CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections
Abstract
1. Role of Chemokines during Viral Infections
2. Structure and Function of CXCL10
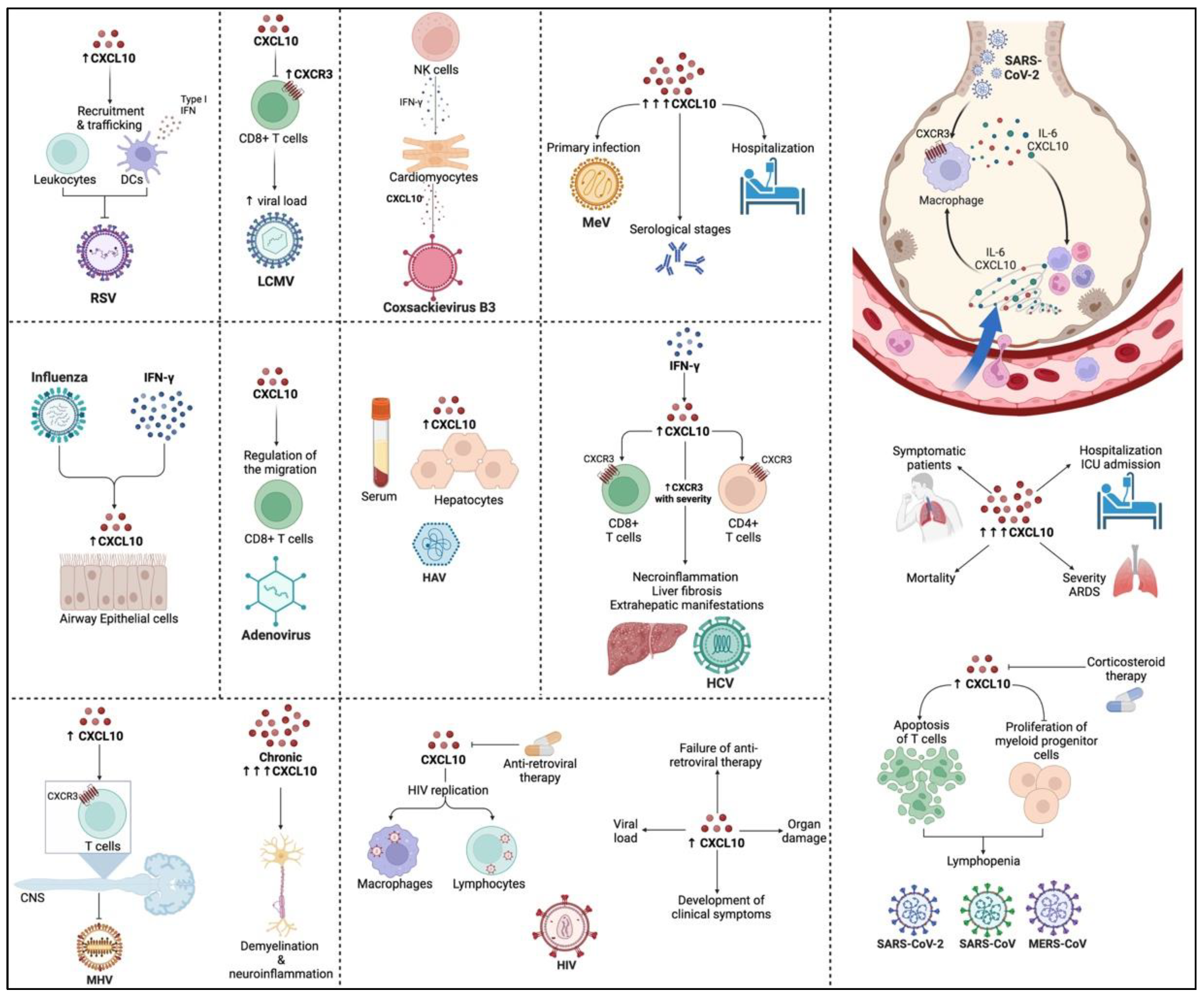
3. Role of CXCL10 in RNA Viral Infections
4. Effects of CXCL10 during DNA Viral Infections
5. Role of CXCL10 in Oncolytic Viruses
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Maghazachi, A.M.A.; Al-Aoukaty, A. Chemokines activate natural killer cells through heterotrimeric G-proteins: Implications for the treatment of AIDS and cancer. FASEB J. 1998, 12, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Nomiyama, H.; Osada, N.; Yoshie, O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. 2010, 21, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Proudfoot, A.E.; Uguccioni, M. Modulation of Chemokine Responses: Synergy and Cooperativity. Front. Immunol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Mellado, M.; Rodríguez-Frade, J.M.; Vila-Coro, A.J.; Fernández, S.; Martín de Ana, A.; Jones, D.R.; Torán, J.L.; Martínez, A.C. Chemokine receptor homo-or heterodimerization activates distinct signaling pathways. EMBO J. 2001, 20, 2497–2507. [Google Scholar] [CrossRef] [PubMed]
- Murphy, P.M.; Baggiolini, M.; Charo, I.F.; Hébert, C.A.; Horuk, R.; Matsushima, K.; Miller, L.H.; Oppenheim, J.J.; Power, C.A. International union of pharmacology. XXII. Nomenclature for chemokine receptors. Pharmacol. Rev. 2000, 52, 145–176. [Google Scholar] [PubMed]
- Kennedy, J.; Kelner, G.S.; Kleyensteuber, S.; Schall, T.J.; Weiss, M.C.; Yssel, H.; Schneider, P.V.; Cocks, B.G.; Bacon, K.B.; Zlotnik, A. Molecular cloning and functional characterization of human lymphotactin. J. Immunol. 1995, 155, 203–209. [Google Scholar] [PubMed]
- Hughes, C.E.; Nibbs, R.J.B. A guide to chemokines and their receptors. FEBS J. 2018, 285, 2944–2971. [Google Scholar] [CrossRef]
- Stone, M.J.; Hayward, J.A.; Huang, C.; Huma, E.Z.; Sanchez, J. Mechanisms of Regulation of the Chemokine-Receptor Network. Int. J. Mol. Sci. 2017, 18, 342. [Google Scholar] [CrossRef]
- Wilson, S.; Wilkinson, G.; Milligan, G. The CXCR1 and CXCR2 receptors form constitutive homo- and heterodimers selectively and with equal apparent affinities. J. Biol. Chem. 2005, 280, 28663–28674. [Google Scholar] [CrossRef]
- Sohy, D.; Yano, H.; de Nadai, P.; Urizar, E.; Guillabert, A.; Javitch, J.A.; Parmentier, M.; Springael, J.Y. Hetero-oligomerization of CCR2, CCR5, and CXCR4 and the protean effects of “selective” antagonists. J. Biol. Chem. 2009, 284, 31270–31279. [Google Scholar] [CrossRef]
- Petkovic, V.; Moghini, C.; Paoletti, S.; Uguccioni, M.; Gerber, B. Eotaxin-3/CCL26 is a natural antagonist for CC chemokine receptors 1 and 5. A human chemokine with a regulatory role. J. Biol. Chem. 2004, 279, 23357–23363. [Google Scholar] [CrossRef] [PubMed]
- Nibbs, R.J.; Graham, G.J. Immune regulation by atypical chemokine receptors. Nat. Rev. Immunol. 2013, 13, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Bachelerie, F.; Ben-Baruch, A.; Burkhardt, A.M.; Combadiere, C.; Farber, J.M.; Graham, G.J.; Horuk, R.; Sparre-Ulrich, A.H.; Locati, M.; Luster, A.D.; et al. International Union of Basic and Clinical Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol. Rev. 2013, 66, 1–79. [Google Scholar] [CrossRef] [PubMed]
- Cardona, A.E.; Sasse, M.E.; Liu, L.; Cardona, S.M.; Mizutani, M.; Savarin, C.; Hu, T.; Ransohoff, R.M. Scavenging roles of chemokine receptors: Chemokine receptor deficiency is associated with increased levels of ligand in circulation and tissues. Blood 2008, 112, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Fukuma, N.; Akimitsu, N.; Hamamoto, H.; Kusuhara, H.; Sugiyama, Y.; Sekimizu, K. A role of the Duffy antigen for the maintenance of plasma chemokine concentrations. Biochem. Biophys. Res. Commun. 2003, 303, 137–139. [Google Scholar] [CrossRef]
- Lee, J.S.; Frevert, C.W.; Wurfel, M.M.; Peiper, S.C.; Wong, V.A.; Ballman, K.K.; Ruzinski, J.T.; Rhim, J.S.; Martin, T.R.; Goodman, R.B. Duffy antigen facilitates movement of chemokine across the endothelium in vitro and promotes neutrophil transmigration in vitro and in vivo. J. Immunol. 2003, 170, 5244–5251. [Google Scholar] [CrossRef] [PubMed]
- Pruenster, M.; Mudde, L.; Bombosi, P.; Dimitrova, S.; Zsak, M.; Middleton, J.; Richmond, A.; Graham, G.J.; Segerer, S.; Nibbs, R.J.; et al. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat. Immunol. 2009, 10, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Khalil, B.A.; Maghazachi, A.A. Chemokines and chemokine receptors. In Encyclopedia of Infection and Immunity; Rezaei, N., Ed.; Elsevier: Oxford, UK, 2022; pp. 193–205. [Google Scholar]
- Kleist, A.B.; Getschman, A.E.; Ziarek, J.J.; Nevins, A.M.; Gauthier, P.A.; Chevigné, A.; Szpakowska, M.; Volkman, B.F. New paradigms in chemokine receptor signal transduction: Moving beyond the two-site model. Biochem. Pharmacol. 2016, 114, 53–68. [Google Scholar] [CrossRef]
- Salazar-Mather, T.P.; Hokeness, K.L. Cytokine and chemokine networks: Pathways to antiviral defense. Curr. Top. Microbiol. Immunol. 2006, 303, 29–46. [Google Scholar] [CrossRef]
- Decalf, J.R.M.; Fernandes, S.; Longman, R.; Ahloulay, M.; Audat, F.O.; Lefrerre, F.O.; Rice, C.M.; Pol, S.; Albert, M.L. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J. Exp. Med. 2007, 204, 2423–2437. [Google Scholar] [CrossRef]
- Mahalingam, S.; Friedland, J.S.; Heise, M.T.; Rulli, N.E.; Meanger, J.; Lidbury, B.A. Chemokines and viruses: Friends or foes? Trends Microbiol. 2003, 11, 383–391. [Google Scholar] [CrossRef]
- Murphy, P.M. Viral exploitation and subversion of the immune system through chemokine mimicry. Nat. Immunol. 2001, 2, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Loetscher, M.; Loetscher, P.; Brass, N.; Meese, E.; Moser, B. Lymphocyte-specific chemokine receptor CXCR3: Regulation, chemokine binding and gene localization. Eur. J. Immunol. 1998, 28, 3696–3705. [Google Scholar] [CrossRef]
- Qin, S.; Rottman, J.B.; Myers, P.; Kassam, N.; Weinblatt, M.; Loetscher, M.; Koch, A.E.; Moser, B.; Mackay, C.R. The chemokine receptors CXCR3 and CCR5 mark subsets of T cells associated with certain inflammatory reactions. J. Clin. Investig. 1998, 101, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Sallusto, F.; Lenig, D.; Mackay, C.R.; Lanzavecchia, A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J. Exp. Med. 1998, 187, 875–883. [Google Scholar] [CrossRef]
