Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Norovirus-like Particles and IgY Targets
2.2. Immunization of Laying Hens
2.3. IgY Extraction and Concentration
2.4. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
2.5. Enzyme-Linked Immunosorbent Assay: IgY in HuNoVLP-Immunized Hens’ Eggs
2.6. HuNoVLP-Targeted IgY: HuNoVLP Adherence Inhibition Assays
2.7. HuNoV Neutralization in Human Intestinal Enteroids
2.7.1. Human Jejunal Enteroid Culture
2.7.2. Human Norovirus Strains
2.7.3. Anti-HuNoV Activity of IgY Antibodies on HIE Cells
2.7.4. Determination of HuNoV Genome Copies in J2 HIE Cells
3. Results
3.1. HuNoVLPs Induce IgY Antibody Response in Hens
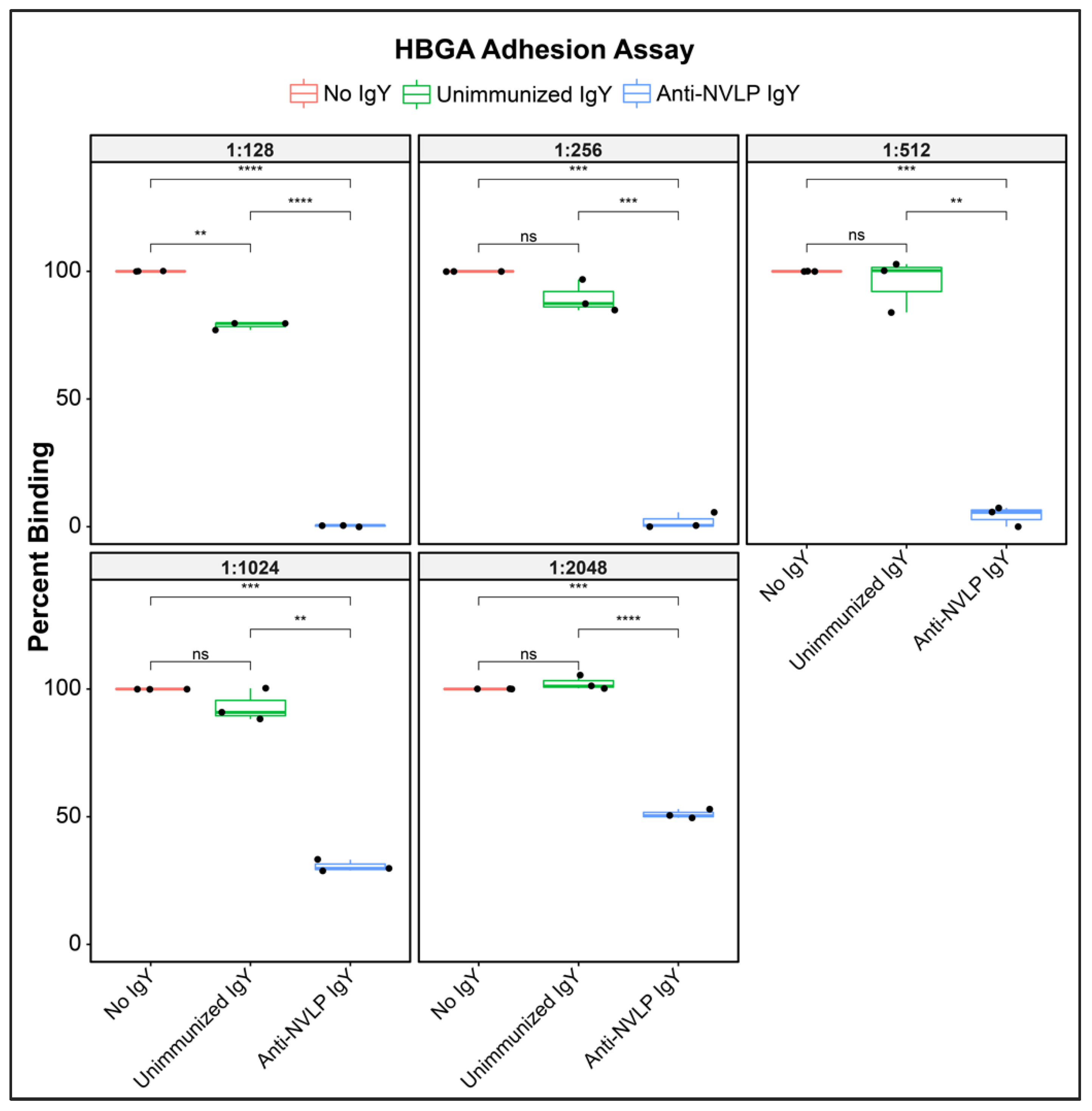
3.2. Anti-HuNoVLP IgY Inhibits Cognate VLP Adhesion to Histo-Blood Group Antigens In Vitro
3.3. Anti-HuNoV GII.4/CHDC2094 IgY Neutralizes Live GII.4[P16] Sydney HuNoV in HIE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luiz, W.B.; Rodrigues, J.F.; Crabb, J.H.; Savarino, S.J.; Ferreira, L.C. Maternal Vaccination with a Fimbrial Tip Adhesin and Passive Protection of Neonatal Mice Against Lethal Human Enterotoxigenic Escherichia coli Challenge. Infect. Immun. 2015, 83, 4555–4564. [Google Scholar] [CrossRef] [Green Version]
- Hallowell, B.D.; Parashar, U.D.; Hall, A.J. Epidemiologic Challenges in Norovirus Vaccine Development. Hum. Vaccines Immunother. 2019, 15, 1279–1283. [Google Scholar] [CrossRef]
- Netzler, N.E.; Enosi Tuipulotu, D.; White, P.A. Norovirus Antivirals: Where Are We Now? Med. Res. Rev. 2019, 39, 860–886. [Google Scholar] [CrossRef] [PubMed]
- Hameed, J.M.; Mccaffrey, R.L.; Mccoy, A.; Brannock, T.; Martin, G.J.; Scouten, W.T.; Brooks, K.; Putnam, S.D.; Riddle, M.S. Incidence, Etiology and Risk Factors for Travelers’ Diarrhea during a Hospital Ship-Based Military Humanitarian Mission: Continuing Promise 2011. PLoS ONE 2016, 11, E0154830. [Google Scholar] [CrossRef] [Green Version]
- Ashbaugh, H.R.; Early, J.M.; Johnson, M.E.; Simons, M.P.; Graf, P.C.F.; Riddle, M.S.; Swierczewski, B.E.; GTD Study Team. A Multisite Network Assessment of the Epidemiology and Etiology of Acquired Diarrhea Among U.S. Military and Western Travelers (Global Travelers’ Diarrhea Study): A Principal Role of Norovirus Among Travelers with Gastrointestinal Illness. Am. J. Trop. Med. Hyg. 2020, 103, 1855–1863. [Google Scholar] [CrossRef]
- Cardemil, C.V.; Parashar, U.D.; Hall, A.J. Norovirus Infection in Older Adults: Epidemiology, Risk Factors, and Opportunities for Prevention and Control. Infect. Dis. Clin. N. Am. 2017, 31, 839–870. [Google Scholar] [CrossRef]
- Green, K.Y. Norovirus Infection in Immunocompromised Hosts. Clin. Microbiol. Infect. 2014, 20, 717–723. [Google Scholar] [CrossRef] [Green Version]
