Interplay between the Lung Microbiome, Pulmonary Immunity and Viral Reservoirs in People Living with HIV under Antiretroviral Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ethical Consideration
2.3. Bronchoalveolar Lavage and Blood Collection
2.4. Flow Cytometry Phenotyping of CD4 and CD8 T-cell Subsets
2.5. HIV-DNA Quantification
2.6. Sequence Data Collection and Analysis
2.6.1. Sequencing
2.6.2. Bioinformatics
2.6.3. Statistics
3. Results
3.1. Participant Characteristics
3.2. Sequence Data Summary
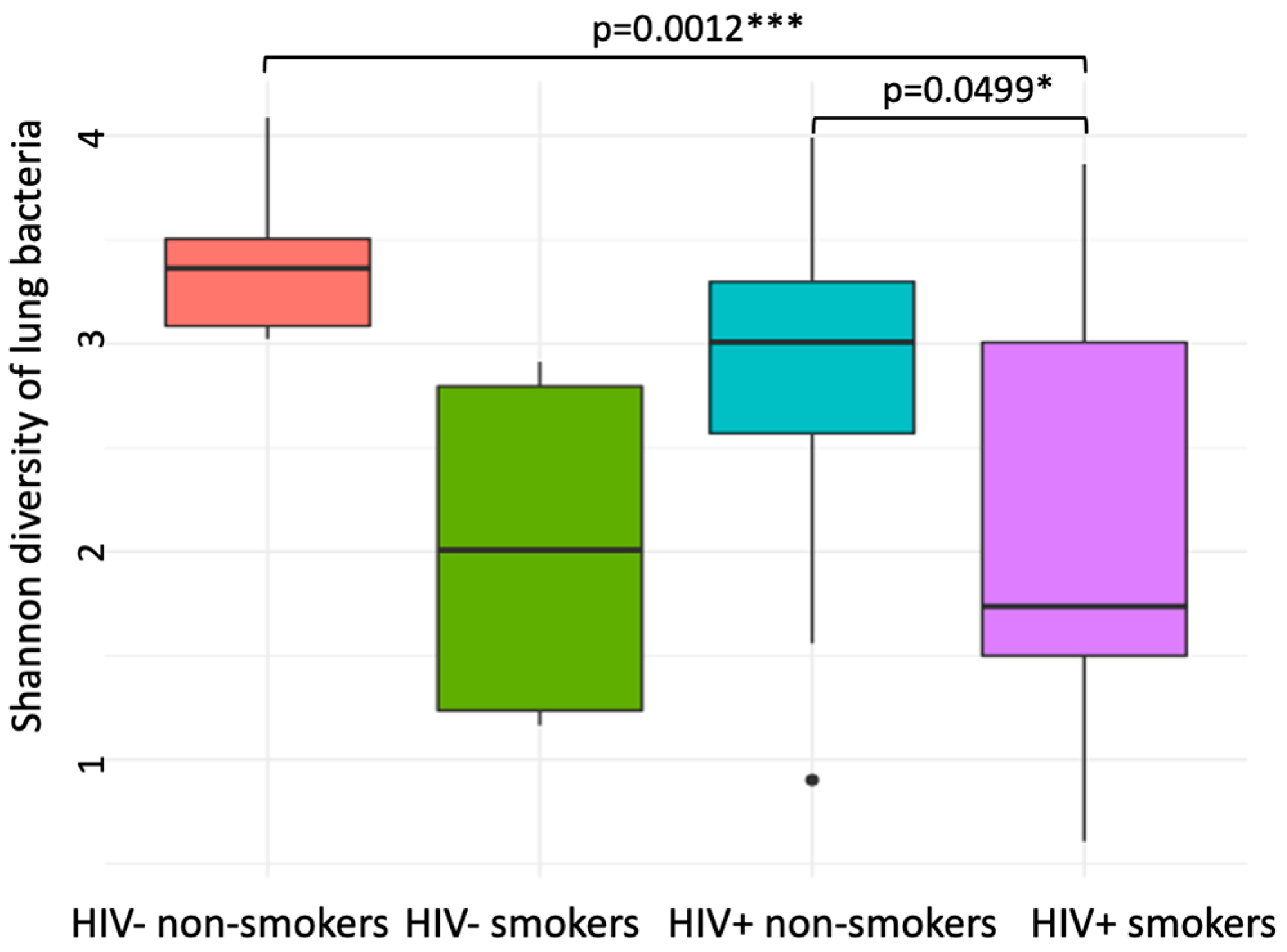
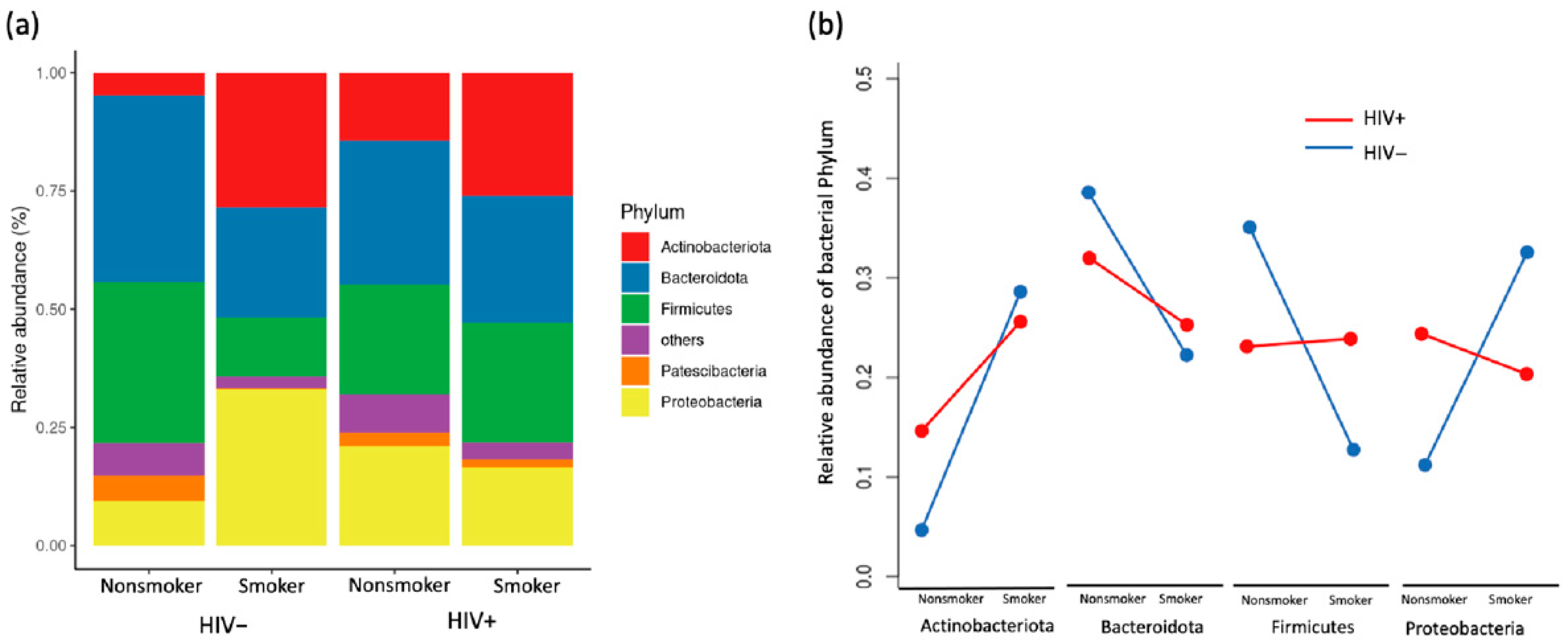
3.3. Compositional Analysis of Pulmonary Microbiota
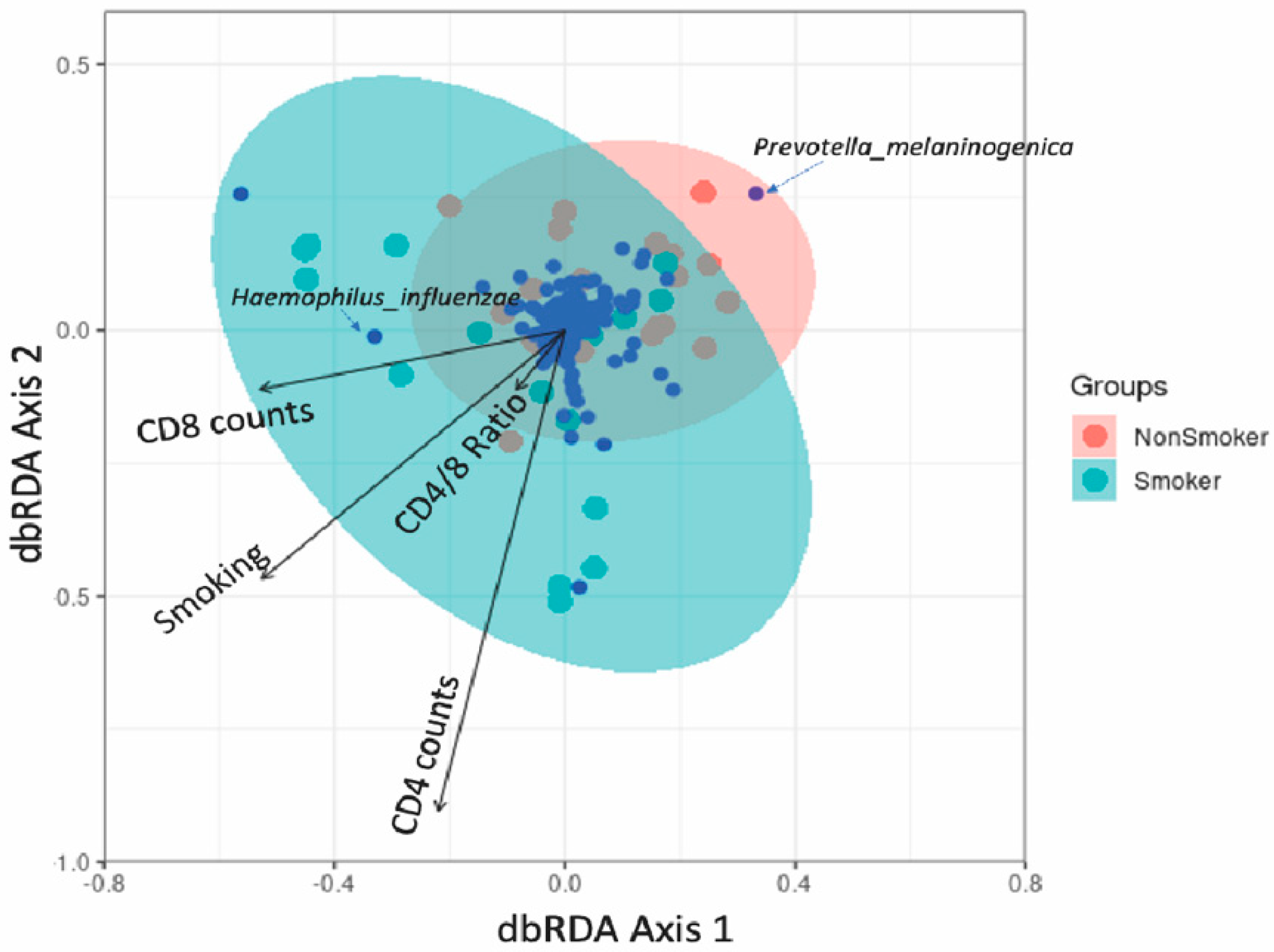
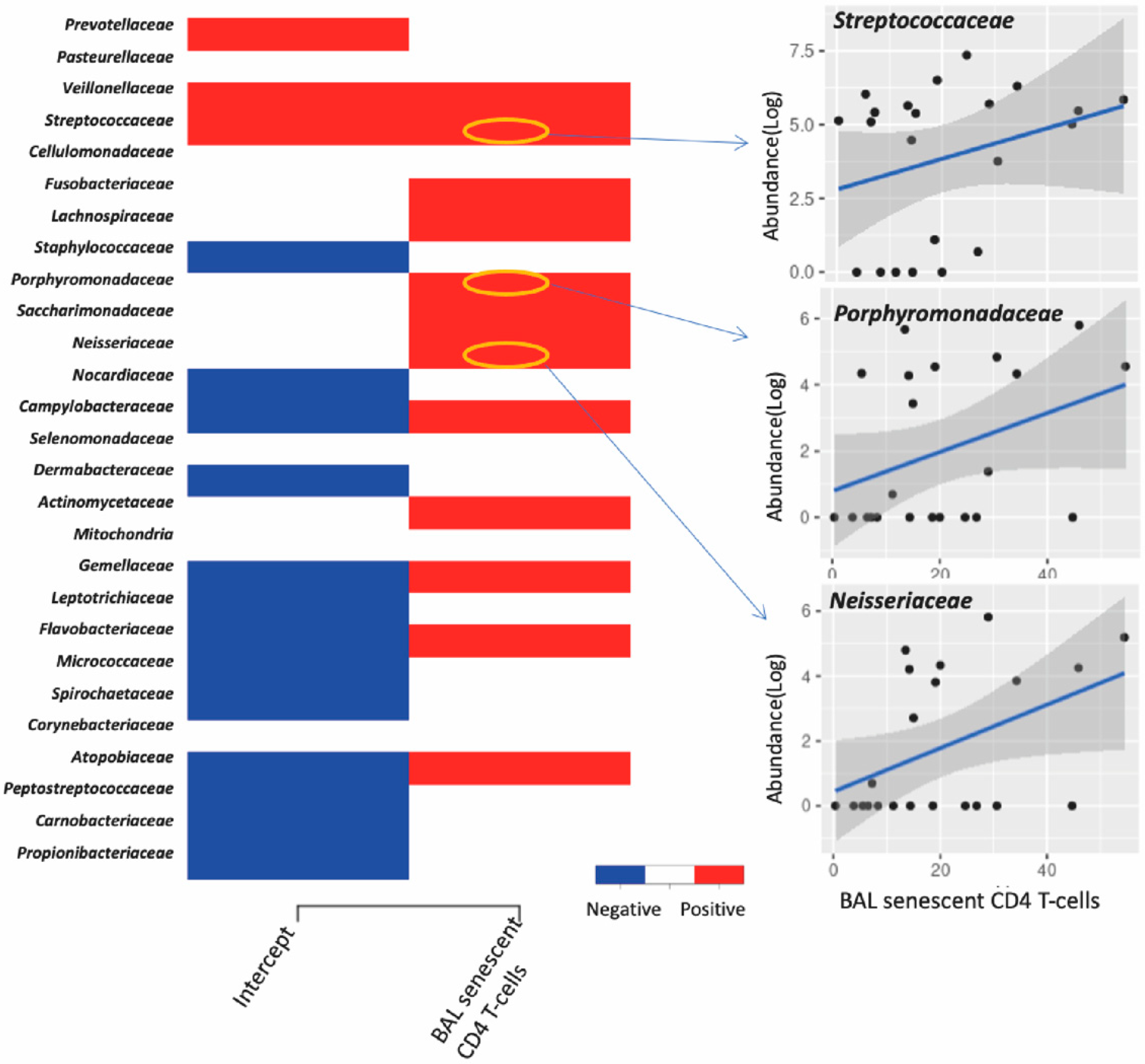
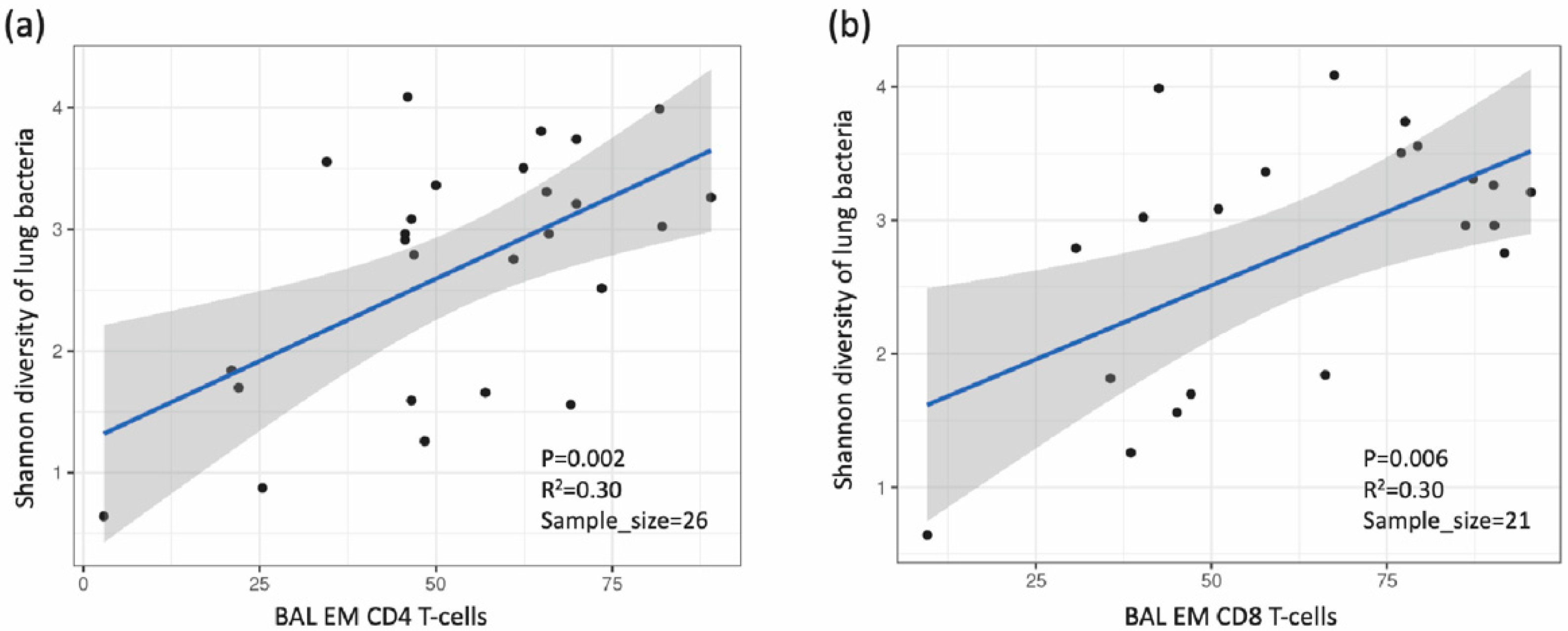
3.4. Association between Lung Microbiota, Pulmonary Immune Activation and Immunosenescence
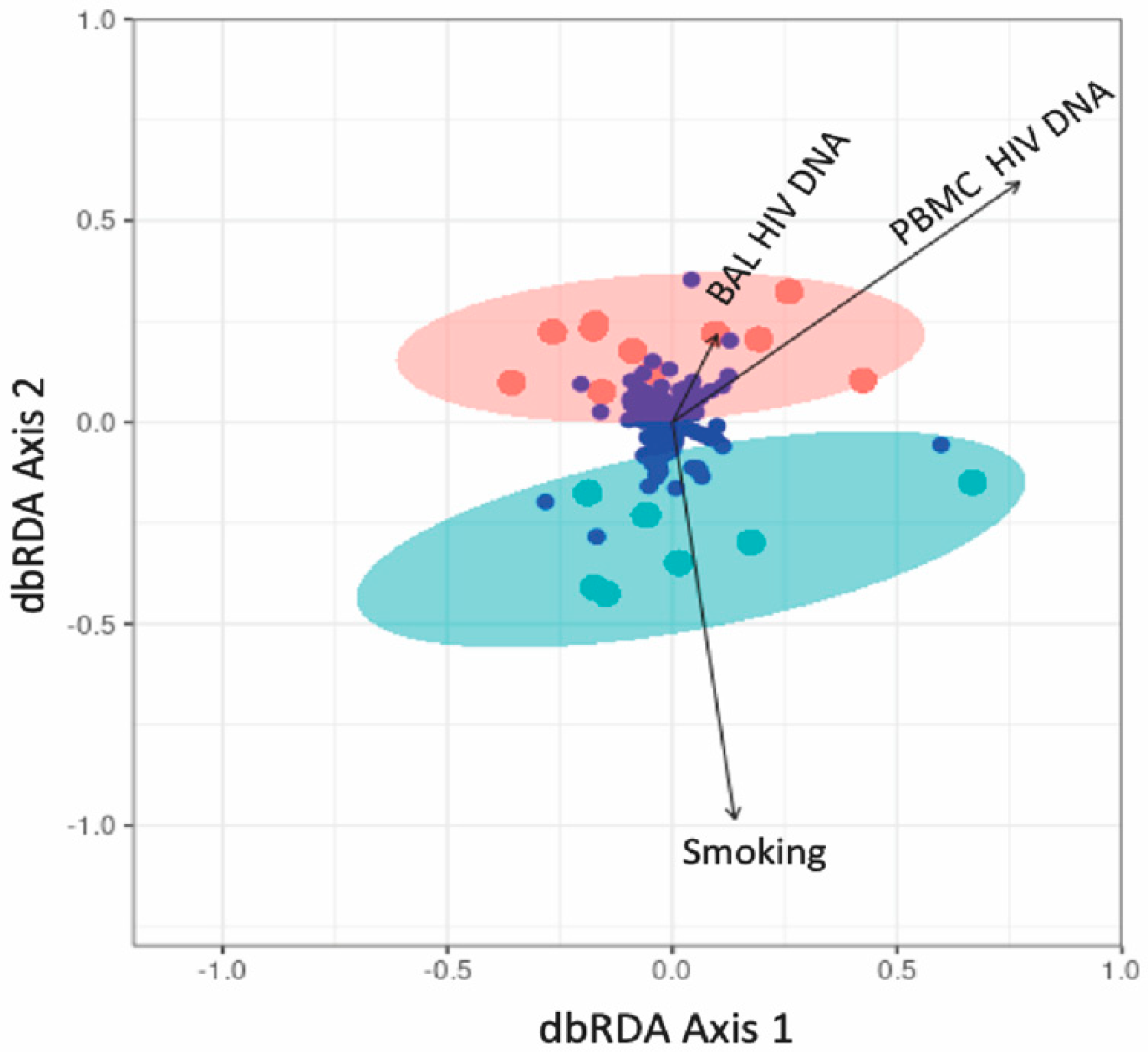
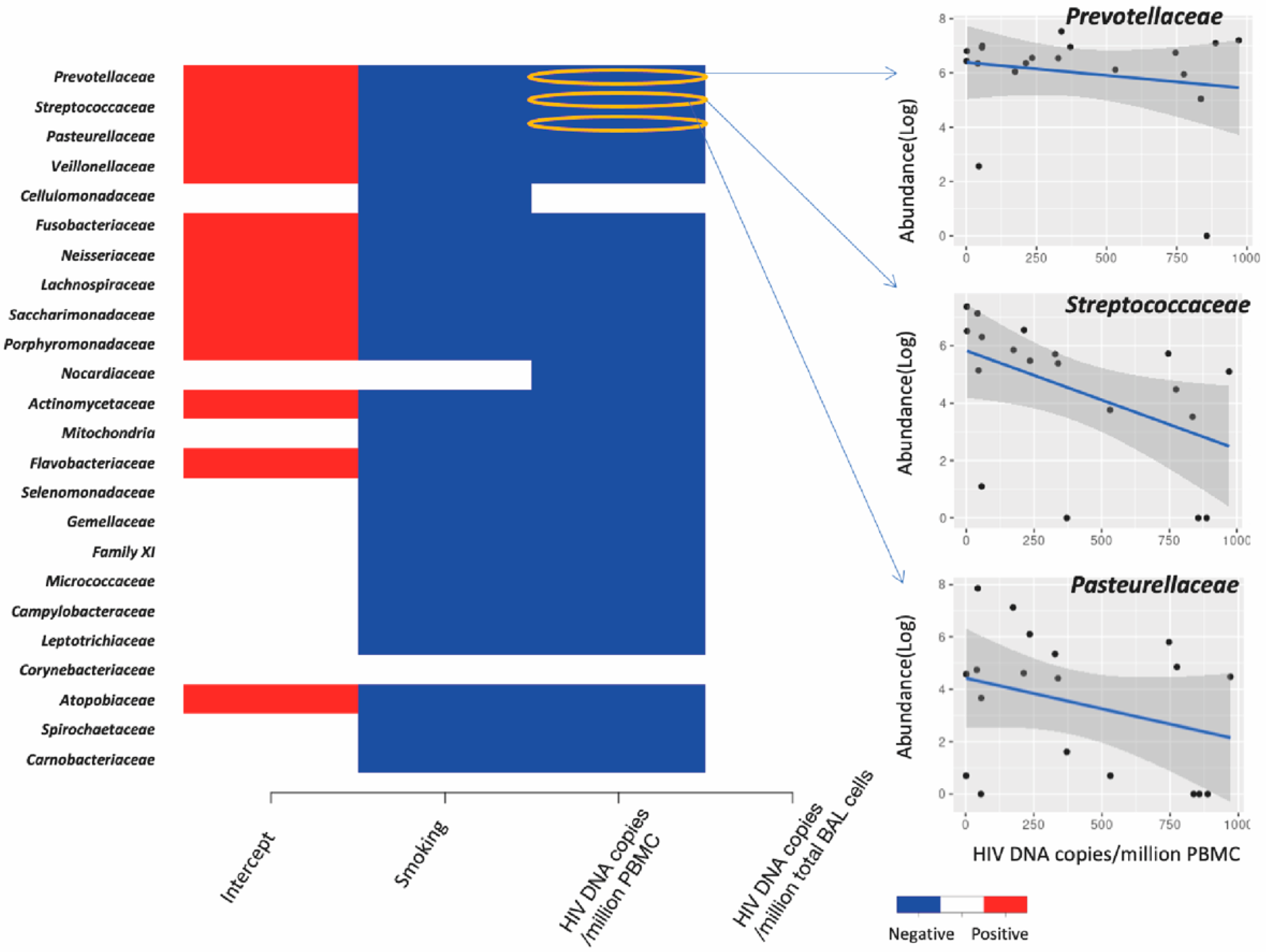
