interactions. Nat. Rev. Microbiol. 2017, 15, 351–364. [Google Scholar] [CrossRef]
- Li, B.; Clohisey, S.M.; Chia, B.S.; Wang, B.; Cui, A.; Eisenhaure, T.; Schweitzer, L.D.; Hoover, P.; Parkinson, N.J.; Nachshon, A.; et al. Genome-Wide CRISPR Screen Identifies Host Dependency Factors for Influenza A Virus Infection. Nat. Commun. 2020, 11, 164. [Google Scholar] [CrossRef]
- Han, J.; Perez, J.T.; Chen, C.; Li, Y.; Benitez, A.; Kandasamy, M.; Lee, Y.; Andrade, J.; tenOever, B.; Manicassamy, B. Genome-Wide CRISPR/Cas9 Screen Identifies Host Factors Essential for Influenza Virus Replication. Cell Rep. 2018, 23, 596–607. [Google Scholar] [CrossRef] [Green Version]
- Grodzki, M.; Bluhm, A.P.; Schaefer, M.; Tagmount, A.; Russo, M.; Sobh, A.; Rafiee, R.; Vulpe, C.D.; Karst, S.M.; Norris, M.H. Genome-Scale CRISPR Screens Identify Host Factors That Promote Human Coronavirus Infection. Genome Med. 2022, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Baggen, J.; Persoons, L.; Vanstreels, E.; Jansen, S.; Van Looveren, D.; Boeckx, B.; Geudens, V.; De Man, J.; Jochmans, D.; Wauters, J.; et al. Genome-Wide CRISPR Screening Identifies TMEM106B as a Proviral Host Factor for SARS-CoV-2. Nat. Genet. 2021, 53, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Lee, K.; Bae, S.; Park, J.; Lee, C.-K.; Kim, M.; Kim, E.; Kim, M.; Kim, S.; Kim, C.; et al. CRISPR/Cas9-Mediated Gene Knockout Screens and Target Identification via Whole-Genome Sequencing Uncover Host Genes Required for Picornavirus Infection. J. Biol. Chem. 2017, 292, 10664–10671. [Google Scholar] [CrossRef] [PubMed]
- Park, R.J.; Wang, T.; Koundakjian, D.; Hultquist, J.F.; Lamothe-Molina, P.; Monel, B.; Schumann, K.; Yu, H.; Krupzcak, K.M.; Garcia-Beltran, W.; et al. A Genome-Wide CRISPR Screen Identifies a Restricted Set of HIV Host Dependency Factors. Nat. Genet. 2017, 49, 193–203. [Google Scholar] [CrossRef]
- Yi, C.; Cai, C.; Cheng, Z.; Zhao, Y.; Yang, X.; Wu, Y.; Wang, X.; Jin, Z.; Xiang, Y.; Jin, M.; et al. Genome-Wide CRISPR-Cas9 Screening Identifies the CYTH2 Host Gene as a Potential Therapeutic Target of Influenza Viral Infection. Cell Rep. 2022, 38, 110559. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, S.; Li, W.; Yau, E.; Hui, H.; Singh, P.K.; Achuthan, V.; Young Karris, M.A.; Engelman, A.N.; Rana, T.M. Genome-Wide CRISPR/Cas9 Transcriptional Activation Screen Identifies a Histone Acetyltransferase Inhibitor Complex as a Regulator of HIV-1 Integration. Nucleic Acids Res. 2022, 50, 6687–6701. [Google Scholar] [CrossRef]