- Wang, L.; Knudsen, E.; Jin, Y.; Gessani, S.; Maghazachi, A.A. Lysophospholipids and chemokines activate distinct signal transduction pathways in T helper 1 and T helper 2 cells. Cell Signal. 2004, 16, 991–1000. [Google Scholar] [CrossRef]
- Kelsen, S.G.; Aksoy, M.O.; Yang, Y.; Shahabuddin, S.; Litvin, J.; Safadi, F.; Rogers, T.J. The chemokine receptor CXCR3 and its splice variant are expressed in human airway epithelial cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L584–L591. [Google Scholar] [CrossRef]
- Lasagni, L.; Francalanci, M.; Annunziato, F.; Lazzeri, E.; Giannini, S.; Cosmi, L.; Sagrinati, C.; Mazzinghi, B.; Orlando, C.; Maggi, E.; et al. An alternatively spliced variant of CXCR3 mediates the inhibition of endothelial cell growth induced by IP-10, Mig, and I-TAC, and acts as functional receptor for platelet factor 4. J. Exp. Med. 2003, 197, 1537–1549. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Yates, C.C.; Wells, A. IP-10 blocks vascular endothelial growth factor-induced endothelial cell motility and tube formation via inhibition of calpain. Circ. Res. 2006, 98, 617–625. [Google Scholar] [CrossRef]
- Bonecchi, R.; Locati, M.; Galliera, E.; Vulcano, M.; Sironi, M.; Fra, A.M.; Gobbi, M.; Vecchi, A.; Sozzani, S.; Haribabu, B.; et al. Differential Recognition and Scavenging of Native and Truncated Macrophage-Derived Chemokine (Macrophage-Derived Chemokine/CC Chemokine Ligand 22) by the D6 Decoy Receptor. J. Immunol. 2004, 172, 4972. [Google Scholar] [CrossRef]
- Chevigné, A.; Janji, B.; Meyrath, M.; Reynders, N.; D’Uonnolo, G.; Uchański, T.; Xiao, M.; Berchem, G.; Ollert, M.; Kwon, Y.-J.; et al. CXCL10 Is an Agonist of the CC Family Chemokine Scavenger Receptor ACKR2/D6. Cancers 2021, 13, 1054. [Google Scholar] [CrossRef] [PubMed]
- Dyer, K.D.; Percopo, C.M.; Fischer, E.R.; Gabryszewski, S.J.; Rosenberg, H.F. Pneumoviruses infect eosinophils and elicit MyD88-dependent release of chemoattractant cytokines and interleukin-6. Blood 2009, 114, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Ravetch, J.V. Biochemical characterization of a gamma interferon-inducible cytokine (IP-10). J. Exp. Med. 1987, 166, 1084–1097. [Google Scholar] [CrossRef]
- Lo, B.K.; Yu, M.; Zloty, D.; Cowan, B.; Shapiro, J.; McElwee, K.J. CXCR3/ligands are significantly involved in the tumorigenesis of basal cell carcinomas. Am. J. Pathol. 2010, 176, 2435–2446. [Google Scholar] [CrossRef]
- Angiolillo, A.L.; Sgadari, C.; Taub, D.D.; Liao, F.; Farber, J.M.; Maheshwari, S.; Kleinman, H.K.; Reaman, G.H.; Tosato, G. Human interferon-inducible protein 10 is a potent inhibitor of angiogenesis in vivo. J. Exp. Med. 1995, 182, 155–162. [Google Scholar] [CrossRef]
- Persano, L.; Crescenzi, M.; Indraccolo, S. Anti-angiogenic gene therapy of cancer: Current status and future prospects. Mol. Aspects Med. 2007, 28, 87–114. [Google Scholar] [CrossRef]
- Belperio, J.A.; Keane, M.P.; Arenberg, D.A.; Addison, C.L.; Ehlert, J.E.; Burdick, M.D.; Strieter, R.M. CXC chemokines in angiogenesis. J. Leukoc. Biol. 2000, 68, 1–8. [Google Scholar] [CrossRef]
- Strieter, R.M.; Polverini, P.J.; Kunkel, S.L.; Arenberg, D.A.; Burdick, M.D.; Kasper, J.; Dzuiba, J.; Van Damme, J.; Walz, A.; Marriott, D.; et al. The functional role of the ELR motif in CXC chemokine-mediated angiogenesis. J. Biol. Chem. 1995, 270, 27348–27357. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, G.J.; Holloway, D.E.; Colvin, R.A.; Campanella, G.K.; Papageorgiou, A.C.; Luster, A.D.; Acharya, K.R. Crystal structures of oligomeric forms of the IP-10/CXCL10 chemokine. Structure 2003, 11, 521–532. [Google Scholar] [CrossRef]
- Ohmori, Y.; Hamilton, T.A. Cooperative interaction between interferon (IFN) stimulus response element and kappa B sequence motifs controls IFN gamma- and lipopolysaccharide-stimulated transcription from the murine IP-10 promoter. J. Biol. Chem. 1993, 268, 6677–6688. [Google Scholar] [CrossRef]
- Majumder, S.; Zhou, L.Z.; Chaturvedi, P.; Babcock, G.; Aras, S.; Ransohoff, R.M. Regulation of human IP-10 gene expression in astrocytoma cells by inflammatory cytokines. J. Neurosci. Res. 1998, 54, 169–180. [Google Scholar] [CrossRef]
- Varley, C.L.; Armitage, S.; Hassanshahiraviz, G.; Dickson, A.J. Regulation of the C-X-C chemokine, mob-1, gene expression in primary rat hepatocytes. Cytokine 2003, 23, 64–75. [Google Scholar] [CrossRef]
- Romagnani, P.; Lazzeri, E.; Lasagni, L.; Mavilia, C.; Beltrame, C.; Francalanci, M.; Rotondi, M.; Annunziato, F.; Maurenzig, L.; Cosmi, L.; et al. IP-10 and Mig production by glomerular cells in human proliferative glomerulonephritis and regulation by nitric oxide. J. Am. Soc. Nephrol. 2002, 13, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Logsdon, C.D. Cholecystokinin induction of mob-1 chemokine expression in pancreatic acinar cells requires NF-kappaB activation. Am. J. Physiol. 1999, 277, C74–C82. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Logsdon, C.D. CCK stimulates mob-1 expression and NF-kappaB activation via protein kinase C and intracellular Ca(2+). Am. J. Physiol. Cell Physiol. 2000, 278, C344–C351. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Lee, Z.H.; Song, Y.W. CXCL10 and autoimmune diseases. Autoimmun. Rev. 2009, 8, 379–383. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Stiles, J.K. The emerging role of CXCL10 in cancer (Review). Oncol. Lett. 2011, 2, 583–589. [Google Scholar] [CrossRef]
- Mee, J.B.; Johnson, C.M.; Morar, N.; Burslem, F.; Groves, R.W. The psoriatic transcriptome closely resembles that induced by interleukin-1 in cultured keratinocytes: Dominance of innate immune responses in psoriasis. Am. J. Pathol. 2007, 171, 32–42. [Google Scholar] [CrossRef]
- Elemam, N.M.; Hannawi, S.; Maghazachi, A.A. Role of chemokines and chemokine receptors in rheumatoid arthritis. Immunotargets Ther. 2020, 9, 43–56. [Google Scholar] [CrossRef]
- Trifilo, M.J.; Montalto-Morrison, C.; Stiles, L.N.; Hurst, K.R.; Hardison, J.L.; Manning, J.E.; Masters, P.S.; Lane, T.E. CXC chemokine ligand 10 controls viral infection in the central nervous system: Evidence for a role in innate immune response through recruitment and activation of natural killer cells. J. Virol. 2004, 78, 585–594. [Google Scholar] [CrossRef]
