Insights on 21 Years of HBV Surveillance in Blood Donors in France
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Population
2.2. HBV Donation Testing
2.3. Definitions
2.4. Residual Risk Estimates
2.5. Lookback Investigations
2.6. Statistical Analysis
3. Results
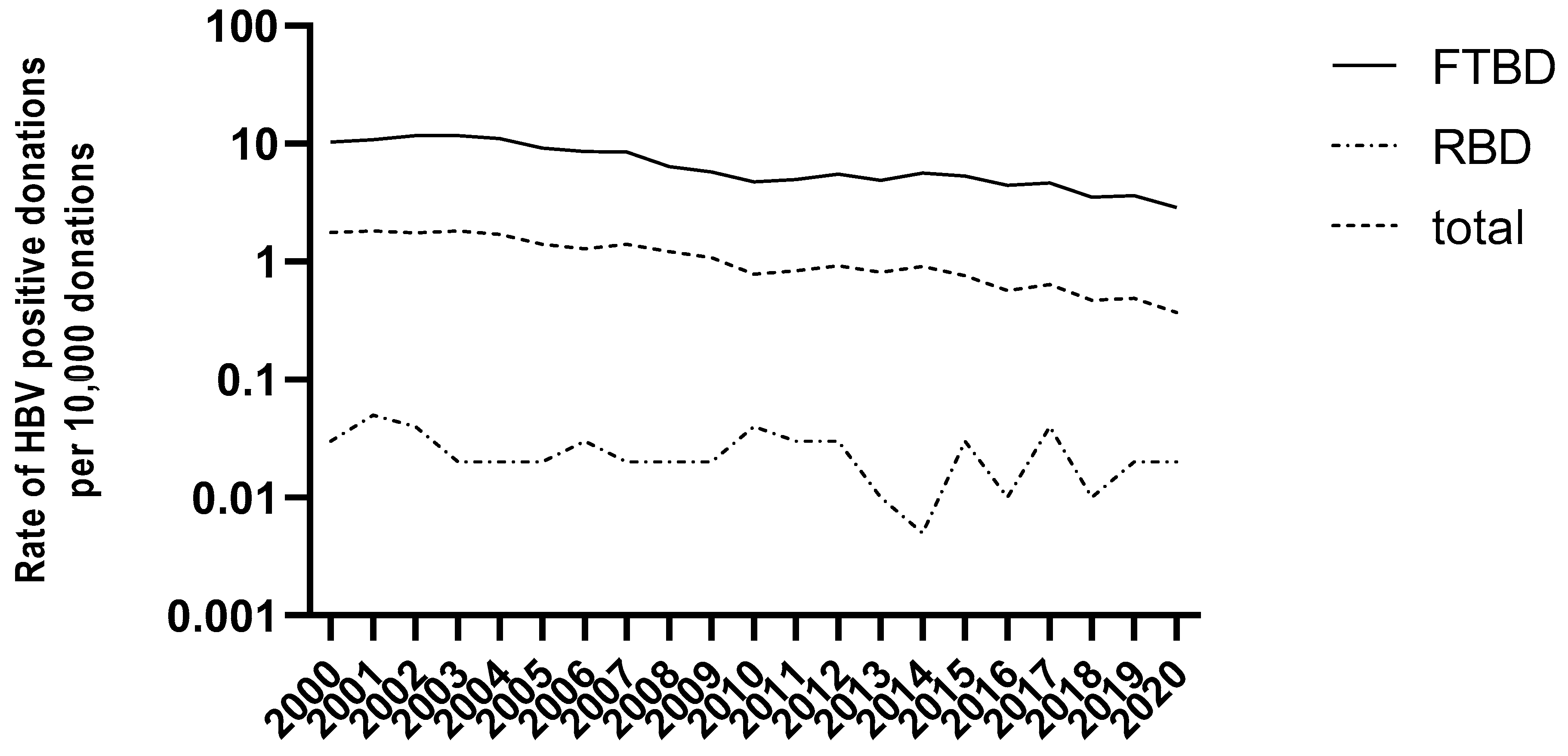
3.1. HBV Epidemiology in Blood Donors in France (2000–2020)
3.2. HBV Molecular Epidemiology (2005–2020)
3.3. Characterization of Donations according to HBV Markers (2005–2020)
3.4. Comprehensive Analysis of Donations Exhibiting Discrepant Molecular and Serological HBV Markers
3.5. Seronegative OBI: A Case Report
3.6. HBV Residual Risk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO HBV Fact Sheet. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 12 September 2022).