- Wiedenheft, B.; Sternberg, S.H.; Doudna, J.A. RNA-Guided Genetic Silencing Systems in Bacteria and Archaea. Nature 2012, 482, 331–338. [Google Scholar] [CrossRef]
- Terns, M.P.; Terns, R.M. CRISPR-Based Adaptive Immune Systems. Curr. Opin. Microbiol. 2011, 14, 321–327. [Google Scholar] [CrossRef]
- Mojica, F.J.M.; Díez-Villaseñor, C.; García-Martínez, J.; Soria, E. Intervening Sequences of Regularly Spaced Prokaryotic Repeats Derive from Foreign Genetic Elements. J. Mol. Evol. 2005, 60, 174–182. [Google Scholar] [CrossRef]
- Jinek, M.; Chylinski, K.; Fonfara, I.; Hauer, M.; Doudna, J.A.; Charpentier, E. A Programmable Dual-RNA-Guided DNA Endonuclease in Adaptive Bacterial Immunity. Science 2012, 337, 816–821. [Google Scholar] [CrossRef]
- Gasiunas, G.; Barrangou, R.; Horvath, P.; Siksnys, V. Cas9-CrRNA Ribonucleoprotein Complex Mediates Specific DNA Cleavage for Adaptive Immunity in Bacteria. Proc. Natl. Acad. Sci. USA 2012, 109, E2579–E2586. [Google Scholar] [CrossRef] [PubMed]
- Shalem, O.; Sanjana, N.E.; Zhang, F. High-Throughput Functional Genomics Using CRISPR-Cas9. Nat. Rev. Genet. 2015, 16, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Horlbeck, M.A.; Adamson, B.; Villalta, J.E.; Chen, Y.; Whitehead, E.H.; Guimaraes, C.; Panning, B.; Ploegh, H.L.; Bassik, M.C.; et al. Genome-Scale CRISPR-Mediated Control of Gene Repression and Activation. Cell 2014, 159, 647–661. [Google Scholar] [CrossRef]
- Vigouroux, A.; Oldewurtel, E.; Cui, L.; Bikard, D.; van Teeffelen, S. Tuning DCas9’s Ability to Block Transcription Enables Robust, Noiseless Knockdown of Bacterial Genes. Mol. Syst. Biol. 2018, 14, e7899. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, C.; Zhang, T.; Li, F.; Yang, W.; Kaminski, R.; Fagan, P.R.; Putatunda, R.; Young, W.-B.; Khalili, K.; et al. CRISPR/GRNA-Directed Synergistic Activation Mediator (SAM) Induces Specific, Persistent and Robust Reactivation of the HIV-1 Latent Reservoirs. Sci. Rep. 2015, 5, 16277. [Google Scholar] [CrossRef] [PubMed]
- Bikard, D.; Jiang, W.; Samai, P.; Hochschild, A.; Zhang, F.; Marraffini, L.A. Programmable Repression and Activation of Bacterial Gene Expression Using an Engineered CRISPR-Cas System. Nucleic Acids Res. 2013, 41, 7429–7437. [Google Scholar] [CrossRef]
- Kampmann, M. CRISPRi and CRISPRa Screens in Mammalian Cells for Precision Biology and Medicine. ACS Chem. Biol. 2018, 13, 406–416. [Google Scholar] [CrossRef]
- Roesch, F.; OhAinle, M. HIV-CRISPR: A CRISPR/Cas9 Screening Method to Identify Genes Affecting HIV Replication. Bio Protoc. 2020, 10, e3614. [Google Scholar] [CrossRef]