- Deng, G.; Zhou, G.; Zhang, R.; Zhai, Y.; Zhao, W.; Yan, Z.; Deng, C.; Yuan, X.; Xu, B.; Dong, X.; et al. Regulatory polymorphisms in the promoter of CXCL10 gene and disease progression in male hepatitis B virus carriers. Gastroenterology 2008, 134, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Haeberle, H.A.; Kuziel, W.A.; Dieterich, H.J.; Casola, A.; Gatalica, Z.; Garofalo, R.P. Inducible expression of inflammatory chemokines in respiratory syncytial virus-infected mice: Role of MIP-1alpha in lung pathology. J. Virol. 2001, 75, 878–890. [Google Scholar] [CrossRef]
- Tripp, R.A.; Jones, L.; Anderson, L.J. Respiratory syncytial virus G and/or SH glycoproteins modify CC and CXC chemokine mRNA expression in the BALB/c mouse. J. Virol. 2000, 74, 6227–6229. [Google Scholar] [CrossRef]
- Schneider, D.; Ganesan, S.; Comstock, A.T.; Meldrum, C.A.; Mahidhara, R.; Goldsmith, A.M.; Curtis, J.L.; Martinez, F.J.; Hershenson, M.B.; Sajjan, U. Increased cytokine response of rhinovirus-infected airway epithelial cells in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2010, 182, 332–340. [Google Scholar] [CrossRef]
- Mihm, S.; Schweyer, S.; Ramadori, G. Expression of the chemokine IP-10 correlates with the accumulation of hepatic IFN-gamma and IL-18 mRNA in chronic hepatitis C but not in hepatitis B. J. Med. Virol. 2003, 70, 562–570. [Google Scholar] [CrossRef]
- Mahanty, S.; Gupta, M.; Paragas, J.; Bray, M.; Ahmed, R.; Rollin, P.E. Protection from lethal infection is determined by innate immune responses in a mouse model of Ebola virus infection. Virology 2003, 312, 415–424. [Google Scholar] [CrossRef]
- Nightingale, Z.D.; Patkar, C.; Rothman, A.L. Viral replication and paracrine effects result in distinct, functional responses of dendritic cells following infection with dengue 2 virus. J. Leukoc. Biol. 2008, 84, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Warke, R.V.; Becerra, A.; Zawadzka, A.; Schmidt, D.J.; Martin, K.J.; Giaya, K.; Dinsmore, J.H.; Woda, M.; Hendricks, G.; Levine, T.; et al. Efficient dengue virus (DENV) infection of human muscle satellite cells upregulates type I interferon response genes and differentially modulates MHC I expression on bystander and DENV-infected cells. J. Gen. Virol. 2008, 89, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Covaleda, L.; Fuller, F.J.; Payne, S.L. EIAV S2 enhances pro-inflammatory cytokine and chemokine response in infected macrophages. Virology 2010, 397, 217–223. [Google Scholar] [CrossRef]
- Glass, W.G.; Subbarao, K.; Murphy, B.; Murphy, P.M. Mechanisms of host defense following severe acute respiratory syndrome-coronavirus (SARS-CoV) pulmonary infection of mice. J. Immunol. 2004, 173, 4030–4039. [Google Scholar] [CrossRef]
- Tsunoda, I.; Lane, T.E.; Blackett, J.; Fujinami, R.S. Distinct roles for IP-10/CXCL10 in three animal models, Theiler’s virus infection, EAE, and MHV infection, for multiple sclerosis: Implication of differing roles for IP-10. Mult. Scler. 2004, 10, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.E.; de Lemos, C.; Moos, T.; Christensen, J.P.; Thomsen, A.R. CXCL10 is the key ligand for CXCR3 on CD8+ effector T cells involved in immune surveillance of the lymphocytic choriomeningitis virus-infected central nervous system. J. Immunol. 2006, 176, 4235–4243. [Google Scholar] [CrossRef] [PubMed]
- Stiles, L.N.; Hosking, M.P.; Edwards, R.A.; Strieter, R.M.; Lane, T.E. Differential roles for CXCR3 in CD4+ and CD8+ T cell trafficking following viral infection of the CNS. Eur. J. Immunol. 2006, 36, 613–622. [Google Scholar] [CrossRef] [PubMed]
- De Lemos, C.; Christensen, J.E.; Nansen, A.; Moos, T.; Lu, B.; Gerard, C.; Christensen, J.P.; Thomsen, A.R. Opposing effects of CXCR3 and CCR5 deficiency on CD8+ T cell-mediated inflammation in the central nervous system of virus-infected mice. J. Immunol. 2005, 175, 1767–1775. [Google Scholar] [CrossRef]
- Li, H.; Gang, Z.; Yuling, H.; Luokun, X.; Jie, X.; Hao, L.; Li, W.; Chunsong, H.; Junyan, L.; Mingshen, J.; et al. Different neurotropic pathogens elicit neurotoxic CCR9-or neurosupportive CXCR3-expressing microglia. J. Immunol. 2006, 177, 3644–3656. [Google Scholar] [CrossRef]
- Gandhi, K.S.; McKay, F.C.; Diefenbach, E.; Crossett, B.; Schibeci, S.D.; Heard, R.N.; Stewart, G.J.; Booth, D.R.; Arthur, J.W. Novel approaches to detect serum biomarkers for clinical response to interferon-β treatment in multiple sclerosis. PLoS ONE 2010, 5, e10484. [Google Scholar] [CrossRef][Green Version]
- Treacy, O.; Ryan, A.E.; Heinzl, T.; O’Flynn, L.; Cregg, M.; Wilk, M.; Odoardi, F.; Lohan, P.; O’Brien, T.; Nosov, M.; et al. Adenoviral transduction of mesenchymal stem cells: In vitro responses and in vivo immune responses after cell transplantation. PLoS ONE 2012, 7, e42662. [Google Scholar] [CrossRef]
- Rubin, S.M. Management of multiple sclerosis: An overview. Dis. Month 2013, 59, 253–260. [Google Scholar] [CrossRef]
- Iwakura, Y.; Ishigame, H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006, 116, 1218–1222. [Google Scholar] [CrossRef]
- Kakimi, K.; Lane, T.E.; Wieland, S.; Asensio, V.C.; Campbell, I.L.; Chisari, F.V.; Guidotti, L.G. Blocking chemokine responsive to gamma-2/interferon (IFN)-gamma inducible protein and monokine induced by IFN-gamma activity in vivo reduces the pathogenetic but not the antiviral potential of hepatitis B virus-specific cytotoxic T lymphocytes. J. Exp. Med. 2001, 194, 1755–1766. [Google Scholar] [CrossRef]
- Loetscher, M.; Gerber, B.; Loetscher, P.; Jones, S.A.; Piali, L.; Clark-Lewis, I.; Baggiolini, M.; Moser, B. Chemokine receptor specific for IP10 and mig: Structure, function, and expression in activated T-lymphocytes. J. Exp. Med. 1996, 184, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Liu, Z.X.; Lane, T.; Dennert, G. IP-10 and Mig facilitate accumulation of T cells in the virus-infected liver. Cell. Immunol. 2002, 219, 48–56. [Google Scholar] [CrossRef]
- Maghazachi, A.A.; Skålhegg, B.S.; Rolstad, B.; Al-Aoukaty, A. Interferon-inducible protein-10 and lymphotactin induce the chemotaxis and mobilization of intracellular calcium in natural killer cells through pertussis toxin-sensitive and-insensitive heterotrimeric G-proteins. FASEB J. 1997, 11, 765–774. [Google Scholar] [CrossRef] [PubMed]