- Fung, S.; Choi, H.S.J.; Gehring, A.; Janssen, H.L.A. Getting to HBV Cure: The Promising Paths Forward. Hepatology 2022, 76, 233–250. [Google Scholar] [CrossRef] [PubMed]
- Bruce, M.G.; Bruden, D.; Hurlburt, D.; Morris, J.; Bressler, S.; Thompson, G.; Lecy, D.; Rudolph, K.; Bulkow, L.; Hennessy, T.; et al. Protection and Antibody Levels 35 Years after Primary Series with Hepatitis B Vaccine and Response to a Booster Dose. Hepatology 2022, 76, 1180–1189. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Guo, G.N.; Li, C. The Impact of Hepatitis B Vaccination in the United States, 1999–2018. Hepatology 2022, 75, 1566–1578. [Google Scholar] [CrossRef]
- Stramer, S.L.; Wend, U.; Candotti, D.; Foster, G.A.; Hollinger, F.B.; Dodd, R.Y.; Allain, J.-P.; Gerlich, W. Nucleic Acid Testing to Detect HBV Infection in Blood Donors. N. Engl. J. Med. 2011, 364, 236–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Servant-Delmas, A.; Mercier, M.; Ghouzzi, M.E.; Girault, A.; Bouchardeau, F.; Pillonel, J.; Laperche, S. National Survey of Hepatitis B Virus (HBV) Polymorphism in Asymptomatic HBV Blood Donors from 1999 to 2007 in France. Transfusion 2010, 50, 2607–2618. [Google Scholar] [CrossRef] [PubMed]
- Thibault, V.; Servant-Delmas, A.; Ly, T.D.; Roque-Afonso, A.-M.; Laperche, S. Performance of HBsAg Quantification Assays for Detection of Hepatitis B Virus Genotypes and Diagnostic Escape-Variants in Clinical Samples. J. Clin. Virol. 2017, 89, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Weber, B. Genetic Variability of the S Gene of Hepatitis B Virus: Clinical and Diagnostic Impact. J. Clin. Virol. 2005, 32, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Bloch, E.M.; Vermeulen, M.; Murphy, E. Blood Transfusion Safety in Africa: A Literature Review of Infectious Disease and Organizational Challenges. Transfus. Med. Rev. 2012, 26, 164–180. [Google Scholar] [CrossRef] [Green Version]
- Allain, J.; Opare-Sem, O.; Sarkodie, F.; Rahman, R.; Owusu-Ofori, S. Deferred Donor Care in a Regional Hospital Blood Center in Ghana. Transfusion 2009, 49, 669–675. [Google Scholar] [CrossRef]
- Gao, Z.; Liu, J.; Fu, P.; Huang, M.; Cao, R.; Wen, X.; Zhang, C.; He, T.; Mao, W.; Liao, D.; et al. Estimation of Hepatitis B–Positive Rates in Chinese Blood Donors by Combining Predonation and Postdonation Screening Results. Transfusion 2019, 59, 1749–1754. [Google Scholar] [CrossRef]
- Van De Laar, T.J.; Der Kreek, T.M.-V.; Backer, M.W.M.-D.; Hogema, B.M.; Zaaijer, H.L. The Yield of Universal Antibody to Hepatitis B Core Antigen Donor Screening in the Netherlands, a Hepatitis B Virus Low-endemic Country. Transfusion 2015, 55, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.K.; Busch, M.P.; Schuller, A.; Ismay, S.; Cheng, A.; Seed, C.R.; Jungbauer, C.; Minsk, P.M.; Sondag-Thull, D.; Wendel, S.; et al. International Survey on NAT Testing of Blood Donations: Expanding Implementation and Yield from 1999 to 2009. Vox Sang 2012, 102, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Fiedler, S.A.; Oberle, D.; Chudy, M.; Scheiblauer, H.; Henseler, O.; Halbauer, J.; Heiden, M.; Funk, M. Effectiveness of Blood Donor Screening by HIV, HCV, HBV-NAT Assays, as Well as HBsAg and Anti-HBc Immunoassays in Germany (2008–2015). Vox Sang 2019, 114, 443–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; The Taormina Workshop on Occult HBV Infection Faculty Members. Update of the Statements on Biology and Clinical Impact of Occult Hepatitis B Virus Infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makvandi, M. Update on Occult Hepatitis B Virus Infection. World J. Gastroenterol. 2016, 22, 8720–8734. [Google Scholar] [CrossRef]