- Bok, K.; Green, K.Y. Norovirus Gastroenteritis in Immunocompromised Patients. N. Engl. J. Med. 2012, 367, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roddie, C.; Paul, J.P.; Benjamin, R.; Gallimore, C.I.; Xerry, J.; Gray, J.J.; Peggs, K.S.; Morris, E.C.; Thomson, K.J.; Ward, K.N. Allogeneic Hematopoietic Stem Cell Transplantation and Norovirus Gastroenteritis: A Previously Unrecognized Cause of Morbidity. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2009, 49, 1061–1068. [Google Scholar] [CrossRef] [Green Version]
- Schorn, R.; Höhne, M.; Meerbach, A.; Bossart, W.; Wüthrich, R.P.; Schreier, E.; Müller, N.J.; Fehr, T. Chronic Norovirus Infection After Kidney Transplantation: Molecular Evidence for Immune-Driven Viral Evolution. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2010, 51, 307–314. [Google Scholar] [CrossRef]
- Troeger, H.; Loddenkemper, C.; Schneider, T.; Schreier, E.; Epple, H.J.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Structural and Functional Changes of the Duodenum in Human Norovirus Infection. Gut 2009, 58, 1070–1077. [Google Scholar] [CrossRef] [PubMed]
- Costantini, V.; Morantz, E.K.; Browne, H.; Ettayebi, K.; Zeng, X.L.; Atmar, R.L.; Estes, M.K.; Vinjé, J. Human Norovirus Replication in Human Intestinal Enteroids As Model to Evaluate Virus Inactivation. Emerg. Infect. Dis. 2018, 24, 1453–1464. [Google Scholar] [CrossRef] [Green Version]
- Tan, M.; Jiang, X. Vaccine Against Norovirus. Hum. Vaccines Immunother. 2014, 10, 1449–1456. [Google Scholar] [CrossRef]
- Carmona-Vicente, N.; Vila-Vicent, S.; Allen, D.; Gozalbo-Rovira, R.; Iturriza-Gómara, M.; Buesa, J.; Rodríguez-Díaz, J. Characterization of a Novel Conformational Gii.4 Norovirus Epitope: Implications for Norovirus-Host Interactions. J. Virol. 2016, 90, 7703–7714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Ma, Y.; Lu, M.; Zhang, Y.; Li, A.; Liang, X.; Li, J. Efficient Production of Human Norovirus-Specific Igy in Egg Yolks by Vaccination of Hens with a Recombinant Vesicular Stomatitis Virus Expressing Vp1 Protein. Viruses 2019, 11, 444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes-Penfield, N.W.; Ramani, S.; Estes, M.K.; Atmar, R.L. Prospects and Challenges in the Development of a Norovirus Vaccine. Clin. Ther. 2017, 39, 1537–1549. [Google Scholar] [CrossRef] [Green Version]
- Pauly, D.; Chacana, P.A.; Calzado, E.G.; Brembs, B.; Schade, R. Igy Technology: Extraction of Chicken Antibodies from Egg Yolk by Polyethylene Glycol (Peg) Precipitation. J. Vis. Exp. Jove 2011, 51, e3084. [Google Scholar] [CrossRef]
- National Centre for the Replacement Refinement and Reduction of Animals in Research. The 3rs. Available online: https://Nc3rs.Org.Uk/The-3rs (accessed on 18 February 2022).