3.5. Association between Lung Microbiota and HIV Reservoir Size within the Lungs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcus, J.L.; Leyden, W.A.; Alexeeff, S.E.; Anderson, A.N.; Hechter, R.C.; Hu, H.; Lam, J.O.; Towner, W.J.; Yuan, Q.; Horberg, M.A.; et al. Comparison of Overall and Comorbidity-Free Life Expectancy Between Insured Adults With and Without HIV Infection, 2000–2016. JAMA Netw. Open 2020, 3, e207954. [Google Scholar] [CrossRef] [PubMed]
- Triplette, M.; Crothers, K.; Attia, E.F. Non-Infectious Pulmonary Diseases and HIV. Curr. HIV/AIDS Rep. 2016, 13, 140–148. [Google Scholar] [CrossRef]
- Hirani, A.; Cavallazzi, R.; Vasu, T.; Pachinburavan, M.; Kraft, W.K.; Leiby, B.; Short, W.; Desimone, J.; Squires, K.E.; Weibel, S.; et al. Prevalence of obstructive lung disease in HIV population: A cross sectional study. Respir. Med. 2011, 105, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Drummond, M.B.; Kirk, G.D. HIV-Associated obstructive lung diseases: Insights and implications for the clinician. Lancet Respir. Med. 2014, 2, 583–592. [Google Scholar] [CrossRef]
