Integrated Omics Reveal Time-Resolved Insights into T4 Phage Infection of E. coli on Proteome and Transcriptome Levels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Strains and Media
2.3. RNA Isolation from T4 Phage Infected E. coli
2.4. Preparation of RNA-Sequencing (RNA-Seq) Libraries and Illumina Sequencing
2.5. Northern Blot Analysis
2.6. Proteome Samples Preparation
2.7. Proteome LC-MS Analysis
2.8. Analysis and Visualization of RNA-Seq and Proteomics Data
3. Results and Discussion
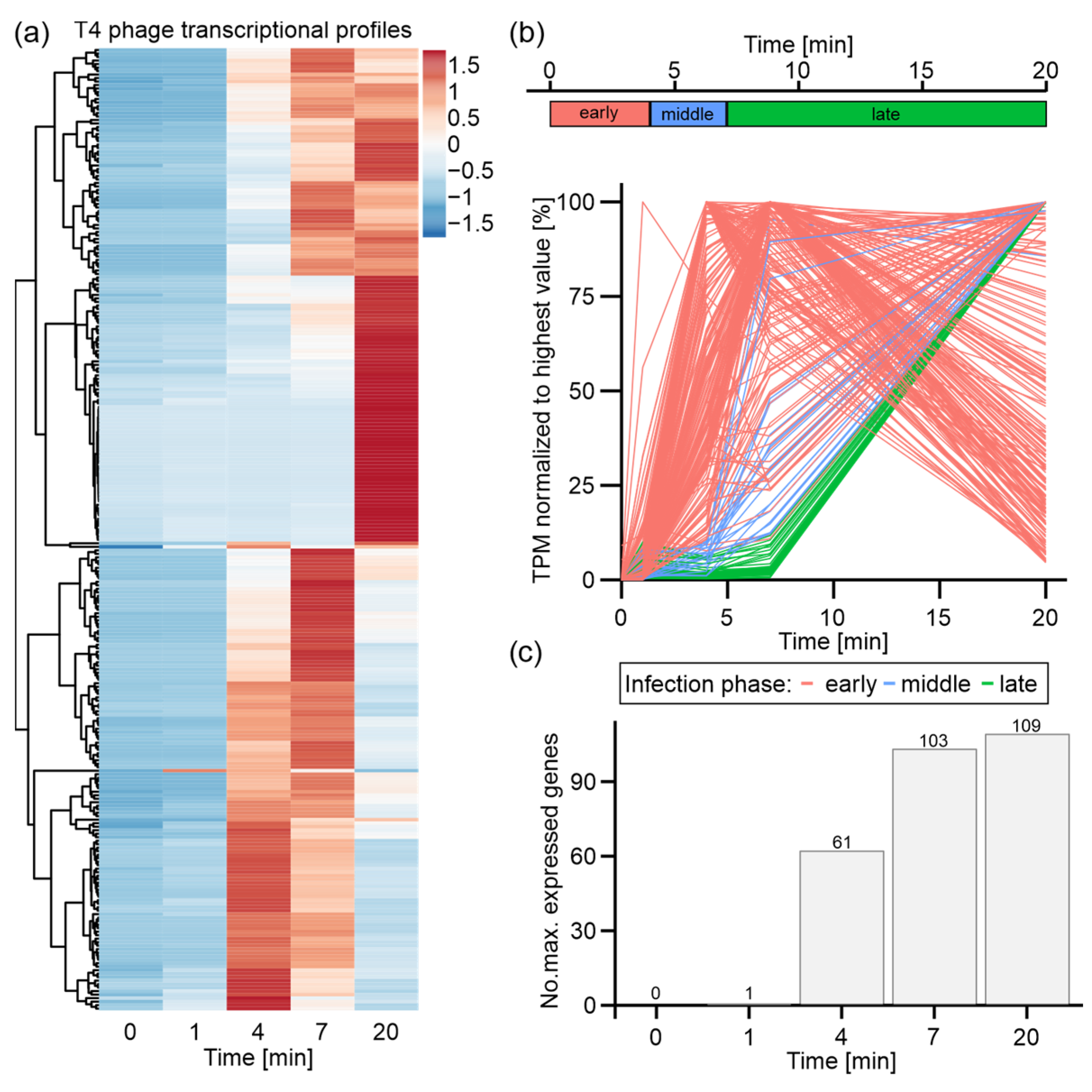
3.1. Time-Resolved Dual-RNA-Seq of T4 Phage Infection
3.2. E. coli Transcript Degradation Is Initiated during the First 4 min of T4 Phage Infection
3.3. T4 Phage Transcription Is Actively Controlled in an Infection-Phase-Dependent Manner
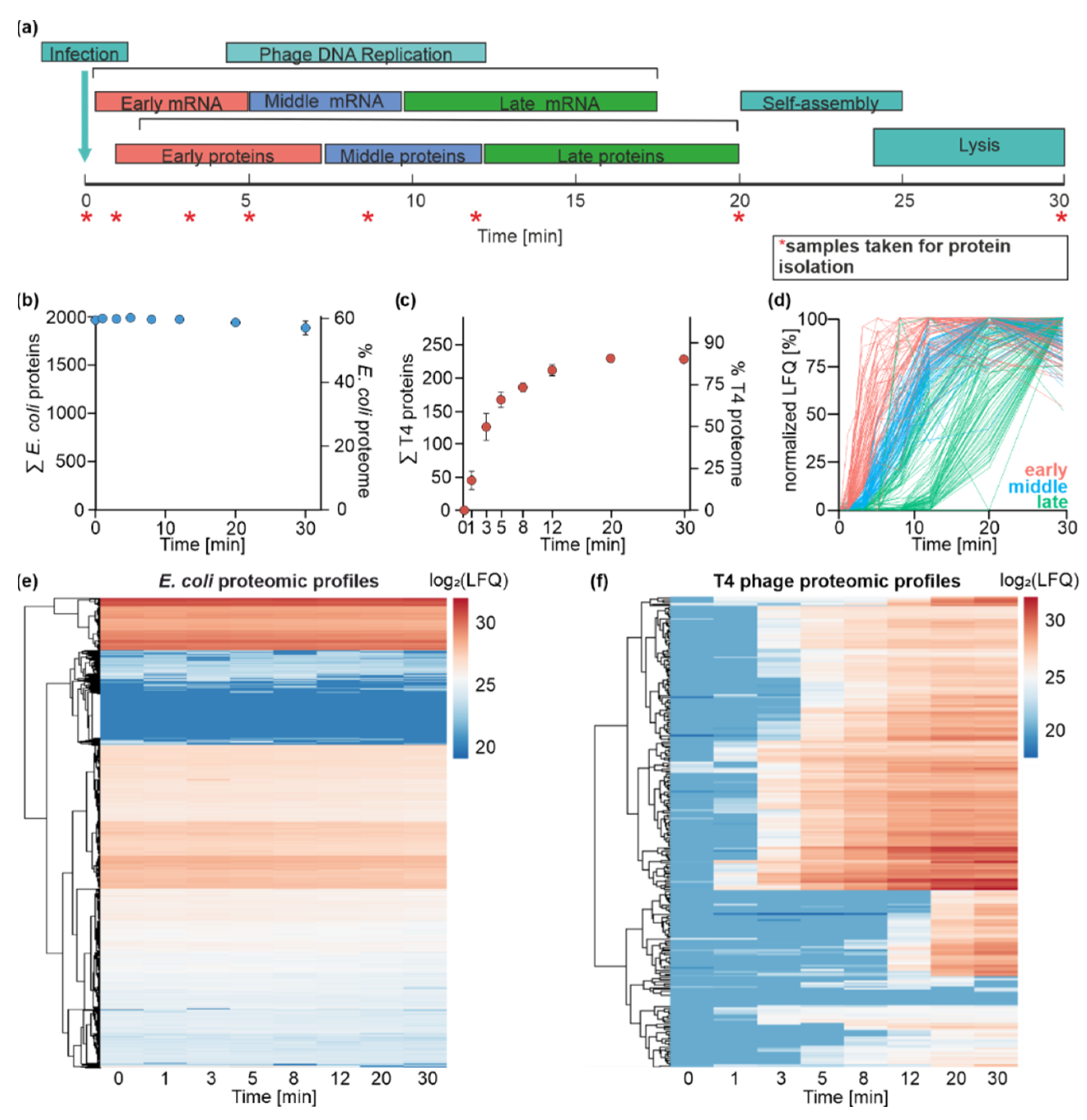
3.4. Time-Resolved Dual-Proteome of T4 Phage Infection
3.5. E. coli Proteome Remains Stable during T4 Phage Infection
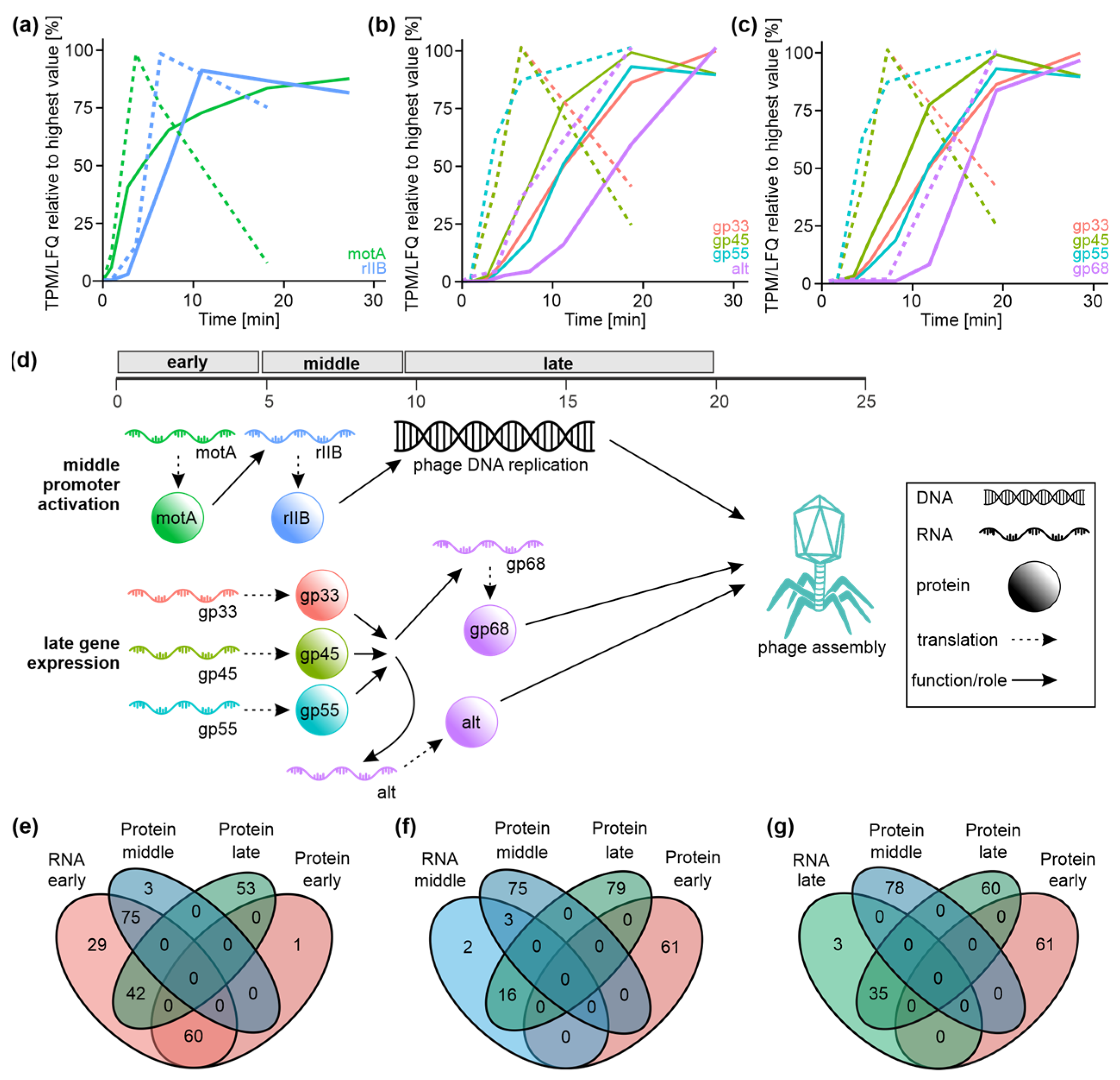
3.6. T4 Phage Protein Synthesis Is Temporally and Functionally Regulated
3.7. Correlation of Transcriptomics and Proteomics Data Implicates Post-Transcriptional Mechanisms Governing T4 Phage Gene Expression
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salmond, G.P.; Fineran, P.C. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Chevallereau, A.; Pons, B.J.; van Houte, S.; Westra, E.R. Interactions between bacterial and phage communities in natural environments. Nat. Rev. Microbiol. 2022, 20, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.K.; Koeris, M.S. The next generation of bacteriophage therapy. Curr. Opin. Microbiol. 2011, 14, 524–531. [Google Scholar] [CrossRef]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgr. Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Miller, E.S.; Kutter, E.; Mosig, G.; Arisaka, F.; Kunisawa, T.; Rüger, W. Bacteriophage T4 genome. Microbiol. Mol. Biol. Rev. 2003, 67, 86–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rittie, L.; Perbal, B. Enzymes used in molecular biology: A useful guide. J. Cell Commun. Signal 2008, 2, 25–45. [Google Scholar] [CrossRef] [Green Version]
- Gamkrelidze, M.; Dabrowska, K. T4 bacteriophage as a phage display platform. Arch. Microbiol. 2014, 196, 473–479. [Google Scholar] [CrossRef] [Green Version]