- Vega, C.; Bok, M.; Chacana, P.; Saif, L.; Fernandez, F.; Parreno, V. Egg Yolk Igy: Protection Against Rotavirus Induced Diarrhea and Modulatory Effect On the Systemic and Mucosal Antibody Responses in Newborn Calves. Vet. Immunol. Immunopathol. 2011, 142, 156–169. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; Casswall, T.H.; Juneja, L.R.; Hoq, E.; Hossain, I.; Fuchs, G.J.; Hammarstrom, L. Randomized, Placebo-Controlled, Clinical Trial of Hyperimmunized Chicken Egg Yolk Immunoglobulin in Children with Rotavirus Diarrhea. J. Pediatr. Gastroenterol. Nutr. 2001, 32, 19–25. [Google Scholar] [CrossRef]
- Guo, R.; Wu, S.; Guan, L.; Xie, Y.; Yang, X.; Li, Z.; Zhang, Z. Dietary Multivalent Anti-Helicobacter Pylori Immunoglobulin Y Significantly Increase the H. Pylori Eradication and Improve the Clinical Symptoms in Patients. Helicobacter 2021, 26, E12843. [Google Scholar] [CrossRef]
- Diraviyam, T.; Zhao, B.; Wang, Y.; Schade, R.; Michael, A.; Zhang, X. Effect of Chicken Egg Yolk Antibodies (Igy) Against Diarrhea in Domesticated Animals: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, E97716. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Zhao, S.; He, D.; Yang, Y.; Li, Y.; Zhu, S. Preparation and Characterization of Egg Yolk Immunoglobulin Y Specific to Influenza B Virus. Antivir. Res. 2012, 93, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Y.H.; Jin, L.J.; Guo, J.; Li, X.Y.; Lu, Y.N.; Chen, J.; Xu, Y.P. Characterization of Specific Egg Yolk Immunoglobulin (Igy) Against Mastitis-Causing Escherichia coli. Vet. Microbiol. 2008, 130, 126–133. [Google Scholar] [CrossRef]
- Zhao, S.Q.; Sun, Y.M.; Zhang, C.Y.; Huang, X.Y.; Zhang, H.R.; Zhu, Z.Y. Studies On Purification of Methamidophos Monoclonal Antibodies and Comparative Immunoactivity of Purified Antibodies. Biomed. Environ. Sci. 2003, 16, 119–125. [Google Scholar] [PubMed]
- Artman, C.; Brumfield, K.D.; Khanna, S.; Goepp, J. Avian Antibodies (Igy) Targeting Spike Glycoprotein of Severe Acute Respiratory Syndrome Coronavirus 2 (Sars-Cov-2) Inhibit Receptor Binding and Viral Replication. PLoS ONE 2021, 16, E0252399. [Google Scholar] [CrossRef] [PubMed]
- Garaicoechea, L.; Aguilar, A.; Parra, G.I.; Bok, M.; Sosnovtsev, S.V.; Canziani, G.; Green, K.Y.; Bok, K.; Parreno, V. Llama Nanoantibodies with Therapeutic Potential Against Human Norovirus Diarrhea. PLoS ONE 2015, 10, E0133665. [Google Scholar] [CrossRef] [PubMed]
- Millard, S. Envstats, 2nd ed.; Springer: New York, NY, USA, 2013. [Google Scholar]
- Estes, M.K.; Ettayebi, K.; Tenge, V.R.; Murakami, K.; Karandikar, U.; Lin, S.C.; Ayyar, B.V.; Cortes-Penfield, N.W.; Haga, K.; Neill, F.H.; et al. Human Norovirus Cultivation in Nontransformed Stem Cell-Derived Human Intestinal Enteroid Cultures: Success and Challenges. Viruses 2019, 11, 638. [Google Scholar] [CrossRef] [Green Version]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of Human Noroviruses in Stem Cell-Derived Human Enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef] [Green Version]