- Minuk, G.Y.; Sun, D.; Uhanova, J.; Zhang, M.; Caouette, S.; Nicolle, L.E.; Gutkin, A.; Doucette, K.; Martin, B.; Giulivi, A. Occult Hepatitis B Virus Infection in a North American Community-Based Population. J. Hepatol. 2005, 42, 480–485. [Google Scholar] [CrossRef]
- Ji, D.; Pang, X.; Shen, D.; Liu, S.; Goyal, H.; Xu, H. Global Prevalence of Occult Hepatitis B: A Systematic Review and Meta-analysis. J. Viral. Hepatitis 2022, 29, 317–329. [Google Scholar] [CrossRef]
- Candotti, D.; Assennato, S.M.; Laperche, S.; Allain, J.-P.; Levicnik-Stezinar, S. Multiple HBV Transfusion Transmissions from Undetected Occult Infections: Revising the Minimal Infectious Dose. Gut 2019, 68, 313–321. [Google Scholar] [CrossRef]
- Vermeulen, M.; Dickens, C.; Lelie, N.; Walker, E.; Coleman, C.; Keyter, M.; Reddy, R.; Crookes, R.; Kramvis, A. Hepatitis B Virus Transmission by Blood Transfusion during 4 Years of Individual-donation Nucleic Acid Testing in South Africa: Estimated and Observed Window Period Risk. Transfusion 2012, 52, 880–892. [Google Scholar] [CrossRef]
- Satake, M.; Taira, R.; Yugi, H.; Hino, S.; Kanemitsu, K.; Ikeda, H.; Tadokoro, K. Infectivity of Blood Components with Low Hepatitis B Virus DNA Levels Identified in a Lookback Program. Transfusion 2007, 47, 1197–1205. [Google Scholar] [CrossRef]
- Levicnik-Stezinar, S.; Rahne-Potokar, U.; Candotti, D.; Lelie, N.; Allain, J.-P. Anti-HBs Positive Occult Hepatitis B Virus Carrier Blood Infectious in Two Transfusion Recipients. J. Hepatol. 2008, 48, 1022–1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuen, M.-F.; Wong, D.K.-H.; Lee, C.-K.; Tanaka, Y.; Allain, J.-P.; Fung, J.; Leung, J.; Lin, C.-K.; Sugiyama, M.; Sugauchi, F.; et al. Transmissibility of Hepatitis B Virus (HBV) Infection through Blood Transfusion from Blood Donors with Occult HBV Infection. Clin. Infect. Dis. 2011, 52, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Allain, J.; Mihaljevic, I.; Gonzalez-Fraile, M.I.; Gubbe, K.; Holm-Harritshøj, L.; Garcia, J.M.; Brojer, E.; Erikstrup, C.; Saniewski, M.; Wernish, L.; et al. Infectivity of Blood Products from Donors with Occult Hepatitis B Virus Infection. Transfusion 2013, 53, 1405–1415. [Google Scholar] [CrossRef] [PubMed]
- Taira, R.; Satake, M.; Momose, S.; Hino, S.; Suzuki, Y.; Murokawa, H.; Uchida, S.; Tadokoro, K. Residual Risk of Transfusion-transmitted Hepatitis B Virus (HBV) Infection Caused by Blood Components Derived from Donors with Occult HBV Infection in Japan. Transfusion 2013, 53, 1393–1404. [Google Scholar] [CrossRef]
- Seed, C.R.; Maloney, R.; Kiely, P.; Bell, B.; Keller, A.J.; Pink, J.; Team, B.S.M.S.L. Infectivity of Blood Components from Donors with Occult Hepatitis B Infection–Results from an Australian Lookback Programme. Vox Sang 2015, 108, 113–122. [Google Scholar] [CrossRef]
- Lieshout-Krikke, R.W.; van Kraaij, M.G.J.; Danovic, F.; Zaaijer, H.L. Rare Transmission of Hepatitis B Virus by Dutch Donors with Occult Infection. Transfusion 2016, 56, 691–698. [Google Scholar] [CrossRef]