- Kutter, E.; Gachechiladze, K.; Poglazov, A.; Marusich, E.; Shneider, M.; Aronsson, P.; Napuli, A.; Porter, D.; Mesyanzhinov, V. Evolution of T4-related phages. Virus Genes 1995, 11, 285–297. [Google Scholar] [CrossRef]
- Ueno, H.; Yonesaki, T. Phage-induced change in the stability of mRNAs. Virology 2004, 329, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Depping, R.; Lohaus, C.; Meyer, H.E.; Rüger, W. The mono-ADP-ribosyltransferases Alt and ModB of bacteriophage T4: Target proteins identified. Biochem. Biophys. Res. Commun. 2005, 335, 1217–1223. [Google Scholar] [CrossRef]
- Tiemann, B.; Depping, R.; Gineikiene, E.; Kaliniene, L.; Nivinskas, R.; Rüger, W. ModA and ModB, two ADP-ribosyltransferases encoded by bacteriophage T4: Catalytic properties and mutation analysis. J. Bacteriol. 2004, 186, 7262–7272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrer, H.; Zillig, W.; Mailhammer, R. ADP-ribosylation of DNA-dependent RNA polymerase of Escherichia coli by an NAD+: Protein ADP-ribosyltransferase from bacteriophage T4. Eur. J. Biochem. 1975, 60, 227–238. [Google Scholar] [CrossRef]
- Koch, T.; Raudonikiene, A.; Wilkens, K.; Rüger, W. Overexpression, purification, and characterization of the ADP-ribosyltransferase (gpAlt) of bacteriophage T4: ADP-ribosylation of E. coli RNA polymerase modulates T4 “early” transcription. Gene Expr. 1995, 4, 253–264. [Google Scholar] [PubMed]
- Wilkens, K.; Tiemann, B.; Bazan, F.; Rüger, W. ADP-ribosylation and early transcription regulation by bacteriophage T4. Adv. Exp. Med. Biol. 1997, 419, 71–82. [Google Scholar]
- Uzan, M. RNA processing and decay in bacteriophage T4. Prog. Mol. Biol. Transl. Sci. 2009, 85, 43–89. [Google Scholar] [PubMed]
- Uzan, M.; Miller, E.S. Post-transcriptional control by bacteriophage T4: mRNA decay and inhibition of translation initiation. Virol. J. 2010, 7, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinton, D.M. Transcriptional control in the prereplicative phase of T4 development. Virol. J. 2010, 7, 289. [Google Scholar] [CrossRef] [Green Version]
- Patterson-West, J.; Arroyo-Mendoza, M.; Hsieh, M.L.; Harrison, D.; Walker, M.M.; Knipling, L.; Hinton, D.M. The Bacteriophage T4 MotB Protein, a DNA-Binding Protein, Improves Phage Fitness. Viruses 2018, 10, 343. [Google Scholar] [CrossRef] [Green Version]
