Small-Molecule RAF265 as an Antiviral Therapy Acts against PEDV Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells, Virus, Antibodies and Inhibitors
2.2. Preparation of Cell Lysates and Western Blotting
2.3. Quantitative Real-Time PCR (qRT-PCR)
2.4. Confocal Microscopy Analysis
2.5. Generation of Vero-eIF4E(S209A) Cells
2.6. Production of Spike Pseudotyped Particles and Virus Entry Assay
2.7. Statistics
3. Results and Discussion
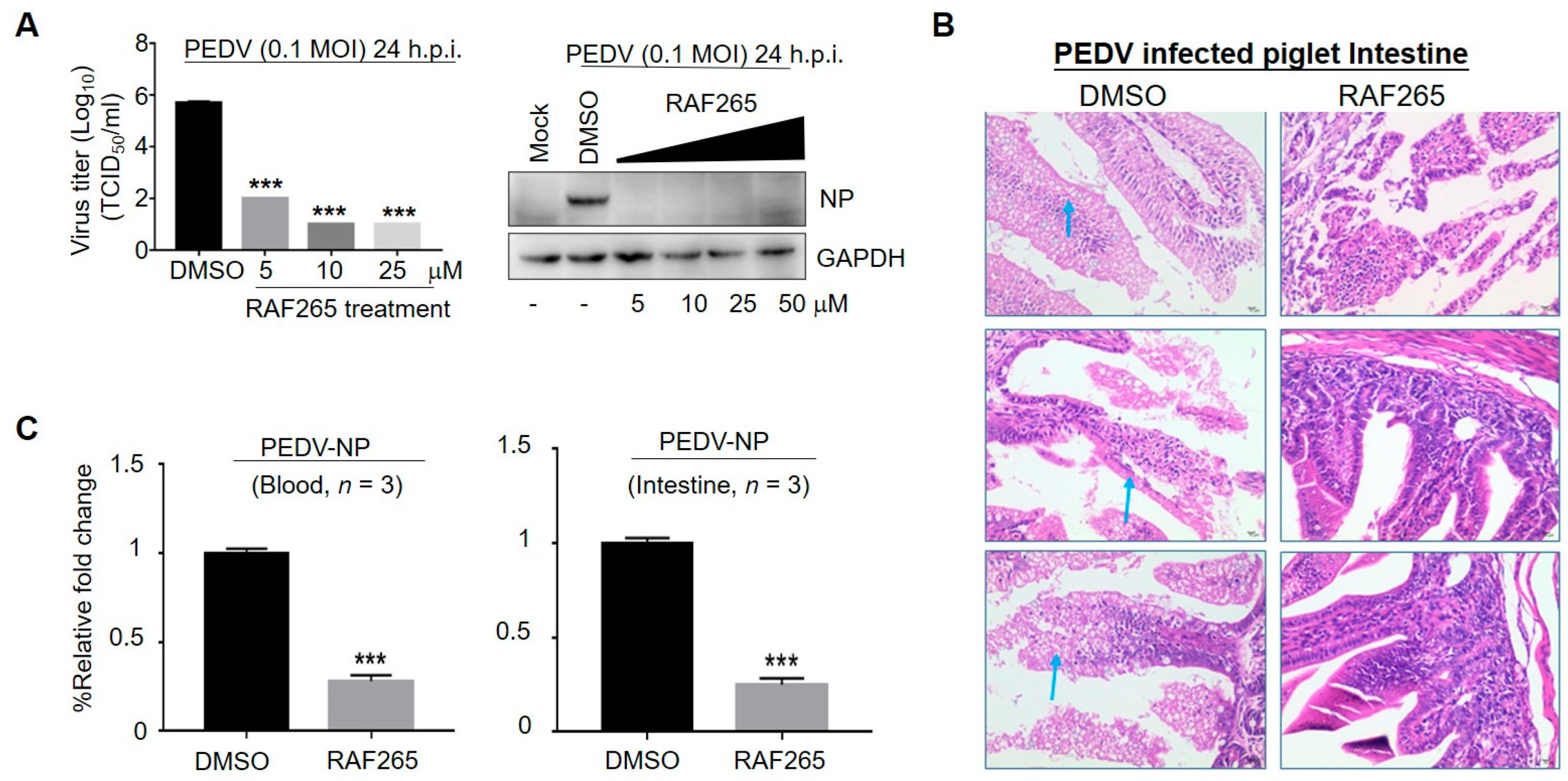
3.1. RAF265 Significantly Reduced Virus Proliferation In Vitro and In Vivo
3.2. RAF265-Mediated Actin–Myosin Arrangement Interfered with PEDV Entry
3.3. RAF265 Blocked Viral Entry of Coronavirus PEDV, SARS-CoV, and SARS-CoV-2
3.4. RAF265 Reduced the Synthesis of Viral Proteins of PEDV by Depressing p-eIF4E
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Nakagawa, K.; Lokugamage, K.G.; Makino, S. Viral and Cellular mRNA Translation in Coronavirus-Infected Cells. Adv. Virus Res. 2016, 96, 165–192. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Saif, L.J.; Wang, Q. Porcine epidemic diarrhea virus (PEDV): An update on etiology, transmission, pathogenesis, and prevention and control. Virus Res. 2020, 286, 198045. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Fang, L.; Xiao, S. Porcine epidemic diarrhea in China. Virus Res. 2016, 226, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Gerdts, V.; Zakhartchouk, A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet. Microbiol. 2017, 206, 45–51. [Google Scholar] [CrossRef]
- Tonelli, M.; Cichero, E. Fight Against H1N1 Influenza A Virus: Recent Insights Towards the Development of Druggable Compounds. Curr. Med. Chem. 2016, 23, 1802–1817. [Google Scholar] [CrossRef]
- Tonelli, M.; Naesens, L.; Gazzarrini, S.; Santucci, M.; Cichero, E.; Tasso, B.; Moroni, A.; Costi, M.P.; Loddo, R. Host dihydrofolate reductase (DHFR)-directed cycloguanil analogues endowed with activity against influenza virus and respiratory syncytial virus. Eur. J. Med. Chem. 2017, 135, 467–478. [Google Scholar] [CrossRef]
- Li, C.C.; Wang, X.J.; Wang, H.R. Repurposing host-based therapeutics to control coronavirus and influenza virus. Drug Discov. Today 2019, 24, 726–736. [Google Scholar] [CrossRef]
- Stuart, D.; Aardalen, K.; Venetsanakos, E.; Nagel, T.; Renhowe, P. RAF265 is a potent Raf kinase inhibitor with selective anti-proliferative activity in vitro and in vivo. Cancer Res. 2008, 68, 4876. [Google Scholar]