- Ettayebi, K.; Tenge, V.R.; Cortes-Penfield, N.W.; Crawford, S.E.; Neill, F.H.; Zeng, X.-L.; Yu, X.; Ayyar, B.V.; Burrin, D.; Ramani, S.; et al. New Insights and Enhanced Human Norovirus Cultivation in Human Intestinal Enteroids. mSphere 2021, 6, E01136-20. [Google Scholar] [CrossRef]
- Mori, K.; Motomura, K.; Somura, Y.; Kimoto, K.; Akiba, T.; Sadamasu, K. Comparison of Genetic Characteristics in the Evolution of Norovirus Gii.4 and Gii.17. J. Med. Virol. 2017, 89, 1480–1484. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. Reporting and Surveillance for Norovirus: Calicinet. Available online: Https://Calicivirustypingtool.Cdc.Gov (accessed on 18 March 2022).
- Hierholzer, J.C.; Killington, R.A. Virus Isolation and Quantitation. In Virology Methods Manual; Elsevier: Amsterdam, The Netherlands, 1996; pp. 25–46. [Google Scholar] [CrossRef]
- Miura, T.; Parnaudeau, S.; Grodzki, M.; Okabe, S.; Atmar, R.L.; Le Guyader, F.S. Environmental Detection of Genogroup I, Ii, and Iv Noroviruses by Using a Generic Real-Time Reverse Transcription-Pcr Assay. Appl. Environ. Microbiol. 2013, 79, 6585–6592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magaña, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinjé, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel Gii.4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef] [Green Version]
- Alvarado, G.; Ettayebi, K.; Atmar, R.L.; Bombardi, R.G.; Kose, N.; Estes, M.K.; Crowe, J.E. Human Monoclonal Antibodies That Neutralize Pandemic Gii.4 Noroviruses. Gastroenterology 2018, 155, 1898–1907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, K.J.; Estes, M.K.; Neill, F.H.; Atmar, R.L. Use of Heat Release and an Internal Rna Standard Control in Reverse Transcription-Pcr Detection of Norwalk Virus from Stool Samples. J. Clin. Microbiol. 1997, 35, 511–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rocha-Pereira, J.; Jochmans, D.; Debing, Y.; Verbeken, E.; Nascimento, M.S.; Neyts, J. the Viral Polymerase Inhibitor 2’-C-Methylcytidine Inhibits Norwalk Virus Replication and Protects Against Norovirus-Induced Diarrhea and Mortality in a Mouse Model. J. Virol. 2013, 87, 11798–11805. [Google Scholar] [CrossRef] [Green Version]
- Atmar, R.L.; Ettayebi, K.; Ayyar, B.V.; Neill, F.H.; Braun, R.P.; Ramani, S.; Estes, M.K. Comparison of Microneutralization and Histo-Blood Group Antigen-Blocking Assays for Functional Norovirus Antibody Detection. J. Infect. Dis. 2020, 221, 739–743. [Google Scholar] [CrossRef]
- Lun, J.H.; Hewitt, J.; Yan, G.J.H.; Enosi Tuipulotu, D.; Rawlinson, W.D.; White, P.A. Recombinant Gii.P16/Gii.4 Sydney 2012 Was the Dominant Norovirus Identified in Australia and New Zealand in 2017. Viruses 2018, 10, 548. [Google Scholar] [CrossRef] [Green Version]