- Mahalingam, S.; Farber, J.M.; Karupiah, G. The interferon-inducible chemokines MuMig and Crg-2 exhibit antiviral activity In vivo. J. Virol. 1999, 73, 1479–1491. [Google Scholar] [CrossRef] [PubMed]
- Cole, A.M.; Ganz, T.; Liese, A.M.; Burdick, M.D.; Liu, L.; Strieter, R.M. Cutting edge: IFN-inducible ELR- CXC chemokines display defensin-like antimicrobial activity. J. Immunol. 2001, 167, 623–627. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.P.; Lu, H.L.; Lai, S.L.; Campanella, G.S.; Sung, J.M.; Lu, M.Y.; Wu-Hsieh, B.A.; Lin, Y.L.; Lane, T.E.; Luster, A.D.; et al. Dengue virus induces expression of CXC chemokine ligand 10/IFN-gamma-inducible protein 10, which competitively inhibits viral binding to cell surface heparan sulfate. J. Immunol. 2006, 177, 3185–3192. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, Z.; Lim, T.; Zhang, H.; He, J.; Walker, E.; Shier, C.; Wang, Y.; Su, Y.; Sall, A.; et al. CXCL10 inhibits viral replication through recruitment of natural killer cells in coxsackievirus B3-induced myocarditis. Circ. Res. 2009, 104, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Cantini, G.; Mello, T.; Francalanci, M.; Gelmini, S.; Cosmi, L.; Santarlasci, V.; Degl’Innocenti, S.; Luciani, P.; Deledda, C.; et al. Molecular mechanisms underlying the pro-inflammatory synergistic effect of tumor necrosis factor alpha and interferon gamma in human microvascular endothelium. Eur. J. Cell Biol. 2009, 88, 731–742. [Google Scholar] [CrossRef]
- Dhillon, N.K.; Peng, F.; Ransohoff, R.M.; Buch, S. PDGF synergistically enhances IFN-γ-induced expression of CXCL10 in blood-derived macrophages: Implications for HIV dementia. J. Immunol. 2007, 179, 2722. [Google Scholar] [CrossRef]
- Hardaker, E.L.; Bacon, A.M.; Carlson, K.; Roshak, A.K.; Foley, J.J.; Schmidt, D.B.; Buckley, P.T.; Comegys, M.; Panettieri, R.A., Jr.; Sarau, H.M.; et al. Regulation of TNF-alpha- and IFN-gamma-induced CXCL10 expression: Participation of the airway smooth muscle in the pulmonary inflammatory response in chronic obstructive pulmonary disease. FASEB J. 2004, 18, 191–193. [Google Scholar] [CrossRef]
- Kanda, N.; Watanabe, S. Substance P enhances the production of interferon-induced protein of 10 kDa by human keratinocytes in synergy with interferon-gamma. J. Investig. Dermatol. 2002, 119, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Loos, T.; Dekeyzer, L.; Struyf, S.; Schutyser, E.; Gijsbers, K.; Gouwy, M.; Fraeyman, A.; Put, W.; Ronsse, I.; Grillet, B.; et al. TLR ligands and cytokines induce CXCR3 ligands in endothelial cells: Enhanced CXCL9 in autoimmune arthritis. Lab. Investig. 2006, 86, 902–916. [Google Scholar] [CrossRef] [PubMed]
- Brentano, F.; Schorr, O.; Gay, R.E.; Gay, S.; Kyburz, D. RNA released from necrotic synovial fluid cells activates rheumatoid arthritis synovial fibroblasts via Toll-like receptor 3. Arthritis Rheum. 2005, 52, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Nazar, A.S.; Shin, H.S.; Vanguri, P.; Shin, M.L. IP-10 gene transcription by virus in astrocytes requires cooperation of ISRE with adjacent kappaB site but not IRF-1 or viral transcription. J. Interferon Cytokine Res. 1998, 18, 987–997. [Google Scholar] [CrossRef] [PubMed]
- Lebre, M.C.; van der Aar, A.M.; van Baarsen, L.; van Capel, T.M.; Schuitemaker, J.H.; Kapsenberg, M.L.; de Jong, E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.E.; Parker, L.C.; Ward, J.R.; Jones, E.C.; Whyte, M.K.; Brightling, C.E.; Bradding, P.; Dower, S.K.; Sabroe, I. Cooperative molecular and cellular networks regulate Toll-like receptor-dependent inflammatory responses. FASEB J. 2006, 20, 2153–2155. [Google Scholar] [CrossRef]
- Taima, K.; Imaizumi, T.; Yamashita, K.; Ishikawa, A.; Fujita, T.; Yoshida, H.; Takanashi, S.; Okumura, K.; Satoh, K. Expression of IP-10/CXCL10 is upregulated by double-stranded RNA in BEAS-2B bronchial epithelial cells. Respiration 2006, 73, 360–364. [Google Scholar] [CrossRef]
- Majumder, S.; Zhou, L.Z.; Chaturvedi, P.; Babcock, G.; Aras, S.; Ransohoff, R.M. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J. Immunol. 1998, 161, 4736–4744. [Google Scholar]
- Oslund, K.L.; Zhou, X.; Lee, B.; Zhu, L.; Duong, T.; Shih, R.; Baumgarth, N.; Hung, L.-Y.; Wu, R.; Chen, Y. Synergistic Up-Regulation of CXCL10 by Virus and IFN γ in Human Airway Epithelial Cells. PLoS ONE 2014, 9, e100978. [Google Scholar] [CrossRef]
- Carpentier, P.A.; Williams, B.R.; Miller, S.D. Distinct roles of protein kinase R and toll-like receptor 3 in the activation of astrocytes by viral stimuli. Glia 2007, 55, 239–252. [Google Scholar] [CrossRef]
- Imaizumi, T.; Kumagai, M.; Taima, K.; Fujita, T.; Yoshida, H.; Satoh, K. Involvement of retinoic acid-inducible gene-I in the IFN-{gamma}/STAT1 signalling pathway in BEAS-2B cells. Eur. Respir. J. 2005, 25, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Berghäll, H.; Sirén, J.; Sarkar, D.; Julkunen, I.; Fisher, P.B.; Vainionpää, R.; Matikainen, S. The interferon-inducible RNA helicase, mda-5, is involved in measles virus-induced expression of antiviral cytokines. Microbes Infect. 2006, 8, 2138–2144. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Subbarao, K. The immunobiology of SARS*. Annu. Rev. Immunol. 2007, 25, 443–472. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.H.; Chen, C.W.; Schmitz, S.F.; King, C.C.; Chen, W.J.; Wu, Y.C.; Ho, M.S. Candidate genes associated with susceptibility for SARS-coronavirus. Bull. Math. Biol. 2010, 72, 122–132. [Google Scholar] [CrossRef]
- Sgadari, C.; Angiolillo, A.L.; Cherney, B.W.; Pike, S.E.; Farber, J.M.; Koniaris, L.G.; Vanguri, P.; Burd, P.R.; Sheikh, N.; Gupta, G.; et al. Interferon-inducible protein-10 identified as a mediator of tumor necrosis in vivo. Proc. Natl. Acad. Sci. USA 1996, 93, 13791–13796. [Google Scholar] [CrossRef]
- Lane, B.R.; King, S.R.; Bock, P.J.; Strieter, R.M.; Coffey, M.J.; Markovitz, D.M. The C-X-C chemokine IP-10 stimulates HIV-1 replication. Virology 2003, 307, 122–134. [Google Scholar] [CrossRef]
- Peterson, K.E.; Robertson, S.J.; Portis, J.L.; Chesebro, B. Differences in cytokine and chemokine responses during neurological disease induced by polytropic murine retroviruses Map to separate regions of the viral envelope gene. J. Virol. 2001, 75, 2848–2856. [Google Scholar] [CrossRef]
