Activation of Interferon-Stimulated Genes following Varicella-Zoster Virus Infection in a Human iPSC-Derived Neuronal In Vitro Model Depends on Exogenous Interferon-α
Abstract
:1. Introduction
2. Materials and Methods
2.1. Neuronal Differentiation Protocol
2.2. Cell Culture and VZV Propagation
2.3. VZV Lysate Preparation
2.4. Immunofluorescence Staining
2.5. Poly(dA:dT) and Poly(I:C) Stimulation and VZV and SeV Inoculation of hiPSC-NSCs and hiPSC Neurons
2.6. Neuronal Differentiation in a Compartmentalized Model, Axonal Infection and IFNα Treatment
2.7. Microscopy and Image Analysis
2.8. Preparation of Neuronal Cell Body Lysates
2.9. gDNA and RNA Extraction and cDNA Synthesis
2.10. qPCR and RTqPCR
2.11. Statistical Analysis
3. Results
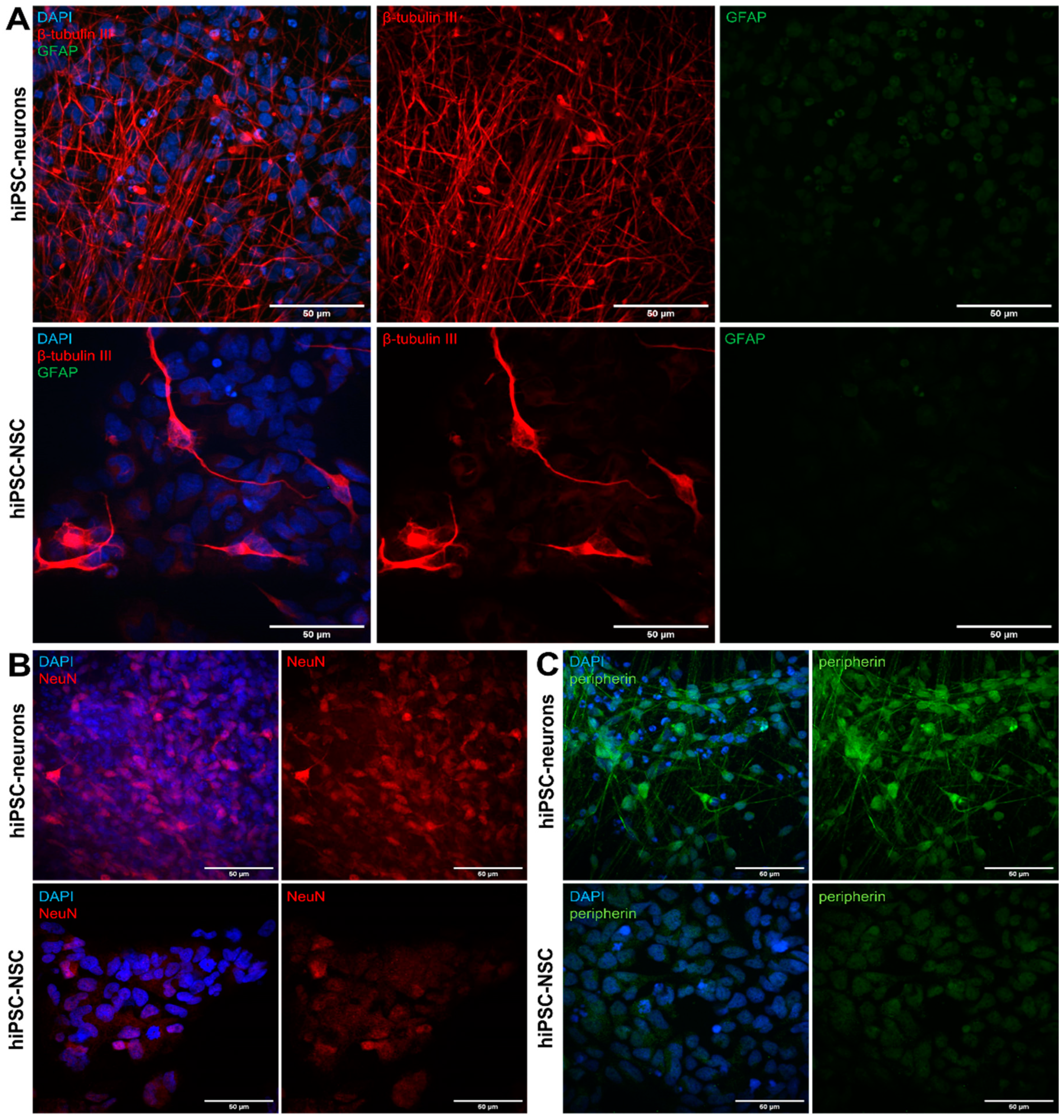
3.1. Differentiation of hiPSC-NSCs into Peripherin-Expressing hiPSC-Neurons
3.2. Generation of A Compartmentalized Neuronal Culture Model Suitable for Axonal VZV Infection
3.3. VZV Infection of and Spreading in Neuronal Cultures Do Not Trigger Type I IFN Production
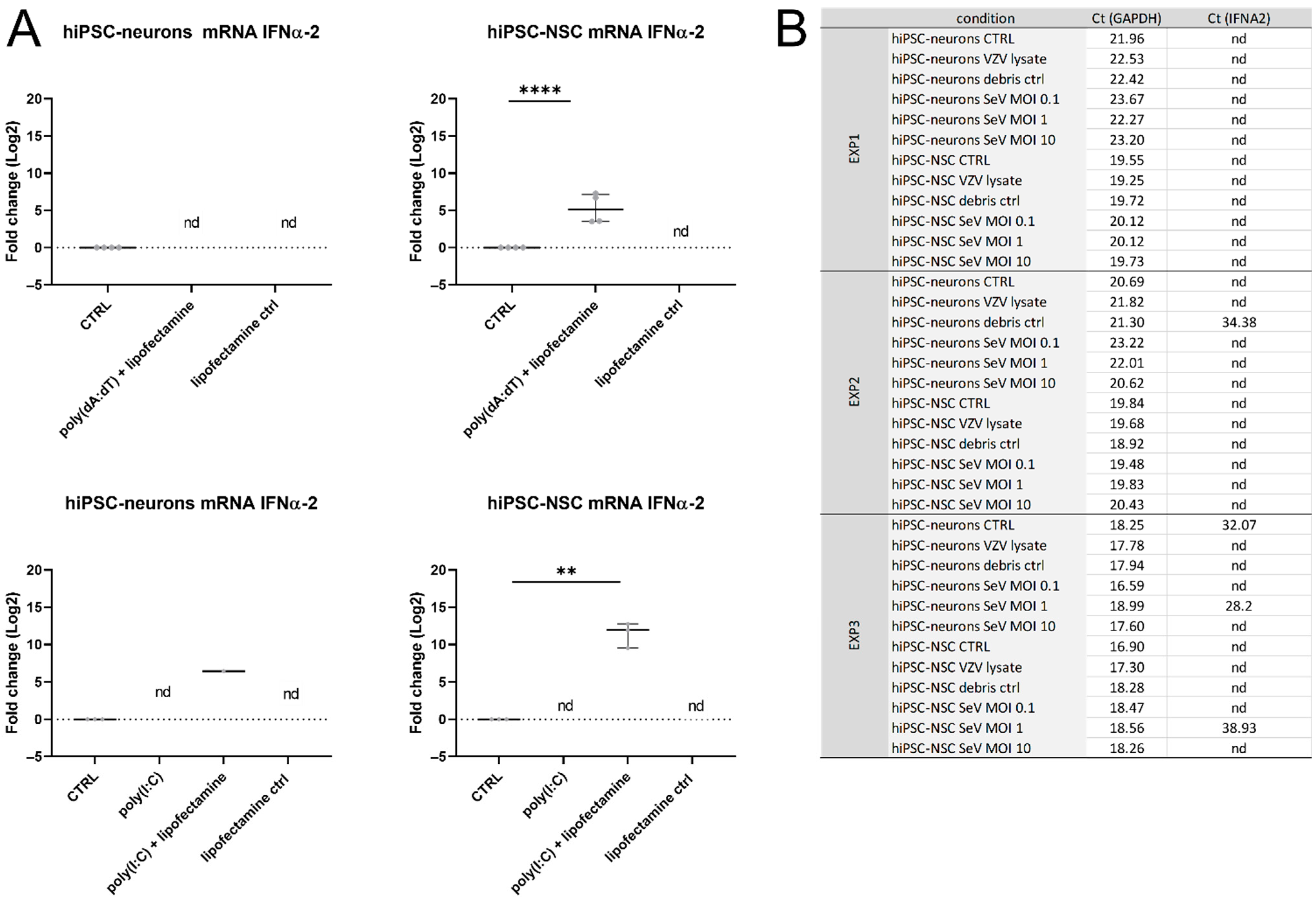
3.4. IFNα-Production Was Not Detected in hiPSC Neurons
3.5. VZV Infection Did Not Result in ISG Upregulation in hiPSC Neurons
3.6. Exogenous IFNα Limited VZV Spread in the Neuronal Cell Body Compartment upon Axonal Infection
3.7. ISGs Were Upregulated in VZV-Infected hiPSC Neurons Treated with IFNα
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brisson, M.; Edmunds, W.J.; Law, B.; Gay, N.J.; Walld, R.; Brownell, M.; Roos, L.L.; DE Serres, G. Epidemiology of varicella zoster virus infection in Canada and the United Kingdom. Epidemiol. Infect. 2001, 127, 305–314. [Google Scholar] [CrossRef] [Green Version]
- Zerboni, L.; Sen, N.; Oliver, S.L.; Arvin, A.M. Molecular mechanisms of varicella zoster virus pathogenesis. Nat. Rev. Genet. 2014, 12, 197–210. [Google Scholar] [CrossRef] [Green Version]
- Moffat, J.; Ku, C.C.; Zerboni, L.; Sommer, M.; Arvin, A. VZV: Pathogenesis and the disease consequences of primary infection. In Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A., Campadelli-Fiume, G., Mocarski, E., Moore, P.S., Roizman, B., Whitley, R., Yamanishi, K., Eds.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Zerboni, L.; Ku, C.-C.; Jones, C.D.; Zehnder, J.L.; Arvin, A.M. Varicella-zoster virus infection of human dorsal root ganglia in vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 6490–6495. [Google Scholar] [CrossRef] [Green Version]