- Heaton, B.E.; Kennedy, E.M.; Dumm, R.E.; Harding, A.T.; Sacco, M.T.; Sachs, D.; Heaton, N.S. A CRISPR Activation Screen Identifies a Pan-Avian Influenza Virus Inhibitory Host Factor. Cell Rep. 2017, 20, 1503–1512. [Google Scholar] [CrossRef]
- Qi, L.S.; Larson, M.H.; Gilbert, L.A.; Doudna, J.A.; Weissman, J.S.; Arkin, A.P.; Lim, W.A. Repurposing CRISPR as an RNA-Guided Platform for Sequence-Specific Control of Gene Expression. Cell 2013, 152, 1173–1183. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, A.; Fujii, W.; Sugiura, K.; Naito, K. High-Fidelity Endonuclease Variant HypaCas9 Facilitates Accurate Allele-Specific Gene Modification in Mouse Zygotes. Commun. Biol. 2019, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Jung, M.-H.; Jeong, E.; Lee, J.K. Using Sniper-Cas9 to Minimize Off-Target Effects of CRISPR-Cas9 Without the Loss of On-Target Activity Via Directed Evolution. J. Vis. Exp. 2019, 144, e59202. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Jeong, E.; Lee, J.; Jung, M.; Shin, E.; Kim, Y.H.; Lee, K.; Jung, I.; Kim, D.; Kim, S.; et al. Directed Evolution of CRISPR-Cas9 to Increase Its Specificity. Nat. Commun. 2018, 9, 3048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, J.; Ge, S.; Lai, L. CRISPR/Cas: Advances, Limitations, and Applications for Precision Cancer Research. Front. Med. 2021, 8, 649896. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.A.; Larson, M.H.; Morsut, L.; Liu, Z.; Brar, G.A.; Torres, S.E.; Stern-Ginossar, N.; Brandman, O.; Whitehead, E.H.; Doudna, J.A.; et al. CRISPR-Mediated Modular RNA-Guided Regulation of Transcription in Eukaryotes. Cell 2013, 154, 442–451. [Google Scholar] [CrossRef] [PubMed]
- Mali, P.; Aach, J.; Stranges, P.B.; Esvelt, K.M.; Moosburner, M.; Kosuri, S.; Yang, L.; Church, G.M. CAS9 Transcriptional Activators for Target Specificity Screening and Paired Nickases for Cooperative Genome Engineering. Nat. Biotechnol. 2013, 31, 833–838. [Google Scholar] [CrossRef]
- Burns, J.C.; Friedmann, T.; Driever, W.; Burrascano, M.; Yee, J.K. Vesicular Stomatitis Virus G Glycoprotein Pseudotyped Retroviral Vectors: Concentration to Very High Titer and Efficient Gene Transfer into Mammalian and Nonmammalian Cells. Proc. Natl. Acad. Sci. USA 1993, 90, 8033–8037. [Google Scholar] [CrossRef]
- Lohmann, V.; Körner, F.; Koch, J.; Herian, U.; Theilmann, L.; Bartenschlager, R. Replication of Subgenomic Hepatitis C Virus RNAs in a Hepatoma Cell Line. Science 1999, 285, 110–113. [Google Scholar] [CrossRef]
- Joung, J.; Konermann, S.; Gootenberg, J.S.; Abudayyeh, O.O.; Platt, R.J.; Brigham, M.D.; Sanjana, N.E.; Zhang, F. Genome-Scale CRISPR-Cas9 Knockout and Transcriptional Activation Screening. Nat. Protoc. 2017, 12, 828–863. [Google Scholar] [CrossRef]