- Candotti, D.; Allain, J.-P. Transfusion-Transmitted Hepatitis B Virus Infection. J. Hepatol. 2009, 51, 798–809. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, S.H.; Lelie, N.; Busch, M.P. Infectivity of Human Immunodeficiency Virus-1, Hepatitis C Virus, and Hepatitis B Virus and Risk of Transmission by Transfusion. Transfusion 2009, 49, 2454–2489. [Google Scholar] [CrossRef]
- Domanović, D.; Ushiro-Lumb, I.; Compernolle, V.; Brusin, S.; Funk, M.; Gallian, P.; Georgsen, J.; Janssen, M.; Jimenez-Marco, T.; Knutson, F.; et al. Pathogen Reduction of Blood Components during Outbreaks of Infectious Diseases in the European Union: An Expert Opinion from the European Centre for Disease Prevention and Control Consultation Meeting. Blood Transfus. Trasfus. Del Sangue 2019, 17, 433–448. [Google Scholar] [CrossRef]
- Azhar, E.I.; Hindawi, S.I.; El-Kafrawy, S.A.; Hassan, A.M.; Tolah, A.M.; Alandijany, T.A.; Bajrai, L.H.; Damanhouri, G.A. Amotosalen and Ultraviolet A Light Treatment Efficiently Inactivates Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) in Human Plasma. Vox Sang 2021, 116, 673–681. [Google Scholar] [CrossRef]
- Lanteri, M.C.; Santa-Maria, F.; Laughhunn, A.; Girard, Y.A.; Picard-Maureau, M.; Payrat, J.; Irsch, J.; Stassinopoulos, A.; Bringmann, P. Inactivation of a Broad Spectrum of Viruses and Parasites by Photochemical Treatment of Plasma and Platelets Using Amotosalen and Ultraviolet A Light. Transfusion 2020, 60, 1319–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prowse, C.V. Component Pathogen Inactivation: A Critical Review. Vox Sang 2013, 104, 183–199. [Google Scholar] [CrossRef] [PubMed]
- Assal, A.; Barlet, V.; Deschaseaux, M.; Dupont, I.; Gallian, P.; Guitton, C.; Morel, P.; David, B.; Micco, P.D. Comparison of the Analytical and Operational Performance of Two Viral Nucleic Acid Test Blood Screening Systems: Procleix Tigris and Cobas s 201. Transfusion 2009, 49, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geno2Pheno. Available online: https://hbv.geno2pheno.org/index.php (accessed on 12 September 2022).
- Zahn, A.; Li, C.; Danso, K.; Candotti, D.; Owusu-Ofori, S.; Temple, J.; Allain, J.-P. Molecular Characterization of Occult Hepatitis B Virus in Genotype E-Infected Subjects. J. Gen. Virol. 2008, 89, 409–418. [Google Scholar] [CrossRef]
- Harvala, H.; Reynolds, C.; Gibney, Z.; Derrick, J.; Ijaz, S.; Davison, K.L.; Brailsford, S. Hepatitis B Infections among Blood Donors in England between 2009 and 2018: Is an Occult Hepatitis B Infection a Risk for Blood Safety? Transfusion 2021, 61, 2402–2413. [Google Scholar] [CrossRef]
- Korelitz, J.J.; Busch, M.P.; Kleinman, S.H.; Williams, A.E.; Gilcher, R.O.; Ownby, H.E.; Schreiber, G.B. A Method for Estimating Hepatitis B Virus Incidence Rates in Volunteer Blood Donors. National Heart, Lung, and Blood Institute Retrovirus Epidemiology Donor Study. Transfusion 1997, 37, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Seed, C.R.; Kiely, P.; Keller, A.J. Residual Risk of Transfusion Transmitted Human Immunodeficiency Virus, Hepatitis B Virus, Hepatitis C Virus and Human T Lymphotrophic Virus. Intern. Med. J. 2005, 35, 592–598. [Google Scholar] [CrossRef]
- Lelie, N.; Vermeulen, M.; van Drimmelen, H.; Coleman, C.; Bruhn, R.; Reddy, R.; Busch, M.; Kleinman, S. Direct comparison of three residual risk models for hepatitis B virus window period infections using updated input parameters. Vox Sang 2020, 115, 133–145. [Google Scholar] [CrossRef]