- Bichara, B.; Routy, J.-P.; Ezer, N.; Costiniuk, C.T. Primary lung cancer diagnoses in people living with HIV in a large clinical centre in Montreal, Canada over 3 decades. AIDS Care 2020, 32, 979–983. [Google Scholar] [CrossRef]
- Mena, A.; Meijide, H.; Marcos, P.J. Lung Cancer in HIV-Infected Patients. Aids Rev. 2016, 18, 138–144. [Google Scholar]
- Costiniuk, C.T.; Salahuddin, S.; Farnos, O.; Olivenstein, R.; Pagliuzza, A.; Orlova, M.; Schurr, E.; De Castro, C.; Bourbeau, J.; Routy, J.-P.; et al. HIV persistence in mucosal CD4+ T cells within the lungs of adults receiving long-term suppressive antiretroviral therapy. AIDS 2018, 32, 2279–2289. [Google Scholar] [CrossRef]
- Meziane, O.; Salahuddin, S.; Pham, T.N.Q.; Farnos, O.; Pagliuzza, A.; Olivenstein, R.; Thomson, E.; Alexandrova, Y.; Orlova, M.; Schurr, E.; et al. HIV Infection and Persistence in Pulmonary Mucosal Double Negative T Cells In Vivo. J. Virol. 2020, 94, e01788-20. [Google Scholar] [CrossRef]