- Su, Y.; Vilgelm, A.E.; Kelley, M.C.; Hawkins, O.E.; Liu, Y.; Boyd, K.L.; Kantrow, S.; Splittgerber, R.C.; Short, S.P.; Sobolik, T.; et al. RAF265 inhibits the growth of advanced human melanoma tumors. Clin. Cancer Res. 2012, 18, 2184–2198. [Google Scholar] [CrossRef]
- Williams, T.E.; Subramanian, S.; Verhagen, J.; McBride, C.M.; Costales, A.; Sung, L.; Antonios-McCrea, W.; McKenna, M.; Louie, A.K.; Ramurthy, S.; et al. Discovery of RAF265: A Potent mut-B-RAF Inhibitor for the Treatment of Metastatic Melanoma. ACS Med. Chem. Lett. 2015, 6, 961–965. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Shi, H.; Guo, D.; Chen, J.; Shi, D.; Zhu, Q.; Zhang, X.; Feng, L. Analysis of protein expression changes of the Vero E6 cells infected with classic PEDV strain CV777 by using quantitative proteomic technique. J. Virol. Methods 2015, 218, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Li, C.C.; Wang, X.J. Three kinds of treatment with Homoharringtonine, Hydroxychloroquine or shRNA and their combination against coronavirus PEDV in vitro. Virol. J. 2020, 17, 71. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wu, Q.; Huang, L.; Yuan, C.; Wang, J.; Yang, Q. An alternative pathway of enteric PEDV dissemination from nasal cavity to intestinal mucosa in swine. Nat. Commun. 2018, 9, 3811. [Google Scholar] [CrossRef]
- Li, J.; Yuan, C.; Liu, P.; Li, Y.; Zhang, P.; Yang, Q. Red blood cells serve as a vehicle for PEDV transmission. Vet. Microbiol. 2021, 257, 109081. [Google Scholar] [CrossRef]
- Nash, T.C.; Buchmeier, M.J. Entry of mouse hepatitis virus into cells by endosomal and nonendosomal pathways. Virology 1997, 233, 1–8. [Google Scholar] [CrossRef]
- Pu, Y.; Zhang, X. Mouse hepatitis virus type 2 enters cells through a clathrin-mediated endocytic pathway independent of Eps15. J. Virol. 2008, 82, 8112–8123. [Google Scholar] [CrossRef]
- Wang, H.; Yang, P.; Liu, K.; Guo, F.; Zhang, Y.; Zhang, G.; Jiang, C. SARS coronavirus entry into host cells through a novel clathrin- and caveolae-independent endocytic pathway. Cell Res. 2008, 18, 290–301. [Google Scholar] [CrossRef]
- Zhao, S.; Gao, J.; Zhu, L.; Yang, Q. Transmissible gastroenteritis virus and porcine epidemic diarrhoea virus infection induces dramatic changes in the tight junctions and microfilaments of polarized IPEC-J2 cells. Virus Res. 2014, 192, 34–45. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, K.; Lan, Y.; Lv, X.; Hu, S.; Guan, J.; Lu, H.; Zhang, J.; Shi, J.; Yang, Y.; et al. Porcine Hemagglutinating Encephalomyelitis Virus Enters Neuro-2a Cells via Clathrin-Mediated Endocytosis in a Rab5-, Cholesterol-, and pH-Dependent Manner. J. Virol. 2017, 91, e01083-17. [Google Scholar] [CrossRef]
