Novel Bacillus-Infecting Bacteriophage B13—The Founding Member of the Proposed New Genus Bunatrivirus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains
2.2. Phage Isolation and Propagation
2.3. Host Range Test
2.4. Killing Assay
2.5. Thermal and pH Stability Tests
2.6. Genome Sequencing, Assembly and Sequence Analysis
2.7. The Genome Packaging Strategy
2.8. Comparative Genomics
2.9. Accession Number
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phage Isolation and Host Range
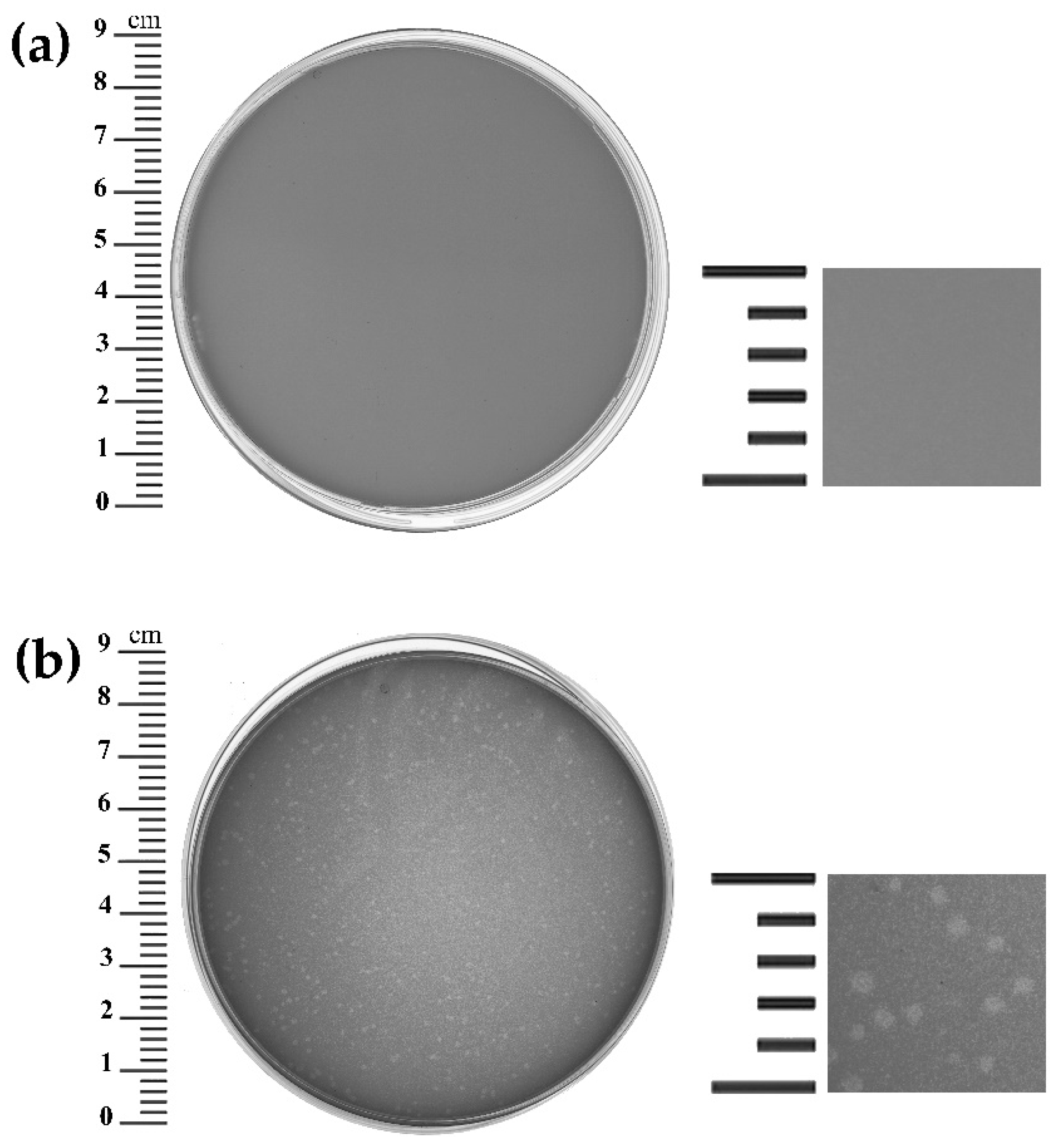
3.2. Killing Assay
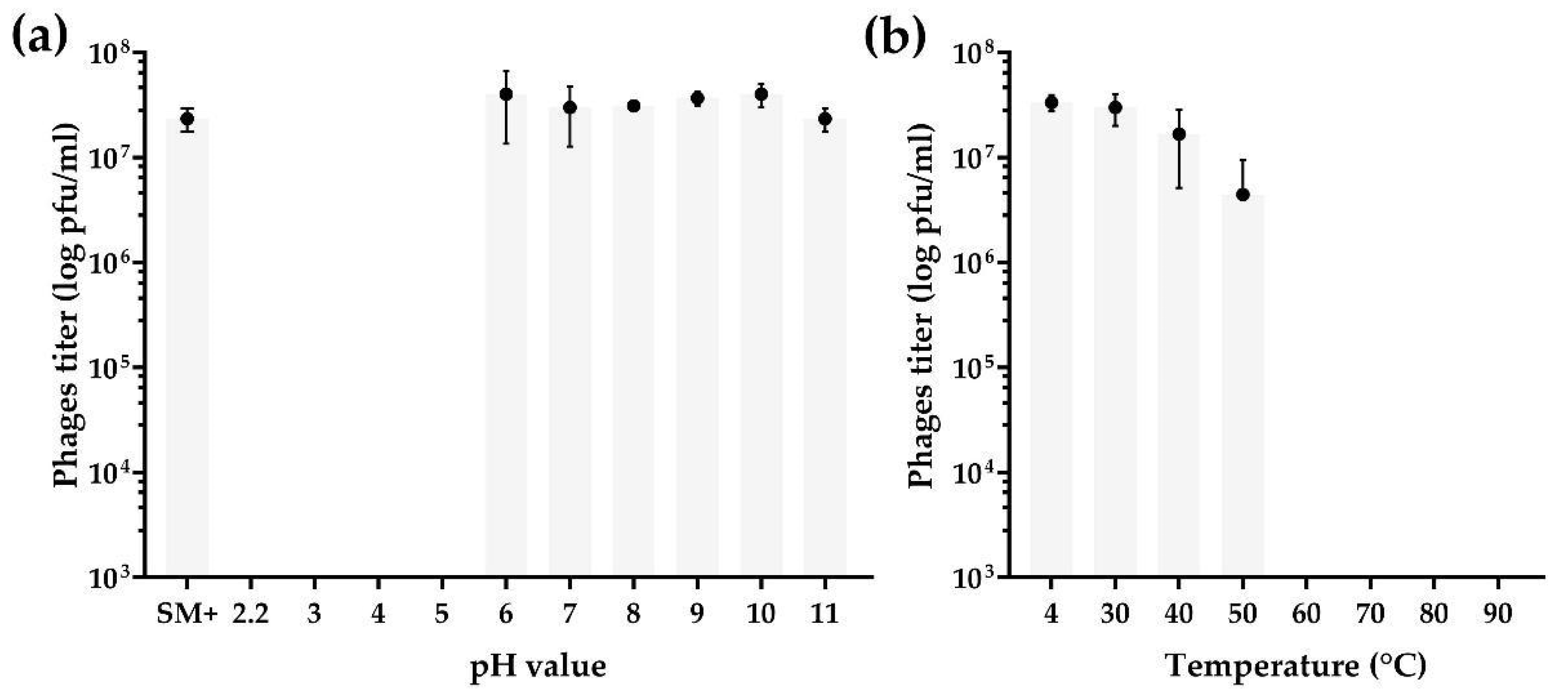
3.3. pH and Thermal Stability Tests
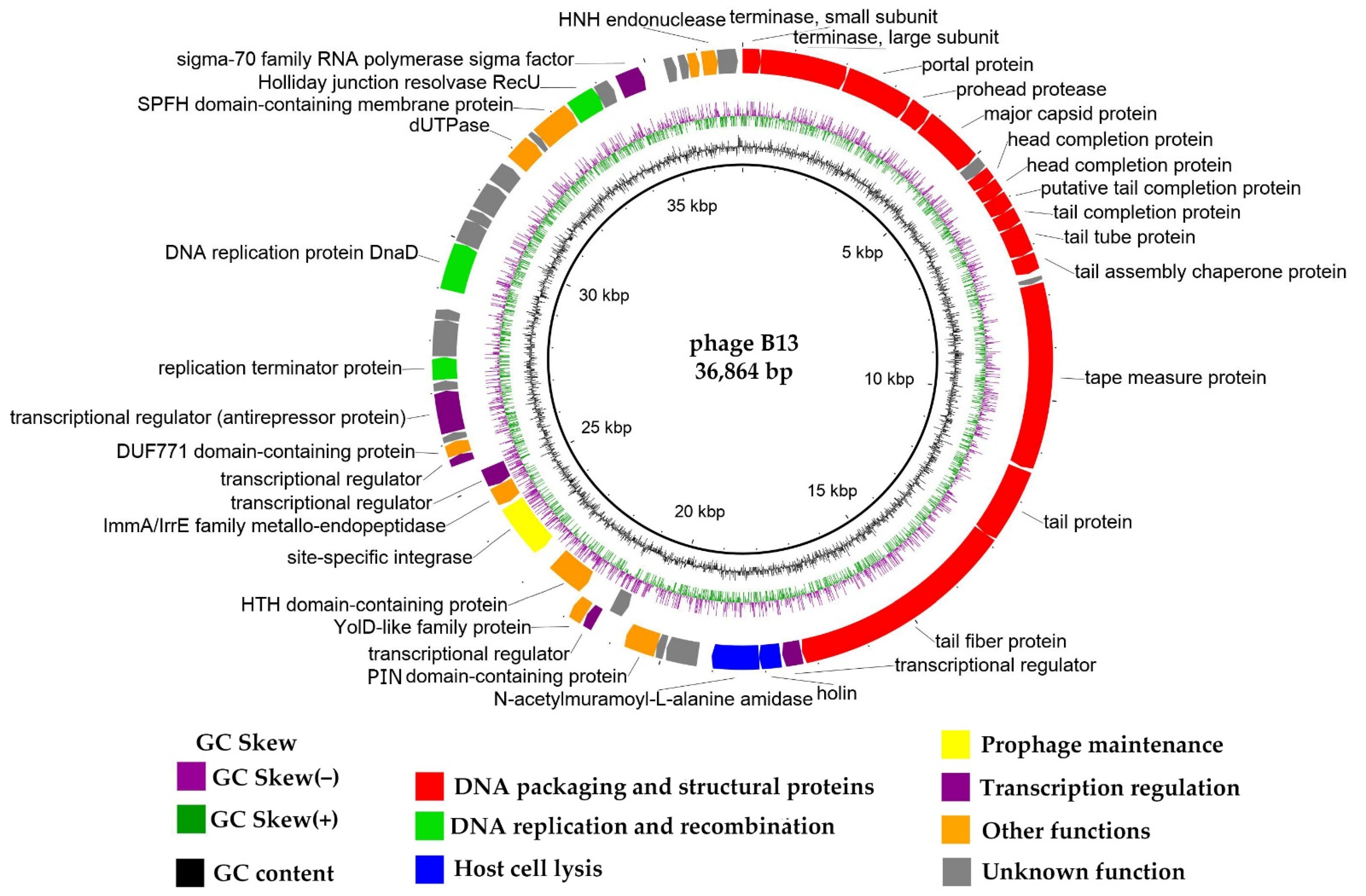
3.4. General Genome Organization
3.4.1. DNA Packaging Genes and Genome Packaging Strategy
3.4.2. Structural, Morphogenesis and Lytic Genes
3.4.3. Replication Genes
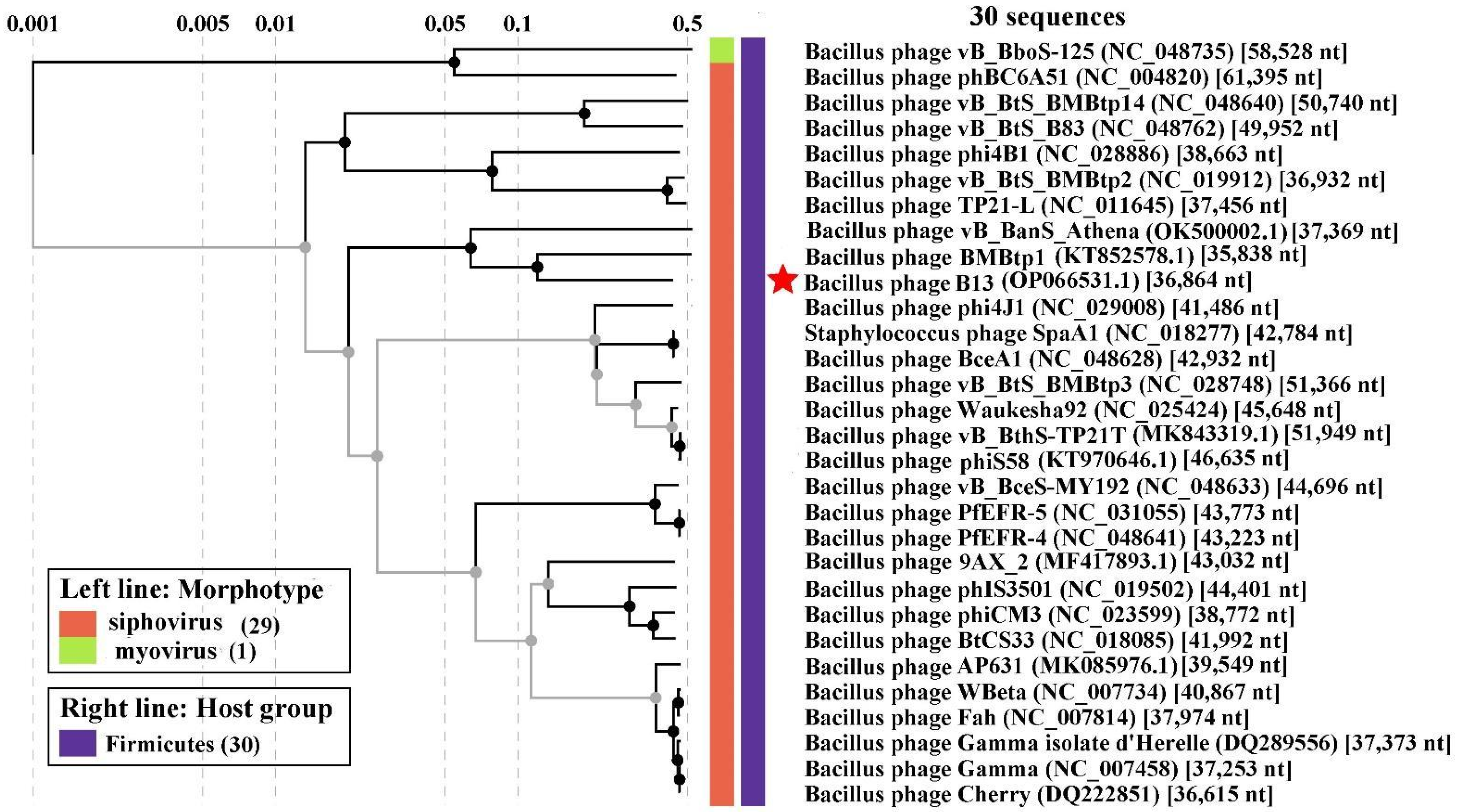
3.5. Comparative Genomics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Comeau, A.M.; Hatfull, G.F.; Krisch, H.M.; Lindell, D.; Mann, N.H.; Prangishvili, D. Exploring the Prokaryotic Virosphere. Res. Microbiol. 2008, 159, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Lefkowitz, E.J.; Dempsey, D.M.; Hendrickson, R.C.; Orton, R.J.; Siddell, S.G.; Smith, D.B. Virus Taxonomy: The Database of the International Committee on Taxonomy of Viruses (ICTV). Nucleic Acids Res. 2018, 46, D708–D717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.; Kim, Y.-C. Genome-Based Virus Taxonomy with the ICTV Database Extension. Genom. Inform. 2018, 16, e22. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.; Kropinski, A.M.; Adriaenssens, E.M. A Roadmap for Genome-Based Phage Taxonomy. Viruses 2021, 13, 506. [Google Scholar] [CrossRef]