- Barclay, L.; Cannon, J.L.; Wikswo, M.E.; Phillips, A.R.; Browne, H.; Montmayeur, A.M.; Tatusov, R.L.; Burke, R.M.; Hall, A.J.; Vinjé, J. Emerging Novel Gii.P16 Noroviruses Associated with Multiple Capsid Genotypes. Viruses 2019, 11, 535. [Google Scholar] [CrossRef] [Green Version]
- Koromyslova, A.D.; Morozov, V.A.; Hefele, L.; Hansman, G.S. Human Norovirus Neutralized by a Monoclonal Antibody Targeting the Histo-Blood Group Antigen Pocket. J. Virol. 2019, 93, e02174-18. [Google Scholar] [CrossRef]
- Vega, C.G.; Bok, M.; Vlasova, A.N.; Chattha, K.S.; Fernandez, F.M.; Wigdorovitz, A.; Parreno, V.G.; Saif, L.J. Igy Antibodies Protect Against Human Rotavirus Induced Diarrhea in the Neonatal Gnotobiotic Piglet Disease Model. PLoS ONE 2012, 7, E42788. [Google Scholar] [CrossRef]
- Dai, Y.C.; Wang, Y.Y.; Zhang, X.F.; Tan, M.; Xia, M.; Wu, X.B.; Jiang, X.; Nie, J. Evaluation of Anti-Norovirus Igy from Egg Yolk of Chickens Immunized with Norovirus P Particles. J. Virol. Methods 2012, 186, 126–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcq, C.; Marlier, D.; Beckers, Y. Improving Adjuvant Systems for Polyclonal Egg Yolk Antibody (Igy) Production in Laying Hens in Terms of Productivity and Animal Welfare. Vet. Immunol. Immunopathol. 2015, 165, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Sears, K.T.; Tennant, S.M.; Reymann, M.K.; Simon, R.; Konstantopoulos, N.; Blackwelder, W.C.; Barry, E.M.; Pasetti, M.F. Bioactive Immune Components of Anti-Diarrheagenic Enterotoxigenic Escherichia coli Hyperimmune Bovine Colostrum Products. Clin. Vaccine Immunol. 2017, 24, e00186-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otto, W.; Najnigier, B.; Stelmasiak, T.; Robins-Browne, R.M. Randomized Control Trials Using a Tablet Formulation of Hyperimmune Bovine Colostrum to Prevent Diarrhea Caused by Enterotoxigenic Escherichia coli in Volunteers. Scand. J. Gastroenterol. 2011, 46, 862–868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gairard-Dory, A.C.; Dégot, T.; Hirschi, S.; Schuller, A.; Leclercq, A.; Renaud-Picard, B.; Gourieux, B.; Kessler, R. Clinical Usefulness of Oral Immunoglobulins in Lung Transplant Recipients with Norovirus Gastroenteritis: A Case Series. Transpl. Proc. 2014, 46, 3603–3605. [Google Scholar] [CrossRef]
- Florescu, D.F.; Hill, L.A.; Mccartan, M.A.; Grant, W. Two Cases of Norwalk Virus Enteritis Following Small Bowel Transplantation Treated with Oral Human Serum Immunoglobulin. Pediatr. Transpl. 2008, 12, 372–375. [Google Scholar] [CrossRef]
- Tashima, T. Delivery of Orally Administered Digestible Antibodies Using Nanoparticles. Int. J. Mol. Sci. 2021, 22, 3349. [Google Scholar] [CrossRef]
- Berry, C.M. Antibody Immunoprophylaxis and Immunotherapy for Influenza Virus Infection: Utilization of Monoclonal Or Polyclonal Antibodies? Hum. Vaccines Immunother. 2018, 14, 796–799. [Google Scholar] [CrossRef]