- Luke, K.; Radek, A.; Liu, X.; Campbell, J.; Uzan, M.; Haselkorn, R.; Kogan, Y. Microarray analysis of gene expression during bacteriophage T4 infection. Virology 2002, 299, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Kashlev, M.; Nudler, E.; Goldfarb, A.; White, T.; Kutter, E. Bacteriophage T4 Alc protein: A transcription termination factor sensing local modification of DNA. Cell 1993, 75, 147–154. [Google Scholar] [CrossRef]
- Drivdahl, R.H.; Kutter, E.M. Inhibition of transcription of cytosine-containing DNA in vitro by the alc gene product of bacteriophage T4. J. Bacteriol. 1990, 172, 2716–2727. [Google Scholar] [CrossRef] [PubMed]
- Brandao, A.; Pires, D.P.; Coppens, L.; Voet, M.; Lavigne, R.; Azeredo, J. Differential transcription profiling of the phage LUZ19 infection process in different growth media. RNA Biol. 2021, 18, 1778–1790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Yin, S.; Li, G.; Wang, J.; Huang, G.; Jiang, B.; You, B.; Gong, Y.; Zhang, C.; Luo, X.; et al. Global Transcriptomic Analysis of the Interactions between Phage phiAbp1 and Extensively Drug-Resistant Acinetobacter baumannii. mSystems 2019, 4, e00068-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mojardin, L.; Salas, M. Global Transcriptional Analysis of Virus-Host Interactions between Phage varphi29 and Bacillus subtilis. J. Virol 2016, 90, 9293–9304. [Google Scholar] [PubMed] [Green Version]
- Leskinen, K.; Blasdel, B.G.; Lavigne, R.; Skurnik, M. RNA-Sequencing Reveals the Progression of Phage-Host Interactions between phiR1-37 and Yersinia enterocolitica. Viruses 2016, 8, 111. [Google Scholar] [CrossRef] [Green Version]
- Kuptsov, N.; Kornienko, M.; Bespiatykh, D.; Gorodnichev, R.; Klimina, K.; Veselovsky, V.; Shitikov, E. Global Transcriptomic Response of Staphylococcus aureus to Virulent Bacteriophage Infection. Viruses 2022, 14, 567. [Google Scholar] [CrossRef]
- Guegler, C.K.; Laub, M.T. Shutoff of host transcription triggers a toxin-antitoxin system to cleave phage RNA and abort infection. Mol. Cell 2021, 81, 2361–2373.e9. [Google Scholar] [CrossRef]
- Cowan, J.; d’Acci, K.; Guttman, B.; Kutter, E. Gel Analysis of T4 Prereplicative Proteins; American Society of Microbiology: Washington, DC, USA, 1994; Volume 4, pp. 520–527. [Google Scholar]