- Santamaría, R.I.; Bustos, P.; van Cauwenberghe, J.; González, V. Hidden Diversity of Double-Stranded DNA Phages in Symbiotic Rhizobium Species. Philos. Trans. R. Soc. B Biol. Sci. 2022, 377, 20200468. [Google Scholar] [CrossRef] [PubMed]
- Benler, S.; Yutin, N.; Antipov, D.; Rayko, M.; Shmakov, S.; Gussow, A.B.; Pevzner, P.; Koonin, E.V. Thousands of Previously Unknown Phages Discovered in Whole-Community Human Gut Metagenomes. Microbiome 2021, 9, 78. [Google Scholar] [CrossRef] [PubMed]
- Dutilh, B.E.; Varsani, A.; Tong, Y.; Simmonds, P.; Sabanadzovic, S.; Rubino, L.; Roux, S.; Muñoz, A.R.; Lood, C.; Lefkowitz, E.J.; et al. Perspective on Taxonomic Classification of Uncultivated Viruses. Curr. Opin. Virol. 2021, 51, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Sansinenea, E. Bacillus thuringiensis Based Biopesticides for Integrated Crop Management. In Biopesticides; Woodhead Publishing: Sawston, UK, 2022. [Google Scholar]
- Lee, N.K.; Kim, W.S.; Paik, H.D. Bacillus Strains as Human Probiotics: Characterization, Safety, Microbiome, and Probiotic Carrier. Food Sci. Biotechnol. 2019, 28, 1297–1305. [Google Scholar] [CrossRef]
- Mingmongkolchai, S.; Panbangred, W. Bacillus Probiotics: An Alternative to Antibiotics for Livestock Production. J. Appl. Microbiol. 2018, 124, 1334–1346. [Google Scholar] [CrossRef]