- A Gilbert, J.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.; Lynch, S.V.; Knight, R. Current understanding of the human microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell. Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef]
- Sulaiman, I.; Wu, B.; Li, Y.; Scott, A.S.; Malecha, P.; Scaglione, B.; Wang, J.; Basavaraj, A.; Chung, S.; Bantis, K.; et al. Evaluation of the airway microbiome in nontuberculous mycobacteria disease. Eur. Respir. J. 2018, 52, 1800810. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lynch, S.V. The emerging relationship between the airway microbiota and chronic respiratory disease: Clinical implications. Expert Rev. Respir. Med. 2011, 5, 809–821. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sze, M.A.; Hogg, J.C.; Sin, D.D. Bacterial microbiome of lungs in COPD. Int. J. Chron. Obs. Pulmon. Dis. 2014, 9, 229–238. [Google Scholar]
- O’Dwyer, D.N.; Ashley, S.L.; Gurczynski, S.J.; Xia, M.; Wilke, C.; Falkowski, N.R.; Norman, K.C.; Arnold, K.B.; Huffnagle, G.B.; Salisbury, M.L.; et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2019, 199, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.-C.J.; Wu, B.G.; Badri, M.H.; Clemente, J.C.; Shen, N.; Meyn, P.; Li, Y.; Yie, T.-A.; Lhakhang, T.; Olsen, E.; et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am. J. Respir. Crit. Care Med. 2018, 198, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Salahuddin, S.; Thomson, E.; Méziane, O.; Farnos, O.; Pagliuzza, A.; Chomont, N.; Olivenstein, R.; Costiniuk, C.; Jenabian, M.-A. Processing of Bronchoalveolar Lavage Fluid and Matched Blood for Alveolar Macrophage and CD4+ T-cell Immunophenotyping and HIV Reservoir Assessment. J. Vis. Exp. 2019, 148, e59427. [Google Scholar] [CrossRef]
- Chelius, M.K.; Triplett, E.W. The Diversity of Archaea and Bacteria in Association with the Roots of Zea mays L. Microb. Ecol. 2001, 41, 252–263. [Google Scholar] [CrossRef]
- Callahan, B.J.; Mcmurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. vegan: Community Ecology Package. R package version 2.5-7. 2020. Available online: https://CRAN.R-project.org/package=vegan (accessed on 17 September 2022).