- Candotti, D.; Grabarczyk, P.; Ghiazza, P.; Roig, R.; Casamitjana, N.; Iudicone, P.; Schmidt, M.; Bird, A.; Crookes, R.; Brojer, E.; et al. Characterization of Occult Hepatitis B Virus from Blood Donors Carrying Genotype A2 or Genotype D Strains. J. Hepatol. 2008, 49, 537–547. [Google Scholar] [CrossRef]
- Vaux, S.; Laporal, S.; Pioche, C.; Bruyand, M.; Lévy-Bruhl, D.; Lot, F.; Brouard, C. Surveillance de l’hépatite B Aiguë Par La Déclaration Obligatoire, France, 2003–2018. Bull. Epidémiol. Hebd. 2019, 2019, 490–495. [Google Scholar]
- Thibault, V.; Laperche, S.; Thiers, V.; Sayon, S.; Letort, M.-J.; Delarocque-Astagneau, E.; Antona, D. Molecular Epidemiology and Clinical Characteristics of Hepatitis B Identified through the French Mandatory Notification System. PLoS ONE 2013, 8, e75267. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.; Jiles, R.; Harris, A.M.; Gupta, N.; Teshale, E. Incidence and Prevalence of Sexually Transmitted Hepatitis B, United States, 2013–2018. Sex Transm. Dis. 2021, 48, 305–309. [Google Scholar] [CrossRef]
- Llaneras, J.; Riveiro-Barciela, M.; Rando-Segura, A.; Marcos-Fosch, C.; Roade, L.; Velázquez, F.; Rodríguez-Frías, F.; Esteban, R.; Buti, M. Etiologies and Features of Acute Viral Hepatitis in Spain. Clin. Gastroenterol. H 2021, 19, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- van Houdt, R.; Bruisten, S.M.; Koedijk, F.D.H.; Dukers, N.H.T.M.; de Coul, E.L.M.O.; Mostert, M.C.; Niesters, H.G.M.; Richardus, J.H.; de Man, R.A.; van Doornum, G.J.J.; et al. Molecular Epidemiology of Acute Hepatitis B in the Netherlands in 2004: Nationwide Survey. J. Med. Virol. 2007, 79, 895–901. [Google Scholar] [CrossRef]
- Basic, M.; Kubesch, A.; Kuhnhenn, L.; Görgülü, E.; Finkelmeier, F.; Dietz, J.; Knabe, M.; Mücke, V.T.; Mücke, M.M.; Berger, A.; et al. Not Uncommon: HBV Genotype G Co-infections among Healthy European HBV Carriers with Genotype A and E Infection. Liver Int. 2021, 41, 1278–1289. [Google Scholar] [CrossRef]
- Sloan, R.D.; Strang, A.L.; Ramsay, M.E.; Teo, C.-G. Genotyping of Acute HBV Isolates from England, 1997–2001. J. Clin. Virol. 2009, 44, 157–160. [Google Scholar] [CrossRef]
- Rosenberg, G.K.; Lattimore, S.; Brailsford, S.R.; Hewitt, P.E.; Tettmar, K.I.; Kitchen, A.D.; Ijaz, S.; Tedder, R.S. Acute Hepatitis B in Blood Donors over a 5-year Period in England and North Wales: Who Is Getting Infected? Transfusion 2014, 54, 1660–1665. [Google Scholar] [CrossRef]
- Teshale, E.H.; Ramachandran, S.; Xia, G.; Roberts, H.; Groeger, J.; Barry, V.; Hu, D.J.; Holmberg, S.D.; Holtzman, D.; Ward, J.W.; et al. Genotypic Distribution of Hepatitis B Virus (HBV) Among Acute Cases of HBV Infection, Selected United States Counties, 1999–2005. Clin. Infect. Dis. 2011, 53, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Bruni, R.; Villano, U.; Taffon, S.; Equestre, M.; Madonna, E.; Chionne, P.; Candido, A.; Dettori, S.; Pisani, G.; Rapicetta, M.; et al. Retrospective Analysis of Acute HBV Infections Occurred in 1978–79 and 1994–95 in North-East Italy: Increasing Prevalence of BCP/Pre-Core Mutants in Sub-Genotype D3. BMC Infect. Dis. 2020, 20, 78. [Google Scholar] [CrossRef] [Green Version]