- Dietrich, R.; Jessberger, N.; Ehling-Schulz, M.; Märtlbauer, E.; Granum, P.E. The Food Poisoning Toxins of Bacillus Cereus. Toxins 2021, 13, 98. [Google Scholar] [CrossRef]
- Abraha, H.B.; Kim, K.P.; Sbhatu, D.B. Bacteriophages for Detection and Control of Foodborne Bacterial Pathogens—The Case of Bacillus Cereus and Their Phages. J. Food Saf. 2021, e12906. [Google Scholar] [CrossRef]
- Ghosh, K.; Kim, K.P. Complete Nucleotide Sequence Analysis of a Novel Bacillus Subtilis-Infecting Phage, BSP38, Possibly Belonging to a New Genus in the Subfamily Spounavirinae. Arch. Virol. 2019, 164, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Furrer, A.D.; Bömeke, M.; Hoppert, M.; Hertel, R. Phage VB_BveM-Goe7 Represents a New Genus in the Subfamily Bastillevirinae. Arch. Virol. 2020, 165, 959–962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piligrimova, E.G.; Kazantseva, O.A.; Nikulin, N.A.; Shadrin, A.M. Bacillus Phage VB_BtS_b83 Previously Designated as a Plasmid May Represent a New Siphoviridae Genus. Viruses 2019, 11, 624. [Google Scholar] [CrossRef] [Green Version]
- Kazantseva, O.A.; Piligrimova, E.G.; Shadrin, A.M. VB_BcM_Sam46 and VB_BcM_Sam112, Members of a New Bacteriophage Genus with Unusual Small Terminase Structure. Sci. Rep. 2021, 11, 12173. [Google Scholar] [CrossRef]
- Cook, R.; Brown, N.; Redgwell, T.; Rihtman, B.; Barnes, M.; Clokie, M.; Stekel, D.J.; Hobman, J.; Jones, M.A.; Millard, A. INfrastructure for a PHAge REference Database: Identification of Large-Scale Biases in the Current Collection of Cultured Phage Genomes. PHAGE Ther. Appl. Res. 2021, 2, 214–223. [Google Scholar] [CrossRef]