- Anderson, M.J.; Ellingsen, K.E.; McArdle, B.H. Multivariate dispersion as a measure of beta diversity. Ecol. Lett. 2006, 9, 683–693. [Google Scholar] [CrossRef]
- Ovaskainen, O.; Tikhonov, G.; Norberg, A.; Blanchet, F.G.; Duan, L.; Dunson, D.; Roslin, T.; Abrego, N. How to make more out of community data? A conceptual framework and its implementation as models and software. Ecol. Lett. 2017, 20, 561–576. [Google Scholar] [CrossRef]
- Hunt, P.W.; Brenchley, J.; Sinclair, E.; McCune, J.M.; Roland, M.; Page-Shafer, K.; Hsue, P.; Emu, B.; Krone, M.; Lampiris, H.; et al. Relationship between T Cell Activation and CD4+ T Cell Count in HIV-Seropositive Individuals with Undetectable Plasma HIV RNA Levels in the Absence of Therapy. J. Infect. Dis. 2008, 197, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.J.; Ruiz, N.L.; León, M.L.; Enríquez, L.M.; Velásquez, M.D.P.M.; Aguirre, J.P.O.; Bohórquez, O.M.R.; Vargas, E.A.V.; Hernández, E.D.; López, C.A.P. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef] [PubMed]
- Aberg, J.A. Aging, inflammation, and HIV infection. Top Antivir. Med. 2012, 20, 101–105. [Google Scholar]
- Budden, K.F.; Gellatly, S.L.; Wood, D.L.A.; Cooper, M.A.; Morrison, M.; Hugenholtz, P.; Hansbro, P.M. Emerging pathogenic links between microbiota and the gut–lung axis. Nat. Rev. Microbiol. 2017, 15, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kunisaki, K.M. Will expanded ART use reduce the burden of HIV-associated chronic lung disease? Curr. Opin. HIV AIDS 2014, 9, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Alexandrova, Y.; Costiniuk, C.T.; Jenabian, M.-A. Pulmonary Immune Dysregulation and Viral Persistence During HIV Infection. Front. Immunol. 2022, 12, 808722. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef]
- Gregory, A.C.; Sullivan, M.B.; Segal, L.N.; Keller, B.C. Smoking is associated with quantifiable differences in the human lung DNA virome and metabolome. Respir. Res. 2018, 19, 174. [Google Scholar] [CrossRef]
- Turek, E.M.; Cox, M.J.; Hunter, M.; Hui, J.; James, P.; Willis-Owen, S.A.; Cuthbertson, L.; James, A.; Musk, A.; Moffatt, M.F.; et al. Airway microbial communities, smoking and asthma in a general population sample. eBioMedicine 2021, 71, 103538. [Google Scholar] [CrossRef]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.; Beck, J.M.; Schloss, P.D.; Campbell, T.B.; Crothers, K.; Curtis, J.L.; Flores, S.C.; Fontenot, A.P.; Ghedin, E.; Huang, L.; et al. Comparison of the respiratory microbiome in healthy nonsmokers and smokers. Am. J. Respir. Crit. Care Med. 2013, 187, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Tsai, A.; Sze, M.A.; Vucic, E.A.; Shaipanich, T.; Harris, M.; Guillemi, S.; Yang, J.; Sinha, S.; Nislow, C.; et al. Decreased microbiome diversity in the HIV small airway epithelium. Respir. Res. 2018, 19, 140. [Google Scholar] [CrossRef] [PubMed]
- Einarsson, G.G.; Comer, D.M.; McIlreavey, L.; Parkhill, J.; Ennis, M.; Tunney, M.M.; Elborn, J.S. Community dynamics and the lower airway microbiota in stable chronic obstructive pulmonary disease, smokers and healthy non-smokers. Thorax 2016, 71, 795–803. [Google Scholar] [CrossRef]
- Flight, W.G.; Smith, A.; Paisey, C.; Marchesi, J.R.; Bull, M.J.; Norville, P.J.; Mutton, K.J.; Webb, A.K.; Bright-Thomas, R.J.; Jones, A.M.; et al. Rapid Detection of Emerging Pathogens and Loss of Microbial Diversity Associated with Severe Lung Disease in Cystic Fibrosis. J. Clin. Microbiol. 2015, 53, 2022–2029. [Google Scholar] [CrossRef]
- Alipour, M.; Zaidi, D.; Valcheva, R.; Jovel, J.; Martínez, I.; Sergi, C.; Walter, J.; Mason, A.; Wong, G.K.-S.; Dieleman, L.A.; et al. Mucosal Barrier Depletion and Loss of Bacterial Diversity are Primary Abnormalities in Paediatric Ulcerative Colitis. J. Crohns Colitis 2015, 10, 462–471. [Google Scholar] [CrossRef]
- Nylund, L.; Nermes, M.; Isolauri, E.; Salminen, S.; De Vos, W.M.; Satokari, R. Severity of atopic disease inversely correlates with intestinal microbiota diversity and butyrate-producing bacteria. Allergy 2015, 70, 241–244. [Google Scholar] [CrossRef]
- Cook, R.; Fulcher, J.A.; Tobin, N.H.; Li, F.; Lee, D.; Javanbakht, M.; Brookmeyer, R.; Shoptaw, S.; Bolan, R.; Aldrovandi, G.M.; et al. Effects of HIV viremia on the gastrointestinal microbiome of young MSM. AIDS 2019, 33, 793–804. [Google Scholar] [CrossRef]