- Kutter, E.; Bryan, D.; Ray, G.; Brewster, E.; Blasdel, B.; Guttman, B. From Host to Phage Metabolism: Hot Tales of Phage T4’s Takeover of E. coli. Viruses 2018, 10, 387. [Google Scholar] [CrossRef] [Green Version]
- Howard-Varona, C.; Hargreaves, K.R.; Solonenko, N.E.; Markillie, L.M.; White, R.A., 3rd; Brewer, H.M.; Ansong, C.; Orr, G.; Adkins, J.N.; Sullivan, M.B. Multiple mechanisms drive phage infection efficiency in nearly identical hosts. ISME J. 2018, 12, 1605–1618. [Google Scholar] [CrossRef] [Green Version]
- Howard-Varona, C.; Lindback, M.M.; Bastien, G.E.; Solonenko, N.; Zayed, A.A.; Jang, H.; Andreopoulos, B.; Brewer, H.M.; Glavina Del Rio, T.; Adkins, J.N.; et al. Phage-specific metabolic reprogramming of virocells. ISME J. 2020, 14, 881–895. [Google Scholar] [CrossRef] [Green Version]
- Gerovac, M.; Wicke, L.; Chihara, K.; Schneider, C.; Lavigne, R.; Vogel, J. A Grad-seq View of RNA and Protein Complexes in Pseudomonas aeruginosa under Standard and Bacteriophage Predation Conditions. mBio 2021, 12, e03454-20. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Peters, T.J.; Yang, P.; Luu, L.D.W.; Vuong, J.; Krycer, J.R.; O’Donoghue, S.I. Temporal ordering of omics and multiomic events inferred from time-series data. NPJ Syst. Biol. Appl. 2020, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Cahova, H.; Winz, M.L.; Hofer, K.; Nubel, G.; Jaschke, A. NAD captureSeq indicates NAD as a bacterial cap for a subset of regulatory RNAs. Nature 2015, 519, 374–377. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Mann, M. MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat. Biotechnol. 2008, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Glatter, T.; Ludwig, C.; Ahrné, E.; Aebersold, R.; Heck, A.J.; Schmidt, A. Large-scale quantitative assessment of different in-solution protein digestion protocols reveals superior cleavage efficiency of tandem Lys-C/trypsin proteolysis over trypsin digestion. J. Proteome Res. 2012, 11, 5145–5156. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Qi, D.; Alawneh, A.M.; Yonesaki, T.; Otsuka, Y. Rapid Degradation of Host mRNAs by Stimulation of RNase E Activity by Srd of Bacteriophage T4. Genetics 2015, 201, 977–987. [Google Scholar] [CrossRef]
- Kim, J.; Shen, R.; Olcott, M.C.; Rajagopal, I.; Mathews, C.K. Adenylate kinase of Escherichia coli, a component of the phage T4 dNTP synthetase complex. J. Biol. Chem. 2005, 280, 28221–28229. [Google Scholar] [CrossRef] [Green Version]