- Arvin, A.M.; Moffat, J.F.; Sommer, M.; Oliver, S.; Che, X.; Vleck, S.; Zerboni, L.; Ku, C.-C. Varicella-Zoster Virus T Cell Tropism and the Pathogenesis of Skin Infection. Curr. Top. Microbiol. Immunol. 2010, 342, 189–209. [Google Scholar] [CrossRef] [Green Version]
- Mahalingam, R.; Messaoudi, I.; Gilden, D. Simian Varicella Virus Pathogenesis. Curr. Top. Microbiol. Immunol. 2010, 342, 309–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, R.W.; Alvarez-Pasquin, M.-J.; Bijl, M.; Franco, E.; Gaillat, J.; Clara, J.G.; Labetoulle, M.; Michel, J.-P.; Naldi, L.; Sanmarti, L.S.; et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: A multidisciplinary perspective. Ther. Adv. Vaccines 2015, 3, 109–120. [Google Scholar] [CrossRef] [Green Version]
- Bilcke, J.; Ogunjimi, B.; Marais, C.; DE Smet, F.; Callens, M.; Callaert, K.; VAN Kerschaver, E.; Ramet, J.; VAN Damme, P.; Beutels, P. The health and economic burden of chickenpox and herpes zoster in Belgium. Epidemiol. Infect. 2012, 140, 2096–2109. [Google Scholar] [CrossRef]
- Panatto, D.; Bragazzi, N.L.; Rizzitelli, E.; Bonanni, P.; Boccalini, S.; Icardi, G.; Gasparini, R.; Amicizia, D. Evaluation of the economic burden of Herpes Zoster (HZ) infection: A systematic literature review. Hum. Vaccines Immunother. 2015, 11, 245–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagel, M.A.; Gilden, D. Complications of Varicella Zoster Virus Reactivation. Curr. Treat. Options Neurol. 2013, 15, 439–453. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Li, Q.; Yang, J.; Zhou, M.; Zhou, N.; He, L. Antiviral treatment for preventing postherpetic neuralgia. Cochrane Database Syst. Rev. 2014, 2, CD006866. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Johnson, R.W.; Breuer, J.; Gnann, J.W.; Levin, M.J.; Backonja, M.; Betts, R.F.; Gershon, A.A.; Haanpää, M.L.; McKendrick, M.W.; et al. Recommendations for the Management of Herpes Zoster. Clin. Infect. Dis. 2007, 44, S1–S26. [Google Scholar] [CrossRef] [PubMed]