- Roe, B.; Coughlan, S.; Hassan, J.; Grogan, A.; Farrell, G.; Norris, S.; Bergin, C.; Hall, W.W. Elevated serum levels of interferon- gamma -inducible protein-10 in patients coinfected with hepatitis C virus and HIV. J. Infect. Dis. 2007, 196, 1053–1057. [Google Scholar] [CrossRef][Green Version]
- Lagging, M.; Romero, A.I.; Westin, J.; Norkrans, G.; Dhillon, A.P.; Pawlotsky, J.-M.; Zeuzem, S.; von Wagner, M.; Negro, F.; Schalm, S.W.; et al. IP-10 predicts viral response and therapeutic outcome in difficult-to-treat patients with HCV genotype 1 infection. Hepatology 2006, 44, 1617–1625. [Google Scholar] [CrossRef]
- Stylianou, E.; Aukrust, P.; Bendtzen, K.; Müller, F.; Frøland, S.S. Interferons and interferon (IFN)-inducible protein 10 during highly active anti-retroviral therapy (HAART)-possible immunosuppressive role of IFN-alpha in HIV infection. Clin. Exp. Immunol. 2000, 119, 479–485. [Google Scholar] [CrossRef]
- Lam, C.W.; Chan, M.H.; Wong, C.K. Severe acute respiratory syndrome: Clinical and laboratory manifestations. Clin. Biochem. Rev. 2004, 25, 121–132. [Google Scholar] [PubMed]
- Wong, C.K.; Lam, C.W.; Wu, A.K.; Ip, W.K.; Lee, N.L.; Chan, I.H.; Lit, L.C.; Hui, D.S.; Chan, M.H.; Chung, S.S.; et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Ozga, A.J.; Chow, M.T.; Lopes, M.E.; Servis, R.L.; Di Pilato, M.; Dehio, P.; Lian, J.; Mempel, T.R.; Luster, A.D. CXCL10 chemokine regulates heterogeneity of the CD8+ T cell response and viral set point during chronic infection. Immunity 2022, 55, 82–97.e88. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Shin, E.C. Clinical implications of chemokines in acute and chronic hepatitis C virus infection. Yonsei Med. J. 2011, 52, 871–878. [Google Scholar] [CrossRef]
- Zeremski, M.; Petrovic, L.M.; Talal, A.H. The role of chemokines as inflammatory mediators in chronic hepatitis C virus infection. J. Viral Hepat. 2007, 14, 675–687. [Google Scholar] [CrossRef]
- Sung, P.S.; Hong, S.-H.; Lee, J.; Park, S.-H.; Yoon, S.K.; Chung, W.J.; Shin, E.-C. CXCL10 is produced in hepatitis A virus-infected cells in an IRF3-dependent but IFN-independent manner. Sci. Rep. 2017, 7, 6387. [Google Scholar] [CrossRef]
- Brownell, J.; Wagoner, J.; Lovelace, E.S.; Thirstrup, D.; Mohar, I.; Smith, W.; Giugliano, S.; Li, K.; Crispe, I.N.; Rosen, H.R.; et al. Independent, parallel pathways to CXCL10 induction in HCV-infected hepatocytes. J. Hepatol. 2013, 59, 701–708. [Google Scholar] [CrossRef]
- Brownell, J.; Bruckner, J.; Wagoner, J.; Thomas, E.; Loo, Y.M.; Gale, M., Jr.; Liang, T.J.; Polyak, S.J. Direct, interferon-independent activation of the CXCL10 promoter by NF-κB and interferon regulatory factor 3 during hepatitis C virus infection. J. Virol. 2014, 88, 1582–1590. [Google Scholar] [CrossRef]
- Murai, M.; Yoneyama, H.; Harada, A.; Yi, Z.; Vestergaard, C.; Guo, B.; Suzuki, K.; Asakura, H.; Matsushima, K. Active participation of CCR5+ CD8+ T lymphocytes in the pathogenesis of liver injury in graft-versus-host disease. J. Clin. Investig. 1999, 104, 49–57. [Google Scholar] [CrossRef]
- Curbishley, S.M.; Eksteen, B.; Gladue, R.P.; Lalor, P.; Adams, D.H. CXCR 3 activation promotes lymphocyte transendothelial migration across human hepatic endothelium under fluid flow. Am. J. Pathol. 2005, 167, 887–899. [Google Scholar] [CrossRef]
- Ajuebor, M.N.; Hogaboam, C.M.; Le, T.; Proudfoot, A.E.; Swain, M.G. CCL3/MIP-1alpha is pro-inflammatory in murine T cell-mediated hepatitis by recruiting CCR1-expressing CD4+ T cells to the liver. Eur. J. Immunol. 2004, 34, 2907–2918. [Google Scholar] [CrossRef] [PubMed]
- Apolinario, A.; Majano, P.L.; Alvarez-Pérez, E.; Saez, A.; Lozano, C.; Vargas, J.; García-Monzón, C. Increased expression of T cell chemokines and their receptors in chronic hepatitis C: Relationship with the histological activity of liver disease. Am. J. Gastroenterol. 2002, 97, 2861–2870. [Google Scholar] [CrossRef] [PubMed]
- Harvey, C.E.; Post, J.J.; Palladinetti, P.; Freeman, A.J.; Ffrench, R.A.; Kumar, R.K.; Marinos, G.; Lloyd, A.R. Expression of the chemokine IP-10 (CXCL10) by hepatocytes in chronic hepatitis C virus infection correlates with histological severity and lobular inflammation. J. Leukoc. Biol. 2003, 74, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Helbig, K.J.; Ruszkiewicz, A.; Semendric, L.; Harley, H.A.J.; McColl, S.R.; Beard, M.R. Expression of the CXCR3 ligand I-TAC by hepatocytes in chronic hepatitis C and its correlation with hepatic inflammation. Hepatology 2004, 39, 1220–1229. [Google Scholar] [CrossRef]
- Zeremski, M.; Petrovic, L.M.; Chiriboga, L.; Brown, Q.B.; Yee, H.T.; Kinkhabwala, M.; Jacobson, I.M.; Dimova, R.; Markatou, M.; Talal, A.H. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology 2008, 48, 1440–1450. [Google Scholar] [CrossRef]
- Yin, X.; Li, X.; Ambardekar, C.; Hu, Z.; Lhomme, S.; Feng, Z. Hepatitis E virus persists in the presence of a type III interferon response. PLoS Pathog. 2017, 13, e1006417. [Google Scholar] [CrossRef]
- Sayed, I.M.; Verhoye, L.; Cocquerel, L.; Abravanel, F.; Foquet, L.; Montpellier, C.; Debing, Y.; Farhoudi, A.; Wychowski, C.; Dubuisson, J.; et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut 2017, 66, 920–929. [Google Scholar] [CrossRef]
- Marion, O.; Lhomme, S.; Nayrac, M.; Dubois, M.; Pucelle, M.; Requena, M.; Migueres, M.; Abravanel, F.; Peron, J.M.; Carrere, N.; et al. Hepatitis E virus replication in human intestinal cells. Gut 2020, 69, 901. [Google Scholar] [CrossRef]
- Devhare, P.; Madiyal, M.; Mukhopadhyay, C.; Shetty, S.; Shastry, S. Interplay between Hepatitis E Virus and Host Cell Pattern Recognition Receptors. Int. J. Mol. Sci. 2021, 22, 9259. [Google Scholar] [CrossRef]
- Peeters, M.; Schenk, J.; De Somer, T.; Roskams, T.; Locus, T.; Klamer, S.; Subissi, L.; Suin, V.; Delwaide, J.; Stärkel, P.; et al. Viral clade is associated with severity of symptomatic genotype 3 Hepatitis E virus infections in Belgium, 2010–2018. J. Hepatol. 2022. [Google Scholar] [CrossRef]
- Ferrari, S.M.; Fallahi, P.; Ruffilli, I.; Elia, G.; Ragusa, F.; Paparo, S.R.; Patrizio, A.; Mazzi, V.; Colaci, M.; Giuggioli, D.; et al. Immunomodulation of CXCL10 Secretion by Hepatitis C Virus: Could CXCL10 Be a Prognostic Marker of Chronic Hepatitis C? J. Immunol. Res. 2019, 2019, 5878960. [Google Scholar] [CrossRef] [PubMed]