- Vigier, P.R.; Marcovich, H. The E. coli mRNA as a precursor for the T4 phage nucleic acids. Mol. Gen. Genet. 1974, 133, 353–362. [Google Scholar] [CrossRef]
- Yang, J.Y.; Fang, W.; Miranda-Sanchez, F.; Brown, J.M.; Kauffman, K.M.; Acevero, C.M.; Bartel, D.P.; Polz, M.F.; Kelly, L. Degradation of host translational machinery drives tRNA acquisition in viruses. Cell Syst. 2021, 12, 771–779.e5. [Google Scholar] [CrossRef]
- Muto, A.; Sato, M.; Tadaki, T.; Fukushima, M.; Ushida, C.; Himeno, H. Structure and function of 10Sa RNA: Trans-translation system. Biochimie 1996, 78, 985–991. [Google Scholar] [CrossRef]
- Christensen, S.K.; Gerdes, K. RelE toxins from bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol. Microbiol. 2003, 48, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Ranquet, C.; Geiselmann, J.; Toussaint, A. The tRNA function of SsrA contributes to controlling repression of bacteriophage Mu prophage. Proc. Natl. Acad. Sci. USA 2001, 98, 10220–10225. [Google Scholar] [CrossRef] [Green Version]
- Keiler, K.C.; Shapiro, L. tmRNA in Caulobacter crescentus is cell cycle regulated by temporally controlled transcription and RNA degradation. J. Bacteriol. 2003, 185, 1825–1830. [Google Scholar] [CrossRef] [Green Version]
- Guerrier-Takada, C.; Gardiner, K.; Marsh, T.; Pace, N.; Altman, S. The RNA moiety of ribonuclease P is the catalytic subunit of the enzyme. Cell 1983, 35 Pt 2, 849–857. [Google Scholar] [CrossRef]
- Jarrous, N.; Reiner, R. Human RNase P: A tRNA-processing enzyme and transcription factor. Nucleic Acids Res. 2007, 35, 3519–3524. [Google Scholar] [CrossRef] [Green Version]
- Babitzke, P.; Romeo, T. CsrB sRNA family: Sequestration of RNA-binding regulatory proteins. Curr. Opin. Microbiol. 2007, 10, 156–163. [Google Scholar] [CrossRef]
- Reichenbach, B.; Maes, A.; Kalamorz, F.; Hajnsdorf, E.; Görke, B. The small RNA GlmY acts upstream of the sRNA GlmZ in the activation of glmS expression and is subject to regulation by polyadenylation in Escherichia coli. Nucleic Acids Res. 2008, 36, 2570–2580. [Google Scholar] [CrossRef]