- Dodd, R.Y.; Nguyen, M.L.; Krysztof, D.E.; Notari, E.P.; Stramer, S.L. Blood Donor Testing for Hepatitis B Virus in the United States: Is There a Case for Continuation of Hepatitis B Surface Antigen Detection? Transfusion 2018, 58, 2166–2170. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, M.; Drimmelen, H.; Coleman, C.; Sykes, W.; Reddy, R.; Busch, M.; Kleinman, S.; Lelie, N. Reassessment of Hepatitis B Virus Window Periods for Two Transcription-mediated Amplification Assays Using Screening Data of South African Blood Donors. Transfusion 2019, 59, 2922–2930. [Google Scholar] [CrossRef] [PubMed]
- Candotti, D.; Laperche, S. Hepatitis B Virus Blood Screening: Need for Reappraisal of Blood Safety Measures? Front. Med. 2018, 5, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lelie, N.; Bruhn, R.; Busch, M.; Vermeulen, M.; Tsoi, W.; Kleinman, S.; Coleman, C.; Reddy, R.; Bird, A.; Cable, R.; et al. Detection of Different Categories of Hepatitis B Virus (HBV) Infection in a Multi-regional Study Comparing the Clinical Sensitivity of Hepatitis B Surface Antigen and HBV-DNA Testing. Transfusion 2017, 57, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Seed, C.R.; Allain, J.; Lozano, M.; Laperche, S.; Gallian, P.; Gross, S.; Kwon, S.; Oh, E.Y.; Kim, J.N.; Chua, S.S.; et al. International Forum on Occult Hepatitis B Infection and Transfusion Safety. Vox Sang 2019, 114, e1–e35. [Google Scholar] [CrossRef]
- Deng, X.; Guo, X.; Gu, H.; Wang, D.; Laperche, S.; Allain, J.; Zang, L.; Candotti, D. Anti-HBc-nonreactive Occult Hepatitis B Infections with HBV Genotypes B and C in Vaccinated Immunocompetent Adults. J. Viral. Hepat. 2022. [Google Scholar] [CrossRef]
- Mosley, J.W.; Stevens, C.E.; Aach, R.A.; Hollinger, F.B.; Mimms, L.T.; Solomon, L.R.; Barbosa, L.H.; Nemo, G.J. Donor Screening for Antibody to Hepatitis B Core Antigen and Hepatitis B Virus Infection in Transfusion Recipients. Transfusion 1995, 35, 5–12. [Google Scholar] [CrossRef]
- Aach, R.D.; Alter, H.J.; Hollinger, F.B.; Holland, P.V.; Lander, J.J.; Melnick, J.L.; Weiler, J.M. Risk of Transfusing Blood Containing Antibody to Hepatitis-B Surface Antigen. Lancet 1974, 304, 190–193. [Google Scholar] [CrossRef]
- Gerlich, W.H. Breakthrough of Hepatitis B Virus Escape Mutants after Vaccination and Virus Reactivation. J. Clin. Virol. 2006, 36, S18–S22. [Google Scholar] [CrossRef]
- Ekiaby, M.E.; Tanaka, J.; Drimmelen, H.; Allain, J.; Lelie, N. Infectivity of Hepatitis B Virus (HBV) Surface Antigen (HBsAg) Positive Plasma with Undetectable HBV-DNA: Can HBsAg Screening Be Discontinued in Egyptian Blood Donors? J. Viral. Hepat. 2022, 29, 330–339. [Google Scholar] [CrossRef]


| 1. DNA+ /HBsAg+/ Anti-HBc+ (CHB) | 2. DNA+ /HBsAg−/ Anti-HBc+ (OBI) | 3. DNA+ /HBsAg+/ Anti-Hbc− (Probable ABI) | 4. DNA+ /HBsAg−/ Anti-Hbc− (Seronegative OBI) | |
|---|---|---|---|---|
| Total | 2079 | 81 | 27 | 25 |
| donor status | ||||
| first-time BD | 2045 (98%) | 69 (85%) | 7 (26%) | 10 (40%) |
| repeat BD | 18 (1%) | 11 (14%) | 18 (63%) | 15 (60%) |
| unknown | 16 (1%) | 1 (1%) | 2 (1%) | 0 (0%) |
| p-value (2 vs. 3) | <0.0001 | |||
| p-value (3 vs. 4) | 0.5512 | |||
| p-value (2 vs. 4) | <0.0001 | |||
| Age [IC95] | ||||
| median | 33 | 54 | 47 | 28 |
| minimum | 18 | 20 | 19 | 18 |
| maximum | 69 | 69 | 68 | 67 |
| p-value (2 vs. 3) | 0.1739 | |||
| p-value (3 vs. 4) | 0.0616 | |||
| p-value (2 vs. 4) | <0.0001 | |||
| Gender | ||||
| males | 1597 | 68 | 20 | 14 |
| females | 479 | 13 | 5 | 11 |
| unknown | 0 | 0 | 2 | 0 |
| sex ratio | 3,3 | 5,2 | 4,0 | 1,3 |
| p-value (2 vs. 3) | 0.7611 | |||
| p-value (3 vs. 4) | 0.1284 | |||