- Hoenen, T.; Groseth, A. Virus–Host Cell Interactions. Cells 2022, 11, 804. [Google Scholar] [CrossRef]
- Marceau, C.D.; Puschnik, A.S.; Majzoub, K.; Ooi, Y.S.; Brewer, S.M.; Fuchs, G.; Swaminathan, K.; Mata, M.A.; Elias, J.E.; Sarnow, P.; et al. Genetic Dissection of Flaviviridae Host Factors through Genome-Scale CRISPR Screens. Nature 2016, 535, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.L.; Cherepanova, N.A.; Bozzacco, L.; MacDonald, M.R.; Gilmore, R.; Tai, A.W. Dengue Virus Hijacks a Noncanonical Oxidoreductase Function of a Cellular Oligosaccharyltransferase Complex. mBio 2017, 8, e00939-17. [Google Scholar] [CrossRef] [PubMed]
- Labeau, A.; Simon-Loriere, E.; Hafirassou, M.-L.; Bonnet-Madin, L.; Tessier, S.; Zamborlini, A.; Dupré, T.; Seta, N.; Schwartz, O.; Chaix, M.-L.; et al. A Genome-Wide CRISPR-Cas9 Screen Identifies the Dolichol-Phosphate Mannose Synthase Complex as a Host Dependency Factor for Dengue Virus Infection. J. Virol. 2020, 94, e01751-19. [Google Scholar] [CrossRef] [PubMed]
- Shue, B.; Chiramel, A.I.; Cerikan, B.; To, T.-H.; Frölich, S.; Pederson, S.M.; Kirby, E.N.; Eyre, N.S.; Bartenschlager, R.F.W.; Best, S.M.; et al. Genome-Wide CRISPR Screen Identifies RACK1 as a Critical Host Factor for Flavivirus Replication. J. Virol. 2021, 95, e00596-21. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Muffat, J.; Omer Javed, A.; Keys, H.R.; Lungjangwa, T.; Bosch, I.; Khan, M.; Virgilio, M.C.; Gehrke, L.; Sabatini, D.M.; et al. Genome-Wide CRISPR Screen for Zika Virus Resistance in Human Neural Cells. Proc. Natl. Acad. Sci. USA 2019, 116, 9527–9532. [Google Scholar] [CrossRef] [PubMed]
- Savidis, G.; McDougall, W.M.; Meraner, P.; Perreira, J.M.; Portmann, J.M.; Trincucci, G.; John, S.P.; Aker, A.M.; Renzette, N.; Robbins, D.R.; et al. Identification of Zika Virus and Dengue Virus Dependency Factors Using Functional Genomics. Cell Rep. 2016, 16, 232–246. [Google Scholar] [CrossRef]
- Ma, H.; Dang, Y.; Wu, Y.; Jia, G.; Anaya, E.; Zhang, J.; Abraham, S.; Choi, J.-G.; Shi, G.; Qi, L.; et al. A CRISPR-Based Screen Identifies Genes Essential for West-Nile-Virus-Induced Cell Death. Cell Rep. 2015, 12, 673–683. [Google Scholar] [CrossRef]
- Zhang, R.; Miner, J.J.; Gorman, M.J.; Rausch, K.; Ramage, H.; White, J.P.; Zuiani, A.; Zhang, P.; Fernandez, E.; Zhang, Q.; et al. A CRISPR Screen Defines a Signal Peptide Processing Pathway Required by Flaviviruses. Nature 2016, 535, 164–168. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, H.; Xiao, T.; Wang, Z.; Nie, X.; Li, X.; Qian, P.; Qin, L.; Han, X.; Zhang, J.; et al. CRISPR Screening of Porcine SgRNA Library Identifies Host Factors Associated with Japanese Encephalitis Virus Replication. Nat. Commun. 2020, 11, 5178. [Google Scholar] [CrossRef]