- Hobbs, E.C.; Astarita, J.L.; Storz, G. Small RNAs and small proteins involved in resistance to cell envelope stress and acid shock in Escherichia coli: Analysis of a bar-coded mutant collection. J. Bacteriol. 2010, 192, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Raleigh, E.A.; Wilson, G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc. Natl. Acad. Sci. USA 1986, 83, 9070–9074. [Google Scholar] [CrossRef] [Green Version]
- Alawneh, A.M.; Qi, D.; Yonesaki, T.; Otsuka, Y. An ADP-ribosyltransferase Alt of bacteriophage T4 negatively regulates the Escherichia coli MazF toxin of a toxin-antitoxin module. Mol. Microbiol. 2016, 99, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Bingham, R.; Ekunwe, S.I.; Falk, S.; Snyder, L.; Kleanthous, C. The major head protein of bacteriophage T4 binds specifically to elongation factor Tu. J. Biol. Chem. 2000, 275, 23219–23226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, M.L.; Rossmann, M.G. Structure and function of bacteriophage T4. Future Microbiol. 2014, 9, 1319–1327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serres, M.H.; Gopal, S.; Nahum, L.A.; Liang, P.; Gaasterland, T.; Riley, M. A functional update of the Escherichia coli K-12 genome. Genome Biol. 2001, 2, research0035.1. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.D.; Tomczak, K.; John, A.C.S. Bacteriophages inhibit degradation of abnormal proteins in E. coli. Nature 1978, 275, 424–428. [Google Scholar] [CrossRef]
- Mailhammer, R.; Yang, H.L.; Reiness, G.; Zubay, G. Effects of bacteriophage T4-induced modification of Escherichia coli RNA polymerase on gene expression in vitro. Proc. Natl. Acad. Sci. USA 1975, 72, 4928–4932. [Google Scholar] [CrossRef] [Green Version]
- Von Gabain, A.; Bujard, H. Interaction of E. coli RNA polymerase with promotors of coliphage T5: The rates of complex formation and decay and their correlation with in vitro and in vivo transcriptional activity. Mol. Gen. Genet. 1977, 157, 301–311. [Google Scholar] [CrossRef]
- Chamberlin, M.; McGrath, J.; Waskell, L. New RNA polymerase from Escherichia coli infected with bacteriophage T7. Nature 1970, 228, 227–231. [Google Scholar] [CrossRef]
- Tiemann, B.; Depping, R.; Rüger, W. Overexpression, purification, and partial characterization of ADP-ribosyltransferases modA and modB of bacteriophage T4. Gene Expr. 1999, 8, 187–196. [Google Scholar]