- Larrubia, J.R.; Benito-Martínez, S.; Calvino, M.; Sanz-de-Villalobos, E.; Parra-Cid, T. Role of chemokines and their receptors in viral persistence and liver damage during chronic hepatitis C virus infection. World J. Gastroenterol. 2008, 14, 7149–7159. [Google Scholar] [CrossRef] [PubMed]
- Skinner, D.; Marro, B.S.; Lane, T.E. Chemokine CXCL10 and coronavirus-induced neurologic disease. Viral Immunol. 2019, 32, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Hayney, M.S.; Henriquez, K.M.; Barnet, J.H.; Ewers, T.; Champion, H.M.; Flannery, S.; Barrett, B. Serum IFN-γ-induced protein 10 (IP-10) as a biomarker for severity of acute respiratory infection in healthy adults. J. Clin. Virol. 2017, 90, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Almansa, R.; Sanchez-Garcia, M.; Herrero, A.; Calzada, S.; Roig, V.; Barbado, J.; Rico, L.; Bobillo, F.; Eiros, J.M.; Iglesias, V.; et al. Host response cytokine signatures in viral and nonviral acute exacerbations of chronic obstructive pulmonary disease. J. Interferon Cytokine Res. 2011, 31, 409–413. [Google Scholar] [CrossRef]
- Quint, J.K.; Donaldson, G.C.; Goldring, J.J.; Baghai-Ravary, R.; Hurst, J.R.; Wedzicha, J.A. Serum IP-10 as a biomarker of human rhinovirus infection at exacerbation of COPD. Chest 2010, 137, 812–822. [Google Scholar] [CrossRef]
- Chen, J.; Lau, Y.F.; Lamirande, E.W.; Paddock, C.D.; Bartlett, J.H.; Zaki, S.R.; Subbarao, K. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 2010, 84, 1289–1301. [Google Scholar] [CrossRef]
- Cameron, M.J.; Ran, L.; Xu, L.; Danesh, A.; Bermejo-Martin, J.F.; Cameron, C.M.; Muller, M.P.; Gold, W.L.; Richardson, S.E.; Poutanen, S.M.; et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007, 81, 8692–8706. [Google Scholar] [CrossRef]
- Tynell, J.; Westenius, V.; Rönkkö, E.; Munster, V.J.; Melén, K.; Österlund, P.; Julkunen, I. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. J. Gen. Virol. 2016, 97, 344–355. [Google Scholar] [CrossRef]
- Zhou, J.; Chu, H.; Li, C.; Wong, B.H.; Cheng, Z.S.; Poon, V.K.; Sun, T.; Lau, C.C.; Wong, K.K.; Chan, J.Y.; et al. Active replication of Middle East respiratory syndrome coronavirus and aberrant induction of inflammatory cytokines and chemokines in human macrophages: Implications for pathogenesis. J. Infect. Dis. 2014, 209, 1331–1342. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Chan, J.F.; Wang, Y.; Yuen, T.T.; Chai, Y.; Hou, Y.; Shuai, H.; Yang, D.; Hu, B.; Huang, X.; et al. Comparative replication and immune activation profiles of SARS-CoV-2 and SARS-CoV in human lungs: An ex vivo study with implications for the pathogenesis of COVID-19. Clin. Infect. Dis. 2020, 71, 1400–1409. [Google Scholar] [CrossRef] [PubMed]
- Chi, Y.; Ge, Y.; Wu, B.; Zhang, W.; Wu, T.; Wen, T.; Liu, J.; Guo, X.; Huang, C.; Jiao, Y.; et al. Serum cytokine and chemokine profile in relation to the severity of coronavirus disease 2019 in China. J. Infect. Dis. 2020, 222, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Elemam, N.M.; Hammoudeh, S.; Salameh, L.; Mahboub, B.; Alsafar, H.; Talaat, I.M.; Habib, P.; Siddiqui, M.; Hassan, K.O.; Al-Assaf, O.Y.; et al. Identifying immunological and clinical predictors of COVID-19 severity and sequelae by mathematical modeling. Front. Immunol. 2022, 13, 865845. [Google Scholar] [CrossRef] [PubMed]
- Hue, S.; Beldi-Ferchiou, A.; Bendib, I.; Surenaud, M.; Fourati, S.; Frapard, T.; Rivoal, S.; Razazi, K.; Carteaux, G.; Delfau-Larue, M.H.; et al. Uncontrolled Innate and Impaired Adaptive Immune Responses in Patients with COVID-19 Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2020, 202, 1509–1519. [Google Scholar] [CrossRef] [PubMed]
- Gudowska-Sawczuk, M.; Mroczko, B. What is currently known about the role of CXCL10 in SARS-CoV-2 infection? Int. J. Mol. Sci. 2022, 23, 3673. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127.e114. [Google Scholar] [CrossRef]
- Altara, R.; Manca, M.; Brandão, R.D.; Zeidan, A.; Booz, G.W.; Zouein, F.A. Emerging importance of chemokine receptor CXCR3 and its ligands in cardiovascular diseases. Clin. Sci. 2016, 130, 463–478. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lorè, N.I.; De Lorenzo, R.; Rancoita, P.M.V.; Cugnata, F.; Agresti, A.; Benedetti, F.; Bianchi, M.E.; Bonini, C.; Capobianco, A.; Conte, C.; et al. CXCL10 levels at hospital admission predict COVID-19 outcome: Hierarchical assessment of 53 putative inflammatory biomarkers in an observational study. Mol. Med. 2021, 27, 129. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Zhang, X.; Wang, S.; Peng, Z.; Guo, J.; Jiang, H.; Liu, J.; Xie, Y.; Wang, J.; et al. Leptin correlates with monocytes activation and severe condition in COVID-19 patients. J. Leukoc. Biol. 2021, 110, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.S.; Kim, J.Y.; Kim, M.C.; Park, S.Y.; Kim, B.N.; Bae, S.; Cha, H.H.; Jung, J.; Kim, M.J.; Lee, M.J.; et al. Factors of severity in patients with COVID-19: Cytokine/chemokine concentrations, viral load, and antibody responses. Am. J. Trop. Med. Hyg. 2020, 103, 2412–2418. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.S.; Shu, T.; Kang, L.; Wu, D.; Zhou, X.; Liao, B.W.; Sun, X.L.; Zhou, X.; Wang, Y.Y. Temporal profiling of plasma cytokines, chemokines and growth factors from mild, severe and fatal COVID-19 patients. Signal Transduct. Target. Ther. 2020, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, Y.; Nilsson-Payant, B.E.; Gupta, V.; Wang, P.; Duan, X.; Tang, X.; Zhu, J.; Zhao, Z.; Jaffré, F.; et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell 2020, 27, 125–136.e127. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020, 181, 1036–1045.e1039. [Google Scholar] [CrossRef] [PubMed]
- Başar, E.Z.; Sönmez, H.E.; Uzuner, H.; Karadenizli, A.; Güngör, H.S.; Akgün, G.; Yetimakman, A.F.; Öncel, S.; Babaoğlu, K. CXCL10/IP10 as a Biomarker Linking Multisystem Inflammatory Syndrome and Left Ventricular Dysfunction in Children with SARS-CoV-2. J. Clin. Med. 2022, 11, 1416. [Google Scholar] [CrossRef]
- Imai, Y.; Kuba, K.; Neely, G.G.; Yaghubian-Malhami, R.; Perkmann, T.; van Loo, G.; Ermolaeva, M.; Veldhuizen, R.; Leung, Y.H.; Wang, H.; et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell 2008, 133, 235–249. [Google Scholar] [CrossRef]