- López-Leal, G.; Camelo-Valera, L.C.; Hurtado-Ramírez, J.M.; Verleyen, J.; Castillo-Ramírez, S.; Reyes-Muñoz, A. Mining of Thousands of Prokaryotic Genomes Reveals High Abundance of Prophages with a Strictly Narrow Host Range. Msystems 2022, 7(4), e00326-22. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A New Genome Assembly Algorithm and Its Applications to Single-Cell Sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Brettin, T.; Davis, J.J.; Disz, T.; Edwards, R.A.; Gerdes, S.; Olsen, G.J.; Olson, R.; Overbeek, R.; Parrello, B.; Pusch, G.D.; et al. RASTtk: A Modular and Extensible Implementation of the RAST Algorithm for Building Custom Annotation Pipelines and Annotating Batches of Genomes. Sci. Rep. 2015, 5, 8365. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic Local Alignment Search Tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Söding, J.; Biegert, A.; Lupas, A.N. The HHpred Interactive Server for Protein Homology Detection and Structure Prediction. Nucleic Acids Res. 2005, 33, W244–W248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laslett, D.; Canback, B. ARAGORN, a Program to Detect TRNA Genes and TmRNA Genes in Nucleotide Sequences. Nucleic Acids Res. 2004, 32, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Alikhan, N.F.; Petty, N.K.; ben Zakour, N.L.; Beatson, S.A. BLAST Ring Image Generator (BRIG): Simple Prokaryote Genome Comparisons. BMC Genom. 