- Dukhovny, A.; Lamkiewicz, K.; Chen, Q.; Fricke, M.; Jabrane-Ferrat, N.; Marz, M.; Jung, J.U.; Sklan, E.H. A CRISPR Activation Screen Identifies Genes That Protect against Zika Virus Infection. J. Virol. 2019, 93, e00211-19. [Google Scholar] [CrossRef] [Green Version]
- Luu, A.P.; Yao, Z.; Ramachandran, S.; Azzopardi, S.A.; Miles, L.A.; Schneider, W.M.; Hoffmann, H.-H.; Bozzacco, L.; Garcia, G.; Gong, D.; et al. A CRISPR Activation Screen Identifies an Atypical Rho GTPase That Enhances Zika Viral Entry. Viruses 2021, 13, 2113. [Google Scholar] [CrossRef] [PubMed]
- Sessions, O.M.; Barrows, N.J.; Souza-Neto, J.A.; Robinson, T.J.; Hershey, C.L.; Rodgers, M.A.; Ramirez, J.L.; Dimopoulos, G.; Yang, P.L.; Pearson, J.L.; et al. Discovery of Insect and Human Dengue Virus Host Factors. Nature 2009, 458, 1047–1050. [Google Scholar] [CrossRef] [PubMed]
- Le Sommer, C.; Barrows, N.J.; Bradrick, S.S.; Pearson, J.L.; Garcia-Blanco, M.A. G Protein-Coupled Receptor Kinase 2 Promotes Flaviviridae Entry and Replication. PLoS Negl. Trop. Dis. 2012, 6, e1820. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, M.N.; Ng, A.; Sukumaran, B.; Gilfoy, F.D.; Uchil, P.D.; Sultana, H.; Brass, A.L.; Adametz, R.; Tsui, M.; Qian, F.; et al. RNA Interference Screen for Human Genes Associated with West Nile Virus Infection. Nature 2008, 455, 242–245. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Q.; Tiwari, S.K.; Lichinchi, G.; Yau, E.H.; Hui, H.; Li, W.; Furnari, F.; Rana, T.M. Integrin Avβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy. Cell Rep. 2020, 30, 969–983.E4. [Google Scholar] [CrossRef]
- Hoffmann, H.-H.; Schneider, W.M.; Rozen-Gagnon, K.; Miles, L.A.; Schuster, F.; Razooky, B.; Jacobson, E.; Wu, X.; Yi, S.; Rudin, C.M.; et al. TMEM41B Is a Pan-Flavivirus Host Factor. Cell 2021, 184, 133–148.E20. [Google Scholar] [CrossRef]
- Scaturro, P.; Stukalov, A.; Haas, D.A.; Cortese, M.; Draganova, K.; Płaszczyca, A.; Bartenschlager, R.; Götz, M.; Pichlmair, A. An Orthogonal Proteomic Survey Uncovers Novel Zika Virus Host Factors. Nature 2018, 561, 253–257. [Google Scholar] [CrossRef]
- Shah, P.S.; Link, N.; Jang, G.M.; Sharp, P.P.; Zhu, T.; Swaney, D.L.; Johnson, J.R.; Von Dollen, J.; Ramage, H.R.; Satkamp, L.; et al. Comparative Flavivirus-Host Protein Interaction Mapping Reveals Mechanisms of Dengue and Zika Virus Pathogenesis. Cell 2018, 175, 1931–1945.E18. [Google Scholar] [CrossRef]