| p-value (2 vs. 4) | 0.006 | |||
| Viral load 1 | ||||
| median (log IU/mL) | 2.75 | 0.70 | 5,24 | 1.04 |
| minimum (log IU/mL) | 0.70 | 0.70 | 2.23 | 0.70 |
| maximum (log IU/mL) | 9.53 | 3.03 | 8.04 | 2.65 |
| p-value (2 vs. 3) | <0.0001 | |||
| p-value (3 vs. 4) | <0.0001 | |||
| p-value (2 vs. 4) | 0.0021 | |||
| anti-HBs (mIU/mL) | ||||
| negative | 1510 (4%)2 | 24 (50%) | 17 (100%) | 8 (40%) |
| positive | 69 (96%) | 24 (50%) | 0 (0%) | 12 (60%) |
| not tested | 497 | 33 | 8 | 5 |
| median | 18 | 55 | 0 | 120 |
| Genotype distribution 3 | ||||
| Total genotyped | 1593 | 44 | 22 | 20 |
| A | 438 (21%) | 10 (23%) | 13 (55%) | 6 (30%) |
| B | 77 (4%) | 2 (5%) | 2 (9%) | 3 (15%) |
| C | 73 (4%) | 1 (2%) | 1 (5%) | 0 (0%) |
| D | 647 (31%) | 21 (48%) | 2 (9%) | 3 (15%) |
| E | 348 (16%) | 9 (20%) | 4 (18%) | 5 (25%) |
| F | 10 (1%) | 1 (2%) | 1 (5%) | 3 (15%) |
| p-value (2 vs. 3) | 0.0266 | |||
| p-value (3 vs. 4) | 0.4276 | |||
| p-value (2 vs. 4) | 0.0674 | |||
| (1) viral load below the quantification level (6 IU/mL) were considered at 5 IU/mL (0.70 log IU/mL) | ||||
| (2) % of the tested samples | ||||
| (3) % of genotyped samples | ||||
| Donation | Follow Up | Donor History | Previous Donation | Conclusion (2) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Year | Donor Status (FTBD/RBD) | Sex | Age | Interdonation Interval (Days) | Viral Load (IU/mL) (1) | Genotype | Anti-HBs (mIU/mL) | HBsAg Seroconversion (Days or Month (M) Post Donation) | Anti-HBc Seroconversion (Days or Month (M) Post Donation) | Anti-HBs (mIU/mL) in Control Samples (Days Post Donation) | Vaccination (Year) | Origin | Risk Factor | ||
| 1 | 2007 | FTBD | M | 28 | - | 8 | A | NT | Yes (Day 20) | No (day 20) | NT | ? | Caribbean | Not investigated | - | Acute |
| 2 | 2010 | RBD | M | 28 | 136 | 44 | E | 414 | NT | NT | NT | ? | France | Not investigated | Anti-HBs negative in 2009 | Probable vaccine breakthrough infection |
| 3 | 2010 | FTBD | M | 24 | - | NT | NT | NT | NT | NT | NT | ? | Not investigated | Not investigated | - | ? |
| 4 | 2011 | RBD | M | 36 | 112 | 5 | A2 | Negative | No (M5) | Yes (IgM) (M5) | Yes (M4) | incomplete (2004) | France | MSM | HBV-DNA negative | Vaccine breakthrough infection |
| 5 | 2011 | FTBD | F | 19 | - | 38 | E | Negative | Yes (Day 25) | No (Day 25) | NT | ? | Andorra | Partner HBV | - | Acute |
| 6 | 2012 | RBD | M | 32 | 238 | 60 | D1 | 380 | NT | NT | NT | Probable (health care professional) | France | Partner from endemic area | HBV-DNA negative | Probable vaccine breakthrough infection |
| 7 | 2012 | RBD | F | 19 | 204 | 456 | E | Negative | Yes (Day 5) | NT | NT | ? | France | Partner from endemic area | Acute | |
| 8 | 2012 | RBD | F | 57 | 56 | NT | E | 13 | No (M4, M6) | Yes (M6) | 6800 (M6) | ? | France | Partner from endemic area | Anti-HBs negative in 2011 | Acute |
| 9 | 2012 | FTBD | F | 18 | - | NT | NT | NT | No (Day 17) | No (Day 17) | NT | ? | France | Piercing | - | ? |
| 10 | 2014 | FTBD | F | 18 | - | NT | A1 | Negative | No (Day 14) | No (Day 14) | 8000 (M10) | Yes (2004) | France | Partner from endemic area | - | Vaccine breakthrough infection |
| 11 | 2014 | FTBD | M | 18 | - | 14 | B | 16 | No (Days 22, 42) | No (Days 22, 42) | 185 (Day 22) 188m (Day 42) | Yes (1997) | France | Partner from endemic area | - | Probable vaccine breakthrough infection |