2011, 12, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merrill, B.D.; Ward, A.T.; Grose, J.H.; Hope, S. Software-Based Analysis of Bacteriophage Genomes, Physical Ends, and Packaging Strategies. BMC Genom. 2016, 17, 679. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547. [Google Scholar] [CrossRef]
- Li, S.; Fan, H.; An, X.; Fan, H.; Jiang, H.; Chen, Y.; Tong, Y. Scrutinizing Virus Genome Termini by High-Throughput Sequencing. PLoS ONE 2014, 9, e85806. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Wang, Y.; Tong, Y. Analyzing Genome Termini of Bacteriophage through High-Throughput Sequencing. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1681. [Google Scholar]
- Garneau, J.R.; Depardieu, F.; Fortier, L.C.; Bikard, D.; Monot, M. PhageTerm: A Tool for Fast and Accurate Determination of Phage Termini and Packaging Mechanism Using next-Generation Sequencing Data. Sci. Rep. 2017, 7, 8292. [Google Scholar] [CrossRef]
- Casjens, S.R.; Gilcrease, E.B. Determining DNA Packaging Strategy by Analysis of the Termini of the Chromosomes in Tailed-Bacteriophage Virions. Methods Mol. Biol. 2009, 502, 91–111. [Google Scholar] [CrossRef]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The Viral Proteomic Tree Server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Moreira, B.; Vinuesa, P. GET_HOMOLOGUES, a Versatile Software Package for Scalable and Robust Microbial Pangenome Analysis. Appl. Environ. Microbiol. 