- Monel, B.; Compton, A.A.; Bruel, T.; Amraoui, S.; Burlaud-Gaillard, J.; Roy, N.; Guivel-Benhassine, F.; Porrot, F.; Génin, P.; Meertens, L.; et al. Zika Virus Induces Massive Cytoplasmic Vacuolization and Paraptosis-like Death in Infected Cells. EMBO J. 2017, 36, 1653–1668. [Google Scholar] [CrossRef]
- Lin, D.L.; Inoue, T.; Chen, Y.-J.; Chang, A.; Tsai, B.; Tai, A.W. The ER Membrane Protein Complex Promotes Biogenesis of Dengue and Zika Virus Non-Structural Multi-Pass Transmembrane Proteins to Support Infection. Cell Rep. 2019, 27, 1666–1674.E4. [Google Scholar] [CrossRef] [Green Version]
- Barrows, N.J.; Anglero-Rodriguez, Y.; Kim, B.; Jamison, S.F.; Le Sommer, C.; McGee, C.E.; Pearson, J.L.; Dimopoulos, G.; Ascano, M.; Bradrick, S.S.; et al. Dual Roles for the ER Membrane Protein Complex in Flavivirus Infection: Viral Entry and Protein Biogenesis. Sci. Rep. 2019, 9, 9711. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Canada, C.; Kelleher, D.J.; Gilmore, R. Cotranslational and Posttranslational N-Glycosylation of Polypeptides by Distinct Mammalian OST Isoforms. Cell 2009, 136, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.M.; Steitz, J.A. HuR and MRNA Stability. Cell. Mol. Life Sci. CMLS 2001, 58, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Gallay, P. Curing a Viral Infection by Targeting the Host: The Example of Cyclophilin Inhibitors. Antivir. Res. 2013, 99, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B.; Ohlson, M.B.; Eitson, J.L.; Kumar, A.; McDougal, M.B.; Boys, I.N.; Mar, K.B.; De La Cruz-Rivera, P.C.; Douglas, C.; Konopka, G.; et al. A CRISPR Screen Identifies IFI6 as an ER-Resident Interferon Effector That Blocks Flavivirus Replication. Nat. Microbiol. 2018, 3, 1214–1223. [Google Scholar] [CrossRef]
- Zhou, P.; Chan, B.K.C.; Wan, Y.K.; Yuen, C.T.L.; Choi, G.C.G.; Li, X.; Tong, C.S.W.; Zhong, S.S.W.; Sun, J.; Bao, Y.; et al. A Three-Way Combinatorial CRISPR Screen for Analyzing Interactions among Druggable Targets. Cell Rep. 2020, 32, 108020. [Google Scholar] [CrossRef]
- Tham, H.-W.; Balasubramaniam, V.R.; Chew, M.-F.; Ahmad, H.; Hassan, S.S. Protein-Protein Interactions between A. Aegypti Midgut and Dengue Virus 2: Two-Hybrid Screens Using the Midgut CDNA Library. J. Infect. Dev. Ctries. 2015, 9, 1338–1349. [Google Scholar] [CrossRef]
- Mairiang, D.; Zhang, H.; Sodja, A.; Murali, T.; Suriyaphol, P.; Malasit, P.; Limjindaporn, T.; Finley, R.L. Identification of New Protein Interactions between Dengue Fever Virus and Its Hosts, Human and Mosquito. PLoS ONE 2013, 8, e53535. [Google Scholar] [CrossRef]