- Otsuka, Y.; Yonesaki, T. Dmd of bacteriophage T4 functions as an antitoxin against Escherichia coli LsoA and RnlA toxins. Mol. Microbiol. 2012, 83, 669–681. [Google Scholar] [CrossRef]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, M. Proteases and protein degradation in Escherichia coli. J. Exp. 1992, 48, 178–201. [Google Scholar]
- Gottesman, S. Proteases and their targets in Escherichia coli. Annu. Rev. Genet. 1996, 30, 465–506. [Google Scholar] [CrossRef] [PubMed]
- Skorupski, K.; Tomaschewski, J.; Rüger, W.; Simon, L.D. A bacteriophage T4 gene which functions to inhibit Escherichia coli Lon protease. J. Bacteriol. 1988, 170, 3016–3024. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Suzuki, C.K. Functional mechanics of the ATP-dependent Lon protease- lessons from endogenous protein and synthetic peptide substrates. Biochim. Biophys. Acta (BBA) Proteins Proteom. 2008, 1784, 727–735. [Google Scholar] [CrossRef] [Green Version]
- Hilliard, J.J.; Simon, L.D.; van Melderen, L.; Maurizi, M.R. PinA inhibits ATP hydrolysis and energy-dependent protein degradation by Lon protease. J. Biol. Chem. 1998, 273, 524–527. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Li, X.; Wang, Z.; Li, G.; Ma, B.; Chen, H.; Li, N.; Yang, H.; Wang, Y.; Liu, B. Systemic Expression, Purification, and Initial Structural Characterization of Bacteriophage T4 Proteins without Known Structure Homologs. Front. Microbiol. 2021, 12, 674415. [Google Scholar] [CrossRef]
- Hinton, D.M. Transcription from a Bacteriophage-T4 Middle Promoter Using T4 Mota Protein and Phage-Modified RNA-Polymerase. J. Biol. Chem. 1991, 266, 18034–18044. [Google Scholar] [CrossRef]
- Guild, N.; Gayle, M.; Sweeney, R.; Hollingsworth, T.; Modeer, T.; Gold, L. Transcriptional activation of bacteriophage T4 middle promoters by the motA protein. J. Mol. Biol. 1988, 199, 241–258. [Google Scholar] [CrossRef]
- Pene, C.; Uzan, M. The bacteriophage T4 anti-sigma factor AsiA is not necessary for the inhibition of early promoters in vivo. Mol. Microbiol. 2000, 35, 1180–1191. [Google Scholar] [CrossRef]
- Kolesky, S.E.; Ouhammouch, M.; Geiduschek, E.P. The mechanism of transcriptional activation by the topologically DNA-linked sliding clamp of bacteriophage T4. J. Mol. Biol. 2002, 321, 767–784. [Google Scholar] [CrossRef]