2013, 79, 7696–7701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, D.M.; Kannan, L.; Coleman, M.K.; Wolf, Y.I.; Sorokin, A.; Koonin, E.V.; Mushegian, A. A Low-Polynomial Algorithm for Assembling Clusters of Orthologous Groups from Intergenomic Symmetric Best Matches. Bioinformatics 2010, 26, 1481–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, D.A. Sequencing, Assembling, and Finishing Complete Bacteriophage Genomes. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; Volume 1681. [Google Scholar]
- Veesler, D.; Cambillau, C. A Common Evolutionary Origin for Tailed-Bacteriophage Functional Modules and Bacterial Machineries. Microbiol. Mol. Biol. Rev. 2011, 75, 423–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skorynina, A.V.; Piligrimova, E.G.; Kazantseva, O.A.; Kulyabin, V.A.; Baicher, S.D.; Ryabova, N.A.; Shadrin, A.M. Bacillus-Infecting Bacteriophage Izhevsk Harbors Thermostable Endolysin with Broad Range Specificity. PLoS ONE 2020, 15, e0242657. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazantseva, O.A.; Piligrimova, E.G.; Shadrin, A.M. Novel Bacillus-Infecting Bacteriophage B13—The Founding Member of the Proposed New Genus Bunatrivirus. Viruses 2022, 14, 2300. https://doi.org/10.3390/v14102300
Kazantseva OA, Piligrimova EG, Shadrin AM. Novel Bacillus-Infecting Bacteriophage B13—The Founding Member of the Proposed New Genus Bunatrivirus. Viruses. 2022; 14(10):2300. https://doi.org/10.3390/v14102300
Chicago/Turabian StyleKazantseva, Olesya A., Emma G. Piligrimova, and Andrey M. Shadrin. 2022. "Novel Bacillus-Infecting Bacteriophage B13—The Founding Member of the Proposed New Genus Bunatrivirus" Viruses 14, no. 10: 2300. https://doi.org/10.3390/v14102300