- Lee, H.K.; Oh, Y.; Hong, J.; Lee, S.H.; Hur, J.K. Development of CRISPR Technology for Precise Single-Base Genome Editing: A Brief Review. BMB Rep. 2021, 54, 98–105. [Google Scholar] [CrossRef]
- Burmistrz, M.; Krakowski, K.; Krawczyk-Balska, A. RNA-Targeting CRISPR–Cas Systems and Their Applications. Int. J. Mol. Sci. 2020, 21, 1122. [Google Scholar] [CrossRef] [Green Version]
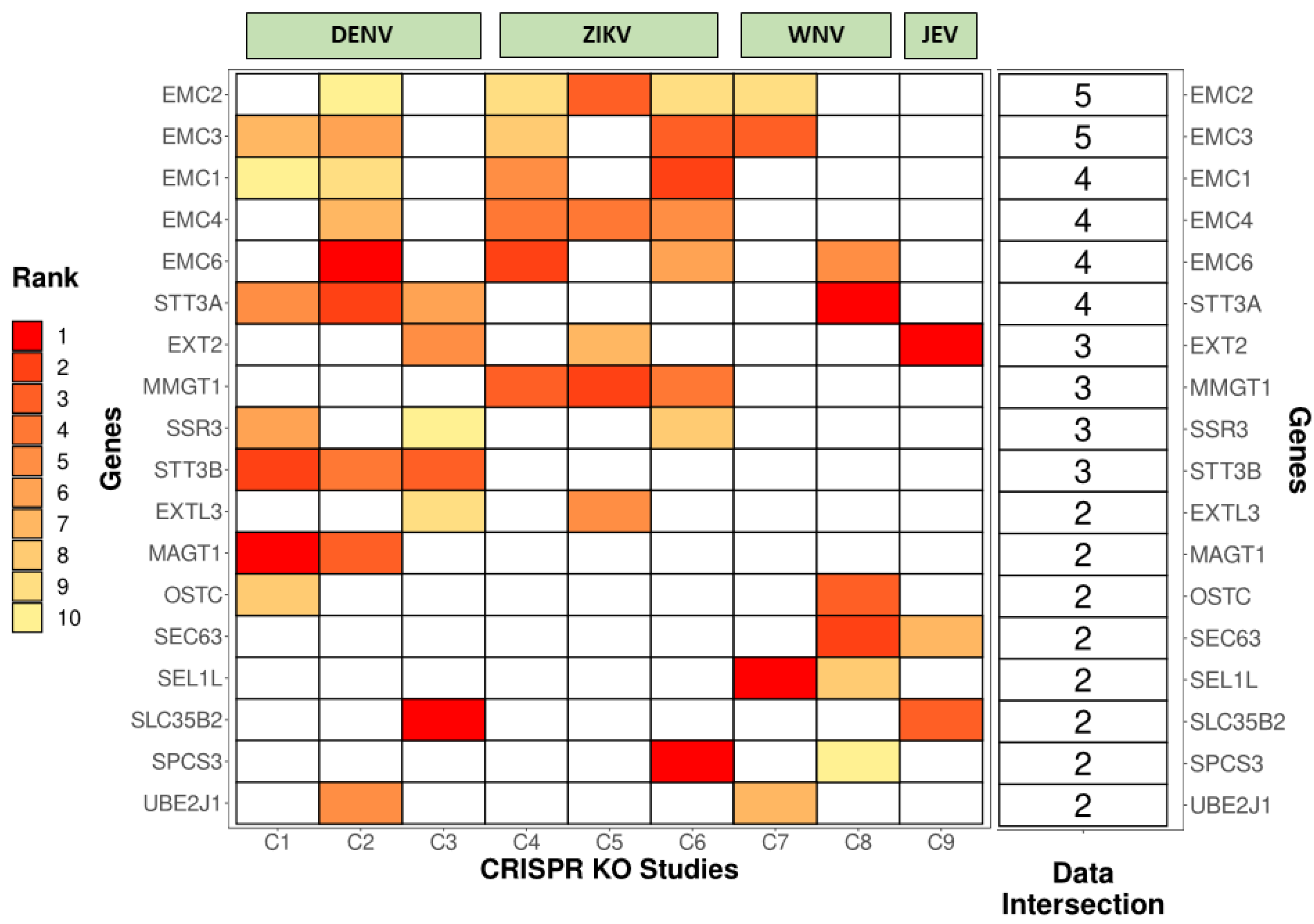

| Type of Genetic Screen | Authors and References | Cell Line | Virus Used for Challenge |
|---|---|---|---|
| CRISPR KO Screens | Caleb D. Marceau et al. (2016) [53] | HuH 7.5.1 | DENV |
| David L Lin et al. (2017) [54] | Huh 7.5.1 | ||
| Athena Labeau et al. (2020) [55] | Haploid HAP1 | ||
| Byron Shue et al. (2021) [56] | Huh 7.5 | ZIKV | |
| Yun Li et al. (2019) [57] | Human pluripotent stem cell (hPSC)-derived neural progenitors (NPs) | ||
| George Savidis et al. (2016) [58] | Huh 7.5 | ||
| H. Ma et al. (2015) [59] | 293FT cells | WNV | |
| Rong Zhang et al. (2016) [60] | 293T-Cas9 cells | ||
| Changzhi Zhao et al. (2020) [61] | Porcine kidney-15 (PK-15) cells | JEV | |
| H.-Heinrich Hoffman et al. (2020) | B3GALT6-deficient human haploid (HAP1) cells | YFV and ZIKV | |
| CRISPRa Screen | Anna Dukhovny et al. (2019) [62] | Huh-7 | ZIKV |
| Anh Phuong Luu (2021) [63] | Human STAT1−/− fibroblasts | ||
| Haploid Genetic Screens | Caleb D. Marceau et al. (2016) [53] | Haploid HAP1 | DENV |
| siRNA | George Savidis et al. (2016) [58] | HeLa | DENV |
| October M. Sessions et al. (2009) [64] | Huh-7 | ||
| Caroline Le Sommer et al. (2012) [65] | Huh-7 | YFV | |
| Manoj N Krishnan et al. (2008) [66] | HeLa | WNV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanojia, A.; Sharma, M.; Shiraz, R.; Tripathi, S. Flavivirus–Host Interaction Landscape Visualized through Genome-Wide CRISPR Screens. Viruses 2022, 14, 2164. https://doi.org/10.3390/v14102164
Kanojia A, Sharma M, Shiraz R, Tripathi S. Flavivirus–Host Interaction Landscape Visualized through Genome-Wide CRISPR Screens. Viruses. 2022; 14(10):2164. https://doi.org/10.3390/v14102164
Chicago/Turabian StyleKanojia, Aditi, Mansi Sharma, Rishad Shiraz, and Shashank Tripathi. 2022. "Flavivirus–Host Interaction Landscape Visualized through Genome-Wide CRISPR Screens" Viruses 14, no. 10: 2164. https://doi.org/10.3390/v14102164
APA StyleKanojia, A., Sharma, M., Shiraz, R., & Tripathi, S. (2022). Flavivirus–Host Interaction Landscape Visualized through Genome-Wide CRISPR Screens. Viruses, 14(10), 2164. https://doi.org/10.3390/v14102164