- Barrenschee, M.; Lex, D.; Uhlig, S. Effects of the TLR2 agonists MALP-2 and Pam3Cys in isolated mouse lungs. PLoS ONE 2010, 5, e13889. [Google Scholar] [CrossRef]
- Ichikawa, A.; Kuba, K.; Morita, M.; Chida, S.; Tezuka, H.; Hara, H.; Sasaki, T.; Ohteki, T.; Ranieri, V.M.; dos Santos, C.C.; et al. CXCL10-CXCR3 enhances the development of neutrophil-mediated fulminant lung injury of viral and nonviral origin. Am. J. Respir. Crit. Care Med. 2013, 187, 65–77. [Google Scholar] [CrossRef]
- Neville, L.F.; Mathiak, G.; Bagasra, O. The immunobiology of interferon-gamma inducible protein 10 kD (IP-10): A novel, pleiotropic member of the C-X-C chemokine superfamily. Cytokine Growth Factor Rev. 1997, 8, 207–219. [Google Scholar] [CrossRef]
- Wang, W.; Yang, P.; Zhong, Y.; Zhao, Z.; Xing, L.; Zhao, Y.; Zou, Z.; Zhang, Y.; Li, C.; Li, T.; et al. Monoclonal antibody against CXCL-10/IP-10 ameliorates influenza A (H1N1) virus induced acute lung injury. Cell Res. 2013, 23, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Cinatl, J., Jr.; Michaelis, M.; Morgenstern, B.; Doerr, H.W. High-dose hydrocortisone reduces expression of the pro-inflammatory chemokines CXCL8 and CXCL10 in SARS coronavirus-infected intestinal cells. Int. J. Mol. Med. 2005, 15, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb. Perspect. Biol. 2014, 6, a016295. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy 2016, 8, 959–970. [Google Scholar] [CrossRef] [PubMed]
- Coperchini, F.; Chiovato, L.; Rotondi, M. Interleukin-6, CXCL10 and infiltrating macrophages in COVID-19-related cytokine storm: Not one for all but all for one! Front. Immunol. 2021, 12, 668507. [Google Scholar] [CrossRef] [PubMed]
- Semmler, G.; Griebler, H.; Aberle, S.W.; Stiasny, K.; Richter, L.; Holzmann, H.; Weseslindtner, L. Elevated CXCL10 serum levels in measles virus primary infection and reinfection correlate with the serological stage and hospitalization status. J. Infect. Dis. 2020, 222, 2030–2034. [Google Scholar] [CrossRef]
- Lin, W.W.; Nelson, A.N.; Ryon, J.J.; Moss, W.J.; Griffin, D.E. Plasma cytokines and chemokines in zambian children with measles: Innate responses and association with HIV-1 coinfection and in-hospital mortality. J. Infect. Dis. 2017, 215, 830–839. [Google Scholar] [CrossRef] [PubMed]
- Moss, W.J. Measles. Lancet 2017, 390, 2490–2502. [Google Scholar] [CrossRef]
- Mina, M.J.; Metcalf, C.J.; de Swart, R.L.; Osterhaus, A.D.; Grenfell, B.T. Long-term measles-induced immunomodulation increases overall childhood infectious disease mortality. Science 2015, 348, 694–699. [Google Scholar] [CrossRef]
- Lindell, D.M.; Lane, T.E.; Lukacs, N.W. CXCL10/CXCR3-mediated responses promote immunity to respiratory syncytial virus infection by augmenting dendritic cell and CD8(+) T cell efficacy. Eur. J. Immunol. 2008, 38, 2168–2179. [Google Scholar] [CrossRef]
- Liu, M.; Guo, S.; Hibbert, J.M.; Jain, V.; Singh, N.; Wilson, N.O.; Stiles, J.K. CXCL10/IP-10 in infectious diseases pathogenesis and potential therapeutic implications. Cytokine Growth Factor Rev. 2011, 22, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Naveca, F.G.; Pontes, G.S.; Chang, A.Y.; Silva, G.; Nascimento, V.A.D.; Monteiro, D.; Silva, M.S.D.; Abdalla, L.F.; Santos, J.H.A.; Almeida, T.A.P.; et al. Analysis of the immunological biomarker profile during acute Zika virus infection reveals the overexpression of CXCL10, a chemokine linked to neuronal damage. Mem. Inst. Oswaldo Cruz 2018, 113, e170542. [Google Scholar] [CrossRef] [PubMed]
- Steain, M.; Gowrishankar, K.; Rodriguez, M.; Slobedman, B.; Abendroth, A. Upregulation of CXCL10 in human dorsal root ganglia during experimental and natural varicella-zoster virus infection. J. Virol. 2011, 85, 626–631. [Google Scholar] [CrossRef]
- Dunn, C.; Brunetto, M.; Reynolds, G.; Christophides, T.; Kennedy, P.T.; Lampertico, P.; Das, A.; Lopes, A.R.; Borrow, P.; Williams, K.; et al. Cytokines induced during chronic hepatitis B virus infection promote a pathway for NK cell-mediated liver damage. J. Exp. Med. 2007, 204, 667–680. [Google Scholar] [CrossRef]
- Bayard, F.; Godon, O.; Nalpas, B.; Costentin, C.; Zhu, R.; Soussan, P.; Vallet-Pichard, A.; Fontaine, H.; Mallet, V.; Pol, S.; et al. T-cell responses to hepatitis B splice-generated protein of hepatitis B virus and inflammatory cytokines/chemokines in chronic hepatitis B patients. ANRS study: HB EP 02 HBSP-FIBRO. J. Viral Hepat. 2012, 19, 872–880. [Google Scholar] [CrossRef] [PubMed]
- Fisicaro, P.; Valdatta, C.; Boni, C.; Massari, M.; Mori, C.; Zerbini, A.; Orlandini, A.; Sacchelli, L.; Missale, G.; Ferrari, C. Early kinetics of innate and adaptive immune responses during hepatitis B virus infection. Gut 2009, 58, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, S.; Sugiyama, M.; Shoji, H.; Mano, Y.; Mita, E.; Okamoto, T.; Matsuura, Y.; Okuno, A.; Takikawa, O.; Mizokami, M.; et al. Indoleamine-2,3-dioxygenase as an effector and an indicator of protective immune responses in patients with acute hepatitis B. Hepatology 2016, 63, 83–94. [Google Scholar] [CrossRef]
- Yoshio, S.; Mano, Y.; Doi, H.; Shoji, H.; Shimagaki, T.; Sakamoto, Y.; Kawai, H.; Matsuda, M.; Mori, T.; Osawa, Y.; et al. Cytokine and chemokine signatures associated with hepatitis B surface antigen loss in hepatitis B patients. JCI Insight 2018, 3, 122268. [Google Scholar] [CrossRef]
- Kakimi, K.; Lane, T.E.; Chisari, F.V.; Guidotti, L.G. Cutting edge: Inhibition of hepatitis B virus replication by activated NK T cells does not require inflammatory cell recruitment to the liver. J. Immunol. 2001, 167, 6701–6705. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.; Ma, J.-W.; Lei, Z.; Zhu, H.-F.; Lei, P.; Yang, Z.-S.; Zhang, B.; Yao, X.-X.; Shi, C.; et al. Hepatitis B virus protein X-induced expression of the CXC chemokine IP-10 is mediated through activation of NF-kB and increases migration of leukocytes. J. Biol. Chem. 2010, 285, 12159–12168. [Google Scholar] [CrossRef]