- Wolf, J.; Abzug, M.J.; Anosike, B.I.; Vora, S.B.; Waghmare, A.; Sue, P.K.; Olivero, R.M.; Oliveira, C.R.; James, S.H.; Morton, T.H.; et al. Updated Guidance On Use and Prioritization of Monoclonal Antibody Therapy for Treatment of COVID-19 in Adolescents. J. Pediatric. Infect. Dis. Soc. 2022, 11, 177–185. [Google Scholar] [CrossRef]
- Rahman, S.; Higo-Moriguchi, K.; Htun, K.W.; Taniguchi, K.; Icatlo, F.C., Jr.; Tsuji, T.; Kodama, Y.; Van Nguyen, S.; Umeda, K.; Oo, H.N.; et al. Randomized Placebo-Controlled Clinical Trial of Immunoglobulin Y as Adjunct to Standard Supportive Therapy for Rotavirus-Associated Diarrhea among Pediatric Patients. Vaccine 2012, 30, 4661–4669. [Google Scholar] [CrossRef]
- Wang, X.; Song, L.; Tan, W.; Zhao, W. Clinical Efficacy of Oral Immunoglobulin Y in Infant Rotavirus Enteritis: Systematic Review and Meta-Analysis. Medicine 2019, 98, E16100. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.L.; Williams, K.L.; Harris, E.; Alvine, T.D.; Henderson, T.; Schiltz, J.; Nilles, M.L.; Bradley, D.S. Dengue Virus Specific Igy Provides Protection Following Lethal Dengue Virus Challenge and Is Neutralizing in the Absence of Inducing Antibody Dependent Enhancement. PLoS Negl. Trop. Dis. 2017, 11, E0005721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantin, C.; Neagu, M.; Diana Supeanu, T.; Chiurciu, V.; Spandidos, D.A. Igy—Turning the Page Toward Passive Immunization in COVID-19 Infection (Review). Exp. Ther. Med. 2020, 20, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Hudson, A.F.; Jia, A.S.; Kunchur, C.R.; Song, A.J.; Tran, E.; Fisher, C.J.; Zanchi, D.; Lee, L.; Kargotich, S.; et al. Affordable Igy-Based Antiviral Prophylaxis for Resource-Limited Settings to Address Epidemic and Pandemic Risks. J. Glob. Health 2022, 12, 05009. [Google Scholar] [CrossRef]
- Bellingeri, R.V.; Picco, N.Y.; Alustiza, F.E.; Canova, J.V.; Molina, M.A.; Acevedo, D.F.; Barbero, C.; Vivas, A.B. Ph-Responsive Hydrogels to Protect Igy from Gastric Conditions: In Vitro Evaluation. J. Food Sci. Technol. 2015, 52, 3117–3122. [Google Scholar] [CrossRef] [Green Version]
- Lei, S.; Ryu, J.; Wen, K.; Twitchell, E.; Bui, T.; Ramesh, A.; Weiss, M.; Li, G.; Samuel, H.; Clark-Deener, S.; et al. Increased and Prolonged Human Norovirus Infection in Rag2/Il2rg Deficient Gnotobiotic Pigs with Severe Combined Immunodeficiency. Sci. Rep. 2016, 6, 25222. [Google Scholar] [CrossRef] [Green Version]
- Kocher, J.; Bui, T.; Giri-Rachman, E.; Wen, K.; Li, G.; Yang, X.; Liu, F.; Tan, M.; Xia, M.; Zhong, W.; et al. Intranasal P Particle Vaccine Provided Partial Cross-Variant Protection against Human Gii.4 Norovirus Diarrhea in Gnotobiotic Pigs. J. Virol. 2014, 88, 9728–9743. [Google Scholar] [CrossRef] [Green Version]