| 12 | 2014 | RBD | F | 43 | 1393 | NT | NT | NT | No (Day 15) | No (Day 15) | NT | ? | Not investigated | Not investigated | Not investigated | ? |
| 13 | 2015 | RBD | F | 25 | 314 | 5 | E | 216 | No (Day 11) | Yes (IgM) (Day 11) | >25000 (Day 11) | Yes (1995) | France | Unknown | Not investigated | Vaccine breakthrough infection |
| 14 | 2015 | RBD | M | 34 | 614 | 108 | A2 | Negative | Yes (Day 18) | No (Day 18) | NT | ? | France | Unknown | Not investigated | Acute |
| 15 | 2015 | RBD | M | 20 | 395 | 5 | D1 | 193 | No (Day 28) | No (Day 28) | Yes (1995) | France | Unknown | Not investigated | Vaccine breakthrough infection | |
| 16 | 2015 | RBD | M | 49 | 104 | 8 | A2 | 20 | No (M 3, M4) | Yes (M 3, M4) | 376 (M4) | Yes (1999) | France | Unknown | HBV-DNA negative | Probable vaccine breakthrough infection |
| 17 | 2015 | FTBD | M | 20 | - | 25 | B4 | 14 | No (M 8) | No (M 8) | NT | Yes (date unknown) | ? | Unknown | - | Vaccine breakthrough infection |
| 18 | 2016 | FTBD | M | 41 | - | 5 | D3 | Negative | No (Day 9) | No (Day 9) | NT | ? | Iran | Sexual | - | ? |
| 19 | 2016 | RBD | F | 46 | 453 | 18 | F1 | Negative | Yes (M 3) | No (M 3) | NT | No | France | Sexual | Not investigated | Acute |
| 20 | 2016 | FTBD | F | 19 | - | NT | 255 | No (Day 13) | Yes (Day 13) | 1177 (Day 13) | ? | France | partner from endemic area | - | Probable vaccine breakthrough infection | |
| 21 | 2017 | RBD | M | 65 | 153 | NT | F1 | NT | No (Day 25) | No (Day 25) | 8 (Day 7) 2824 (Day 25) | ? | Unknown | HBV-DNA negative | Probable vaccine breakthrough infection | |
| 22 | 2019 | RBD | M | 68 | 182 | 5 | A2 | 275 | No (Day 39) | No (Day 39) | 606 (Day 18) | No | France | Unknown | HBV-DNA positive (see OBI case reports) | OBI |
| 23 | 2020 | FTBD | F | 20 | - | 5 | B4 | 47 | No (Day 13) | No (Day 13) | 52 (Day 13) | Yes (chidlhood) | France | Residence in China in early years of life | - | OBI |
| 24 | 2020 | RBD | F | 58 | 112 | 5 | Not typable | Negative | No (Day 67) | Yes (IgM) (Day 67) | NT | No | Partner HBV | HBV-DNA negative | Acute | |
| 25 | 2020 | FTBD | M | 24 | - | 234 | F1 | 11 | No (Day 5) | No (Day 5) | NT | incomplete (7 months before donation) | France | sexual | - | Probable vaccine breakthrough infection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappy, P.; Boizeau, L.; Candotti, D.; Le Cam, S.; Martinaud, C.; Pillonel, J.; Tribout, M.; Maugard, C.; Relave, J.; Richard, P.; et al. Insights on 21 Years of HBV Surveillance in Blood Donors in France. Viruses 2022, 14, 2507. https://doi.org/10.3390/v14112507
Cappy P, Boizeau L, Candotti D, Le Cam S, Martinaud C, Pillonel J, Tribout M, Maugard C, Relave J, Richard P, et al. Insights on 21 Years of HBV Surveillance in Blood Donors in France. Viruses. 2022; 14(11):2507. https://doi.org/10.3390/v14112507
Chicago/Turabian StyleCappy, Pierre, Laure Boizeau, Daniel Candotti, Sophie Le Cam, Christophe Martinaud, Josiane Pillonel, Martin Tribout, Claude Maugard, Josiane Relave, Pascale Richard, and et al. 2022. "Insights on 21 Years of HBV Surveillance in Blood Donors in France" Viruses 14, no. 11: 2507. https://doi.org/10.3390/v14112507
APA StyleCappy, P., Boizeau, L., Candotti, D., Le Cam, S., Martinaud, C., Pillonel, J., Tribout, M., Maugard, C., Relave, J., Richard, P., Morel, P., & Laperche, S. (2022). Insights on 21 Years of HBV Surveillance in Blood Donors in France. Viruses, 14(11), 2507. https://doi.org/10.3390/v14112507