- Taylor, M.P.; Koyuncu, O.O.; Enquist, L.W. Subversion of the actin cytoskeleton during viral infection. Nat. Rev. Microbiol. 2011, 9, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z.; Zhang, Y.; Lin, Z.; Shi, K.; Jiu, Y. Cytoskeleton-a crucial key in host cell for coronavirus infection. J. Mol. Cell Biol. 2020, 12, 968–979. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, C. Extracellular signal-regulated kinase (ERK) activation is required for porcine epidemic diarrhea virus replication. Virology 2015, 484, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Poulikakos, P.I.; Zhang, C.; Bollag, G.; Shokat, K.M.; Rosen, N. RAF inhibitors transactivate RAF dimers and ERK signalling in cells with wild-type BRAF. Nature 2010, 464, 427–430. [Google Scholar] [CrossRef]
- Lehmann, M.J.; Sherer, N.M.; Marks, C.B.; Pypaert, M.; Mothes, W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J. Cell Biol. 2005, 170, 317–325. [Google Scholar] [CrossRef]
- Dewerchin, H.L.; Desmarets, L.M.; Noppe, Y.; Nauwynck, H.J. Myosins 1 and 6, myosin light chain kinase, actin and microtubules cooperate during antibody-mediated internalisation and trafficking of membrane-expressed viral antigens in feline infectious peritonitis virus infected monocytes. Vet. Res. 2014, 45, 17. [Google Scholar] [CrossRef]
- De Regge, N.; Nauwynck, H.J.; Geenen, K.; Krummenacher, C.; Cohen, G.H.; Eisenberg, R.J.; Mettenleiter, T.C.; Favoreel, H.W. Alpha-herpesvirus glycoprotein D interaction with sensory neurons triggers formation of varicosities that serve as virus exit sites. J. Cell Biol. 2006, 174, 267–275. [Google Scholar] [CrossRef]
- Chou, Y.Y.; Cuevas, C.; Carocci, M.; Stubbs, S.H.; Ma, M.; Cureton, D.K.; Chao, L.; Evesson, F.; He, K.; Yang, P.L.; et al. Identification and Characterization of a Novel Broad-Spectrum Virus Entry Inhibitor. J. Virol. 2016, 90, 4494–4510. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, Y.M.; Stevens, B.M.; Noble, M. Inhibition of redox/Fyn/c-Cbl pathway function by Cdc42 controls tumour initiation capacity and tamoxifen sensitivity in basal-like breast cancer cells. EMBO Mol. Med. 2013, 5, 723–736. [Google Scholar] [CrossRef]
- Park, J.E.; Cruz, D.J.; Shin, H.J. Clathrin- and serine proteases-dependent uptake of porcine epidemic diarrhea virus into Vero cells. Virus Res. 2014, 191, 21–29. [Google Scholar] [CrossRef]