- Twigg, H.L., III; Knox, K.S.; Zhou, J.; Crothers, K.A.; Nelson, D.E.; Toh, E.; Day, R.B.; Lin, H.; Gao, X.; Dong, Q.; et al. Effect of Advanced HIV Infection on the Respiratory Microbiome. Am. J. Respir. Crit. Care Med. 2016, 194, 226–235. [Google Scholar] [CrossRef]
- Hatano, H.; Jain, V.; Hunt, P.W.; Lee, T.-H.; Sinclair, E.; Do, T.D.; Hoh, R.; Martin, J.N.; McCune, J.M.; Hecht, F.; et al. Cell-Based Measures of Viral Persistence Are Associated With Immune Activation and Programmed Cell Death Protein 1 (PD-1)–Expressing CD4+ T cells. J. Infect. Dis. 2012, 208, 50–56. [Google Scholar] [CrossRef]
- Borgognone, A.; Noguera-Julian, M.; Oriol, B.; Noël-Romas, L.; Ruiz-Riol, M.; Guillén, Y.; Parera, M.; Casadellà, M.; Duran, C.; Puertas, M.C.; et al. Gut microbiome signatures linked to HIV-1 reservoir size and viremia control. Microbiome 2022, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Osborne, B.J.W.; Hungate, B.A.; Shahabi, K.; Huibner, S.; Lester, R.; Dwan, M.G.; Kovacs, C.; Contente-Cuomo, T.L.; Benko, E.; et al. The Semen Microbiome and Its Relationship with Local Immunology and Viral Load in HIV Infection. PLOS Pathog. 2014, 10, e1004262. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, A.R.; Zheng, Y.; Bosch, R.J.; Krishnan, S.; Rodriguez, B.; Hunt, P.W.; Plants, J.; Seth, A.; Wilson, C.C.; Deeks, S.G.; et al. Soluble Markers of Inflammation and Coagulation but Not T-Cell Activation Predict Non–AIDS-Defining Morbid Events During Suppressive Antiretroviral Treatment. J. Infect. Dis. 2014, 210, 1248–1259. [Google Scholar] [CrossRef] [PubMed]
- Pepper, M.; Jenkins, M. Origins of CD4+ effector and central memory T cells. Nat. Immunol. 2011, 12, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Chomont, N.; El-Far, M.; Ancuta, P.; Trautmann, L.; Procopio, F.A.; Yassine-Diab, B.; Boucher, G.; Boulassel, M.-R.; Ghattas, G.; Brenchley, J.M.; et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat. Med. 2009, 15, 893–900. [Google Scholar] [CrossRef]
- Kulpa, D.A.; Talla, A.; Brehm, J.H.; Ribeiro, S.P.; Yuan, S.; Bebin-Blackwell, A.-G.; Miller, M.; Barnard, R.; Deeks, S.; Hazuda, D.; et al. Differentiation into an Effector Memory Phenotype Potentiates HIV-1 Latency Reversal in CD4 + T Cells. J. Virol. 2019, 93, e00969-19. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, N.; Han, Y.; Zhu, T.; Xie, J.; Qiu, Z.; Song, X.; Li, Y.; Routy, J.-P.; Wang, J. A higher CD4/CD8 ratio correlates with an ultralow cell-associated HIV-1 DNA level in chronically infected patients on antiretroviral therapy: A case control study. BMC Infect. Dis. 2017, 17, 771. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Cellular Senescence as a Mechanism and Target in Chronic Lung Diseases. Am. J. Respir. Crit. Care Med. 2019, 200, 556–564. [Google Scholar] [CrossRef]
- Koo, H.J.; Do, K.-H.; Lee, J.B.; Alblushi, S.; Lee, S.M. Lung Cancer in Combined Pulmonary Fibrosis and Emphysema: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0161437. [Google Scholar] [CrossRef]
- Eriksson, S.; Graf, E.H.; Dahl, V.; Strain, M.C.; Yukl, S.A.; Lysenko, E.S.; Bosch, R.J.; Lai, J.; Chioma, S.; Emad, F.; et al. Comparative Analysis of Measures of Viral Reservoirs in HIV-1 Eradication Studies. PLOS Pathog. 2013, 9, e1003174. [Google Scholar] [CrossRef]
- Morris, A.M.; Huang, L.; Bacchetti, P.; Turner, J.; Hopewell, P.C.; Wallace, J.M.; Kvale, P.A.; Rosen, M.J.; Glassroth, J.; Reichman, L.B.; et al. Permanent Declines in Pulmonary Function Following Pneumonia in Human Immunodeficiency Virus-Infected Persons. Am. J. Respir. Crit. Care Med. 2000, 162, 612–616. [Google Scholar] [CrossRef] [PubMed]
- Norris, K.A.; Morris, A.; Patil, S.; Fernandes, E. Pneumocystis Colonization, Airway Inflammation, and Pulmonary Function Decline in Acquired Immunodeficiency Syndrome. Immunol. Res. 2006, 36, 175–188. [Google Scholar] [CrossRef]
- Cohen, J.; D’Agostino, L.; Wilson, J.; Tuzer, F.; Torres, C. Astrocyte Senescence and Metabolic Changes in Response to HIV Antiretroviral Therapy Drugs. Front. Aging Neurosci. 2017, 9, 281. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.; D’Agostino, L.; Tuzer, F.; Torres, C. HIV antiretroviral therapy drugs induce premature senescence and altered physiology in HUVECs. Mech. Ageing Dev. 2018, 175, 74–82. [Google Scholar] [CrossRef]