- Ecarnot, F.; Bernabei, R.; Gabutti, G.; Giuffrida, S.; Michel, J.-P.; Rezza, G.; Maggi, S. Adult vaccination as the cornerstone of successful ageing: The case of herpes zoster vaccination. A European Interdisciplinary Council on Ageing (EICA) expert focus group. Aging 2019, 31, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Williams, W.W.; Lu, P.J.; O’Halloran, A.; Bridges, C.B.; Kim, D.K.; Pilishvili, T.; Hales, C.M.; Markowitz, L.E. Vaccination coverage among adults, excluding influenza vaccination—United States, 2013. Morb. Mortal. Wkly. Rep. 2015, 64, 95–102. [Google Scholar]
- Williams, W.W.; Lu, P.-J.; O’Halloran, A.; Kim, D.K.; Grohskopf, L.A.; Pilishvili, T.; Skoff, T.H.; Nelson, N.P.; Harpaz, R.; Markowitz, L.E.; et al. Surveillance of Vaccination Coverage among Adult Populations—United States, 2015. MMWR. Surveill. Summ. 2017, 66, 1–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunjimi, B.; Willem, L.; Beutels, P.; Hens, N. Integrating between-host transmission and within-host immunity to analyze the impact of varicella vaccination on zoster. eLife 2015, 4, e07116. [Google Scholar] [CrossRef] [PubMed]
- Arvin, A.M.G.D. Fields Virology; Knipe, D.H.P., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 2015–2057. [Google Scholar]
- Lebrun, M.; Thelen, N.; Thiry, M.; Riva, L.; Ote, I.; Condé, C.; Vandevenne, P.; Di Valentin, E.; Bontems, S.; Sadzot-Delvaux, C. Varicella-zoster virus induces the formation of dynamic nuclear capsid aggregates. Virology 2014, 454–455, 311–327. [Google Scholar] [CrossRef]
- Chaudhuri, V.; Sommer, M.; Rajamani, J.; Zerboni, L.; Arvin, A.M. Functions of Varicella-Zoster Virus ORF23 Capsid Protein in Viral Replication and the Pathogenesis of Skin Infection. J. Virol. 2008, 82, 10231–10246. [Google Scholar] [CrossRef] [Green Version]
- Reichelt, M.; Brady, J.; Arvin, A.M. The Replication Cycle of Varicella-Zoster Virus: Analysis of the Kinetics of Viral Protein Expression, Genome Synthesis, and Virion Assembly at the Single-Cell Level. J. Virol. 2009, 83, 3904–3918. [Google Scholar] [CrossRef] [Green Version]