- Lv, X.; Li, Z.; Guan, J.; Hu, S.; Zhang, J.; Lan, Y.; Zhao, K.; Lu, H.; Song, D.; He, H.; et al. Porcine Hemagglutinating Encephalomyelitis Virus Activation of the Integrin alpha5beta1-FAK-Cofilin Pathway Causes Cytoskeletal Rearrangement To Promote Its Invasion of N2a Cells. J. Virol. 2019, 93, e01736-18. [Google Scholar] [CrossRef]
- Yang, R.; Huang, B.; A, R.; Li, W.; Wang, W.; Deng, Y.; Tan, W. Development and effectiveness of pseudotyped SARS-CoV-2 system as determined by neutralizing efficiency and entry inhibition test in vitro. Biosaf. Health 2020, 2, 226–231. [Google Scholar] [CrossRef]
- Royall, E.; Doyle, N.; Abdul-Wahab, A.; Emmott, E.; Morley, S.J.; Goodfellow, I.; Roberts, L.O.; Locker, N. Murine norovirus 1 (MNV1) replication induces translational control of the host by regulating eIF4E activity during infection. J. Biol. Chem. 2015, 290, 4748–4758. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Y.; Yu, S.; Yang, S.; Qiu, X.; Meng, C.; Tan, L.; Song, C.; Liao, Y.; Liu, W.; Sun, Y.; et al. Newcastle Disease virus infection activates PI3K/Akt/mTOR and p38 MAPK/Mnk1 pathways to benefit viral mRNA translation via interaction of the viral NP protein and host eIF4E. PLoS Pathog. 2020, 16, e1008610. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mohr, I. Phosphorylation of eIF4E by Mnk-1 enhances HSV-1 translation and replication in quiescent cells. Genes Dev. 2004, 18, 660–672. [Google Scholar] [CrossRef] [PubMed]
- Hay, N. Mnk earmarks eIF4E for cancer therapy. Proc. Natl. Acad. Sci. USA 2010, 107, 13975–13976. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Tian, W.-J.; Li, C.-C.; Zhang, X.-Z.; Fan, K.; Li, S.-L.; Wang, X.-J. Small-Molecule RAF265 as an Antiviral Therapy Acts against PEDV Infection. Viruses 2022, 14, 2261. https://doi.org/10.3390/v14102261
Wang J, Tian W-J, Li C-C, Zhang X-Z, Fan K, Li S-L, Wang X-J. Small-Molecule RAF265 as an Antiviral Therapy Acts against PEDV Infection. Viruses. 2022; 14(10):2261. https://doi.org/10.3390/v14102261
Chicago/Turabian StyleWang, Jing, Wen-Jun Tian, Cui-Cui Li, Xiu-Zhong Zhang, Kai Fan, Song-Li Li, and Xiao-Jia Wang. 2022. "Small-Molecule RAF265 as an Antiviral Therapy Acts against PEDV Infection" Viruses 14, no. 10: 2261. https://doi.org/10.3390/v14102261
APA StyleWang, J., Tian, W.-J., Li, C.-C., Zhang, X.-Z., Fan, K., Li, S.-L., & Wang, X.-J. (2022). Small-Molecule RAF265 as an Antiviral Therapy Acts against PEDV Infection. Viruses, 14(10), 2261. https://doi.org/10.3390/v14102261