- Bui, T.; Kocher, J.; Li, Y.; Wen, K.; Li, G.; Liu, F.; Yang, X.; Leroith, T.; Tan, M.; Xia, M.; et al. Median Infectious Dose of Human Norovirus Gii.4 in Gnotobiotic Pigs Is Decreased by Simvastatin Treatment and Increased by Age. J. Gen. Virol. 2013, 94, 2005–2016. [Google Scholar] [CrossRef]
- Kovacs-Nolan, J.; Mine, Y. Microencapsulation for the Gastric Passage and Controlled Intestinal Release of Immunoglobulin Y. J. Immunol. Methods 2005, 296, 199–209. [Google Scholar] [CrossRef]
- Li, X.Y.; Jin, L.J.; Mcallister, T.A.; Stanford, K.; Xu, J.Y.; Lu, Y.N.; Zhen, Y.H.; Sun, Y.X.; Xu, Y.P. Chitosan-Alginate Microcapsules for Oral Delivery of Egg Yolk Immunoglobulin (Igy). J. Agric. Food Chem. 2007, 55, 2911–2917. [Google Scholar] [CrossRef]
- Ghosh, S.; Kumar, M.; Santiana, M.; Mishra, A.; Zhang, M.; Labayo, H.; Chibly, A.M.; Nakamura, H.; Tanaka, T.; Henderson, W.; et al. Enteric Viruses Replicate in Salivary Glands and Infect through Saliva. Nature 2022, 607, 345–350. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.K.; Parreño, V.; Schmidt, P.J.; Lei, S.; Zhong, W.; Jiang, X.; Emelko, M.B.; Yuan, L. Evaluation of the 50% Infectious Dose of Human Norovirus Cin-2 in Gnotobiotic Pigs: A Comparison of Classical and Contemporary Methods for Endpoint Estimation. Viruses 2020, 12, 955. [Google Scholar] [CrossRef] [PubMed]

| Genogroup | Sequence Name a | Sequence 5′–3′ b |
|---|---|---|
| GII.4 c | QNIFS (probe) | /56-FAM/AGC ACG TGG /ZEN/GAG GGC GAT CG/3IABkFQ/ |
| GII.4 c | QNIF2d (+) | ATG TTC AGR TGG ATG AGR TTC TCW GA |
| GII.4 c | COG2R (-) | TCG ACG CCA TCT TCA TTC ACA |
| GI d | MON 432 (+) | TGG ACI CGY GGI CCY AAY CA |
| GI d | G1SKR (-) | CCA ACC CAR CCA TTR TAC A |
| GII d | MON 431 (+) | TGG ACI AGR GGI CCY AAY CA |
| GII d | G2SKR (-) | CCR CCN GCA TRH CCR TTR TAC AT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Artman, C.; Idegwu, N.; Brumfield, K.D.; Lai, K.; Hauta, S.; Falzarano, D.; Parreño, V.; Yuan, L.; Geyer, J.D.; Goepp, J.G. Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection. Viruses 2022, 14, 2371. https://doi.org/10.3390/v14112371
Artman C, Idegwu N, Brumfield KD, Lai K, Hauta S, Falzarano D, Parreño V, Yuan L, Geyer JD, Goepp JG. Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection. Viruses. 2022; 14(11):2371. https://doi.org/10.3390/v14112371
Chicago/Turabian StyleArtman, Chad, Nnebuefe Idegwu, Kyle D. Brumfield, Ken Lai, Shirley Hauta, Darryl Falzarano, Viviana Parreño, Lijuan Yuan, James D. Geyer, and Julius G. Goepp. 2022. "Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection" Viruses 14, no. 11: 2371. https://doi.org/10.3390/v14112371
APA StyleArtman, C., Idegwu, N., Brumfield, K. D., Lai, K., Hauta, S., Falzarano, D., Parreño, V., Yuan, L., Geyer, J. D., & Goepp, J. G. (2022). Feasibility of Polyclonal Avian Immunoglobulins (IgY) as Prophylaxis against Human Norovirus Infection. Viruses, 14(11), 2371. https://doi.org/10.3390/v14112371