- Kinchington, P.R.; Bookey, D.; Turse, S.E. The transcriptional regulatory proteins encoded by varicella-zoster virus open reading frames (ORFs) 4 and 63, but not ORF 61, are associated with purified virus particles. J. Virol. 1995, 69, 4274–4282. [Google Scholar] [CrossRef] [Green Version]
- Kinchington, P.R.; Hougland, J.K.; Arvin, A.M.; Ruyechan, W.T.; Hay, J. The varicella-zoster virus immediate-early protein IE62 is a major component of virus particles. J. Virol. 1992, 66, 359–366. [Google Scholar] [CrossRef] [Green Version]
- Ouwendijk, W.J.D.; Depledge, D.P.; Rajbhandari, L.; Rovis, T.L.; Jonjic, S.; Breuer, J.; Venkatesan, A.; Verjans, G.M.G.M.; Sadaoka, T. Varicella-zoster virus VLT-ORF63 fusion transcript induces broad viral gene expression during reactivation from neuronal latency. Nat. Commun. 2020, 11, 6324. [Google Scholar] [CrossRef]
- Depledge, D.P.; Ouwendijk, W.J.; Sadaoka, T.; Braspenning, S.; Mori, Y.; Cohrs, R.J.; Verjans, G.M.G.M.; Breuer, J. A spliced latency-associated VZV transcript maps antisense to the viral transactivator gene 61. Nat. Commun. 2018, 9, 1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biron, C.A. Role of early cytokines, including alpha and beta interferons (IFN-α\β), in innate and adaptive immune responses to viral infections. Semin. Immunol. 1998, 10, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Carter-Timofte, M.E.; Paludan, S.R.; Mogensen, T.H. RNA Polymerase III as a Gatekeeper to Prevent Severe VZV Infections. Trends Mol. Med. 2018, 24, 904–915. [Google Scholar] [CrossRef] [PubMed]
- Paludan, S.R.; Bowie, A.G.; Horan, K.A.; Fitzgerald, K.A. Recognition of herpesviruses by the innate immune system. Nat. Rev. Immunol. 2011, 11, 143–154. [Google Scholar] [CrossRef]
- Sironi, M.; Peri, A.M.; Cagliani, R.; Forni, D.; Riva, S.; Biasin, M.; Clerici, M.; Gori, A. TLR3 Mutations in Adult Patients With Herpes Simplex Virus and Varicella-Zoster Virus Encephalitis. J. Infect. Dis. 2017, 215, 1430–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogunjimi, B.; Zhang, S.-Y.; Sørensen, K.B.; Skipper, K.A.; Carter-Timofte, M.; Kerner, G.; Luecke, S.; Prabakaran, T.; Cai, Y.; Meester, J.; et al. Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections. J. Clin. Investig. 2017, 127, 3543–3556. [Google Scholar] [CrossRef] [Green Version]