- Sitia, G.; Isogawa, M.; Kakimi, K.; Wieland, S.F.; Chisari, F.V.; Guidotti, L.G. Depletion of neutrophils blocks the recruitment of antigen-nonspecific cells into the liver without affecting the antiviral activity of hepatitis B virus-specific cytotoxic T lymphocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 13717–13722. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.P.; Zerbato, J.M.; Zhao, W.; Braat, S.; Deleage, C.; Tennakoon, G.S.; Mason, H.; Dantanarayana, A.; Rhodes, A.; Rhodes, J.W.; et al. Intrahepatic CXCL10 is strongly associated with liver fibrosis in HIV-Hepatitis B co-infection. PLoS Pathog. 2020, 16, e1008744. [Google Scholar] [CrossRef] [PubMed]
- Wuest, T.R.; Carr, D.J.J. Dysregulation of CXCR3 signaling due to CXCL10 deficiency impairs the antiviral response to herpes simplex virus 1 infection. J. Immunol. 2008, 181, 7985. [Google Scholar] [CrossRef]
- Srivastava, R.; Khan, A.A.; Chilukuri, S.; Syed, S.A.; Tran, T.T.; Furness, J.; Bahraoui, E.; BenMohamed, L. CXCL10/CXCR3-dependent mobilization of herpes simplex virus-specific CD8+ T(EM) and CD8+ T(RM) cells within infected tissues allows efficient protection against recurrent herpesvirus infection and disease. J. Virol. 2017, 91, e00278-17. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.J.; Tomanek, L. Herpes simplex virus and the chemokines that mediate the inflammation. Curr. Top. Microbiol. Immunol. 2006, 303, 47–65. [Google Scholar] [CrossRef]
- Wickham, S.; Lu, B.; Ash, J.; Carr, D.J. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J. Neuroimmunol. 2005, 162, 51–59. [Google Scholar] [CrossRef]
- Barbi, J.; Oghumu, S.; Lezama-Davila, C.M.; Satoskar, A.R. IFN-gamma and STAT1 are required for efficient induction of CXC chemokine receptor 3 (CXCR3) on CD4+ but not CD8+ T cells. Blood 2007, 110, 2215–2216. [Google Scholar] [CrossRef]
- Cook, W.J.; Kramer, M.F.; Walker, R.M.; Burwell, T.J.; Holman, H.A.; Coen, D.M.; Knipe, D.M. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol. J. 2004, 1, 5. [Google Scholar] [CrossRef]
- O’Garra, A.; McEvoy, L.M.; Zlotnik, A. T-cell subsets: Chemokine receptors guide the way. Curr. Biol. 1998, 8, R646–R649. [Google Scholar] [CrossRef]
- Sin, J.; Kim, J.J.; Pachuk, C.; Satishchandran, C.; Weiner, D.B. DNA vaccines encoding interleukin-8 and RANTES enhance antigen-specific Th1-type CD4+ T-cell-mediated protective immunity against herpes simplex virus type 2 in vivo. J. Virol. 2000, 74, 11173–11180. [Google Scholar] [CrossRef]
- Thapa, M.; Carr Daniel, J.J. Herpes simplex virus type 2-induced mortality following genital infection is blocked by anti-tumor necrosis factor alpha antibody in CXCL10-deficient mice. J. Virol. 2008, 82, 10295–10301. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, Y.; Lu, B.; Gerard, C.; Iwasaki, A. CD8+ T lymphocyte mobilization to virus-infected tissue requires CD4+ T-cell help. Nature 2009, 462, 510–513. [Google Scholar] [CrossRef]
- Thapa, M.; Welner, R.S.; Pelayo, R.; Carr, D.J.J. CXCL9 and CXCL10 expression are critical for control of genital herpes simplex virus type 2 infection through mobilization of HSV-specific CTL and NK cells to the nervous system. J. Immunol. 2008, 180, 1098. [Google Scholar] [CrossRef]
- Lind, L.; Studahl, M.; Persson Berg, L.; Eriksson, K. CXCL11 production in cerebrospinal fluid distinguishes herpes simplex meningitis from herpes simplex encephalitis. J. Neuroinflamm. 2017, 14, 134. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Deng, X.; Guan, X.; Geng, L.; Fu, M.; Zhang, B.; Chen, R.; Hu, H.; Hu, K.; Zhang, D.; et al. Herpes simplex virus type 2 infection-induced expression of CXCR3 ligands promotes CD4+ T cell migration and is regulated by the viral immediate-early protein ICP4. Front. Immunol. 2018, 9, 32–57. [Google Scholar] [CrossRef] [PubMed]
- Pol, J.G.; Workenhe, S.T.; Konda, P.; Gujar, S.; Kroemer, G. Cytokines in oncolytic virotherapy. Cytokine Growth Factor Rev. 2020, 56, 4–27. [Google Scholar] [CrossRef] [PubMed]
- Abraham, R.; Mudaliar, P.; Padmanabhan, A.; Sreekumar, E. Induction of Cytopathogenicity in Human Glioblastoma Cells by Chikungunya Virus. PLoS ONE 2013, 8, e75854. [Google Scholar] [CrossRef]
- Carew, J.S.; Espitia, C.M.; Zhao, W.; Mita, M.M.; Mita, A.C.; Nawrocki, S.T. Oncolytic reovirus inhibits angiogenesis through induction of CXCL10/IP-10 and abrogation of HIF activity in soft tissue sarcomas. Oncotarget 2017, 8, 49. [Google Scholar] [CrossRef]
- Fu, X.; Rivera, A.; Tao, L.; Zhang, X. An HSV-2 based oncolytic virus can function as an attractant to guide migration of adoptively transferred T cells to tumor sites. Oncotarget 2014, 6, 2. [Google Scholar] [CrossRef]
- Eckert, E.C.; Nace, R.A.; Tonne, J.M.; Evgin, L.; Vile, R.G.; Russell, S.J. Generation of a Tumor-Specific Chemokine Gradient Using Oncolytic Vesicular Stomatitis Virus Encoding CXCL9. Mol. Therapy-Oncol. 2020, 16, 63–74. [Google Scholar] [CrossRef]
- Li, X.; Lu, M.; Yuan, M.; Ye, J.; Zhang, W.; Xu, L.; Wu, X.; Hui, B.; Yang, Y.; Wei, B.; et al. CXCL10-armed oncolytic adenovirus promotes tumor-infiltrating T-cell chemotaxis to enhance anti-PD-1 therapy. Oncoimmunology 2022, 11, 2118210. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Wu, Y.; Albers, A.; Fang, M.; Qian, X. Strategies for Advanced Oncolytic Virotherapy: Current Technology Innovations and Clinical Approaches. Pharmaceutics 2022, 14, 1811. [Google Scholar] [CrossRef] [PubMed]
- Champion, B.R.; Besneux, M.; Patsalidou, M.; Silva, A.; Zonca, M.; Marino, N.; Genova, G.D.; Illingworth, S.; Fedele, S.; Slater, L.; et al. Abstract 5013: NG-641: An oncolytic T-SIGn virus targeting cancer-associated fibroblasts in the stromal microenvironment of human carcinomas. Cancer Res. 2019, 79, 5013. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elemam, N.M.; Talaat, I.M.; Maghazachi, A.A. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses 2022, 14, 2445. https://doi.org/10.3390/v14112445
Elemam NM, Talaat IM, Maghazachi AA. CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses. 2022; 14(11):2445. https://doi.org/10.3390/v14112445
Chicago/Turabian StyleElemam, Noha Mousaad, Iman Mamdouh Talaat, and Azzam A. Maghazachi. 2022. "CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections" Viruses 14, no. 11: 2445. https://doi.org/10.3390/v14112445
APA StyleElemam, N. M., Talaat, I. M., & Maghazachi, A. A. (2022). CXCL10 Chemokine: A Critical Player in RNA and DNA Viral Infections. Viruses, 14(11), 2445. https://doi.org/10.3390/v14112445





_Talaat.jpg)