- Nechaev, S.; Kamali-Moghaddam, M.; Andre, E.; Leonetti, J.P.; Geiduschek, E.P. The bacteriophage T4 late-transcription coactivator gp33 binds the flap domain of Escherichia coli RNA polymerase. Proc. Natl. Acad. Sci. USA 2004, 101, 17365–17370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moarefi, I.; Jeruzalmi, D.; Turner, J.; O’Donnell, M.; Kuriyan, J. Crystal structure of the DNA polymerase processivity factor of T4 bacteriophage. J. Mol. Biol 2000, 296, 1215–1223. [Google Scholar] [CrossRef]
- Nossal, N.G. Protein-protein interactions at a DNA replication fork: Bacteriophage T4 as a model. FASEB J. 1992, 6, 871–878. [Google Scholar] [CrossRef]
- Mullaney, J.M.; Black, L.W. Capsid targeting sequence targets foreign proteins into bacteriophage T4 and permits proteolytic processing. J. Mol. Biol. 1996, 261, 372–385. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.; Sengstag, C.; Kellenberger, E.; Bickle, T.A. Gene 68, a new bacteriophage T4 gene which codes for the 17K prohead core protein is involved in head size determination. J. Mol. Biol. 1984, 179, 415–430. [Google Scholar] [CrossRef]
- Sanson, B.; Hu, R.M.; Troitskayadagger, E.; Mathy, N.; Uzan, M. Endoribonuclease RegB from bacteriophage T4 is necessary for the degradation of early but not middle or late mRNAs. J. Mol. Biol. 2000, 297, 1063–1074. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wolfram-Schauerte, M.; Pozhydaieva, N.; Viering, M.; Glatter, T.; Höfer, K. Integrated Omics Reveal Time-Resolved Insights into T4 Phage Infection of E. coli on Proteome and Transcriptome Levels. Viruses 2022, 14, 2502. https://doi.org/10.3390/v14112502
Wolfram-Schauerte M, Pozhydaieva N, Viering M, Glatter T, Höfer K. Integrated Omics Reveal Time-Resolved Insights into T4 Phage Infection of E. coli on Proteome and Transcriptome Levels. Viruses. 2022; 14(11):2502. https://doi.org/10.3390/v14112502
Chicago/Turabian StyleWolfram-Schauerte, Maik, Nadiia Pozhydaieva, Madita Viering, Timo Glatter, and Katharina Höfer. 2022. "Integrated Omics Reveal Time-Resolved Insights into T4 Phage Infection of E. coli on Proteome and Transcriptome Levels" Viruses 14, no. 11: 2502. https://doi.org/10.3390/v14112502
APA StyleWolfram-Schauerte, M., Pozhydaieva, N., Viering, M., Glatter, T., & Höfer, K. (2022). Integrated Omics Reveal Time-Resolved Insights into T4 Phage Infection of E. coli on Proteome and Transcriptome Levels. Viruses, 14(11), 2502. https://doi.org/10.3390/v14112502