- Correa-Macedo, W.; Fava, V.M.; Orlova, M.; Cassart, P.; Olivenstein, R.; Sanz, J.; Xu, Y.Z.; Dumaine, A.; Sindeaux, R.H.; Yotova, V.; et al. Alveolar macrophages from persons living with HIV show impaired epigenetic response to Mycobacterium tuberculosis. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef]
- Phelan, J.J.; Sheedy, F.J. Phagocyte metabolism: Neutrophils have their cake but don’t eat it. Trends Immunol. 2021, 42, 846–848. [Google Scholar] [CrossRef]
- Aegerter, H.; Lambrecht, B.N.; Jakubzick, C.V. Biology of lung macrophages in health and disease. Immunity 2022, 55, 1564–1580. [Google Scholar] [CrossRef]


| Parameter | HIV+ (n = 28) | p-Value | HIV- (n = 9) | p-Value | ||
|---|---|---|---|---|---|---|
| Blood T Cell Markers and HIV-DNA | Smoker (n = 14) | Non-Smoker (n = 14) | Smoker (n = 4) | Non-Smoker (n = 5) | ||
| CD4+ T-cell count (cells/mm3; mean ± SD) | 635.0 ± 260.9 | 556.2 ± 196.6 | p = 0.6 | 753.5 ± 284.9 | 409.4 ± 151.0 | p = 0.06 |
| CD4+/8+ T-cell ratio | 0.81 ± 0.41 | 0.75 ± 0.36 | p = 0.8 | 3.50 ± 1.23 | 2.14 ± 0.42 | p = 0.2 |
| Nadir CD4+ T-cells (mean ± SD) | 185.8 ± 88.58 | 218.0 ± 109.3 | p = 0.4 | - | - | |
| %CD4+ HLADR+ CD38+ | 1.21 ± 0.88 | 1.60 ± 0.64 | p = 0.2 | 0.95 ± 0.68 | 1.67 ± 2.67 | p = 0.6 |
| %CD4+ CD57+ CD28- | 1.85 ± 2.06 | 10.21 ± 19.92 | p = 0.6 | 0.62 ± 0.37 | 0.29 ± 0.34 | p = 0.2 |
| %CD8+ HLADR+ CD38+ | 0.92 ± 0.45 | 1.77 ± 2.12 | p = 0.6 | 1.65 ± 0.78 | 2.41 ± 2.30 | p > 0.9 |
| %CD8+ CD57+ CD28- | 32.93 ± 7.92 | 22.17 ± 15.93 | p = 0.3 | 5.75 ± 1.50 | 15.92 ± 13.99 | p = 0.4 |
| PMBC HIV DNA | 198.5 ± 295.0 | 534.5 ± 322.1 | p = 0.02 | - | - | |
| BAL T cell markers and HIV-DNA | ||||||
| % CD4+ T-cells | 47.33 ± 16.63 | 52.44 ± 12.25 | p = 0.3 | 32.78 ± 22.59 | 63.30 ± 25.49 | p = 0.1 |
| % CD8+ T cells | 39.86 ± 15.46 | 40.15 ± 12.30 | p = 0.9 | 49.03 ± 21.60 | 20.44 ± 14.49 | p = 0.06 |
| %CD4+ HLADR+ CD38+ | 2.57 ± 2.22 | 4.07 ± 5.6 | p = 0.6 | 1.42 ± 2.32 | 1.63 ± 2.02 | p = 0.5 |
| %CD4+ CD57+ CD28- | 15.39 ± 10.02 | 28.01 ± 17.28 | p = 0.1 | 12.81 ± 6.54 | 19.47 ± 17.28 | p = 0.7 |
| %CD8+ HLADR+ CD38+ | 2.46 ± 1.44 | 4.13 ± 3.71 | p = 0.6 | 1.66 ± 1.77 | 11.68 ± 7.63 | p = 0.1 |
| %CD8+ CD57+ CD28- | 17.77 ± 2.66 | 35.26 ± 16.67 | p = 0.08 | 14.13 ± 10.14 | 15.90 ± 5.39 | p = 0.5 |
| BAL HIV DNA | 1070 ± 1220 | 8061 ± 14119 | p = 0.1 | - | - | |
| Microbial community data | ||||||
| Shannon index (mean ± SD) | 2.04 ± 1.10 | 2.82 ± 0.88 | p = 0.049 | 2.02 ± 0.93 | 3.41 ± 0.43 | p = 0.052 |
| Richness index (mean ± SD) | 36.35 ± 38.04 | 52.07 ± 30.79 | p = 0.24 | 27.75 ± 7.13 | 61.40 ± 36.65 | p = 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Jenabian, M.-A.; Alexandrova, Y.; Pagliuzza, A.; Olivenstein, R.; Samarani, S.; Chomont, N.; Kembel, S.W.; Costiniuk, C.T. Interplay between the Lung Microbiome, Pulmonary Immunity and Viral Reservoirs in People Living with HIV under Antiretroviral Therapy. Viruses 2022, 14, 2395. https://doi.org/10.3390/v14112395
Wang Z, Jenabian M-A, Alexandrova Y, Pagliuzza A, Olivenstein R, Samarani S, Chomont N, Kembel SW, Costiniuk CT. Interplay between the Lung Microbiome, Pulmonary Immunity and Viral Reservoirs in People Living with HIV under Antiretroviral Therapy. Viruses. 2022; 14(11):2395. https://doi.org/10.3390/v14112395
Chicago/Turabian StyleWang, Zihui, Mohammad-Ali Jenabian, Yulia Alexandrova, Amélie Pagliuzza, Ron Olivenstein, Suzanne Samarani, Nicolas Chomont, Steven W. Kembel, and Cecilia T. Costiniuk. 2022. "Interplay between the Lung Microbiome, Pulmonary Immunity and Viral Reservoirs in People Living with HIV under Antiretroviral Therapy" Viruses 14, no. 11: 2395. https://doi.org/10.3390/v14112395
APA StyleWang, Z., Jenabian, M.-A., Alexandrova, Y., Pagliuzza, A., Olivenstein, R., Samarani, S., Chomont, N., Kembel, S. W., & Costiniuk, C. T. (2022). Interplay between the Lung Microbiome, Pulmonary Immunity and Viral Reservoirs in People Living with HIV under Antiretroviral Therapy. Viruses, 14(11), 2395. https://doi.org/10.3390/v14112395