- Katze, M.G.; He, Y.; Gale, M., Jr. Viruses and interferon: A fight for supremacy. Nat. Rev. Immunol. 2002, 2, 675–687. [Google Scholar] [CrossRef]
- Gerada, C.; Campbell, T.M.; Kennedy, J.; McSharry, B.; Steain, M.; Slobedman, B.; Abendroth, A. Manipulation of the Innate Immune Response by Varicella Zoster Virus. Front. Immunol. 2020, 11, 1. [Google Scholar] [CrossRef] [Green Version]
- Sadler, A.J.; Williams, B.R.G. Interferon-inducible antiviral effectors. Nat. Rev. Immunol. 2008, 8, 559–568. [Google Scholar] [CrossRef]
- Haberthur, K.; Messaoudi, I. Animal Models of Varicella Zoster Virus Infection. Pathogens 2013, 2, 364–382. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.E.; Rovnak, J.; Badani, H.; Cohrs, R.J. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J. Gen. Virol. 2015, 96, 1581–1602. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Zhou, W.; Scott-McKean, J.J.; Emmerling, K.L.; Cai, G.-Y.; Krah, D.L.; Costa, A.C.; Freed, C.R.; Levin, M.J. Human Sensory Neurons Derived from Induced Pluripotent Stem Cells Support Varicella-Zoster Virus Infection. PLoS ONE 2012, 7, e53010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadaoka, T.; Schwartz, C.L.; Rajbhandari, L.; Venkatesan, A.; Cohen, J.I. Human Embryonic Stem Cell-Derived Neurons Are Highly Permissive for Varicella-Zoster Virus Lytic Infection. J. Virol. 2018, 92, e01108-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markus, A.; Grigoryan, S.; Sloutskin, A.; Yee, M.B.; Zhu, H.; Yang, I.H.; Thakor, N.V.; Sarid, R.; Kinchington, P.R.; Goldstein, R.S. Varicella-Zoster Virus (VZV) Infection of Neurons Derived from Human Embryonic Stem Cells: Direct Demonstration of Axonal Infection, Transport of VZV, and Productive Neuronal Infection. J. Virol. 2011, 85, 6220–6233. [Google Scholar] [CrossRef] [Green Version]
- Markus, A.; Lebenthal-Loinger, I.; Yang, I.H.; Kinchington, P.R.; Goldstein, R.S. An In Vitro Model of Latency and Reactivation of Varicella Zoster Virus in Human Stem Cell-Derived Neurons. PLoS Pathog. 2015, 11, e1004885. [Google Scholar] [CrossRef] [Green Version]
- Sadaoka, T.; Depledge, D.P.; Rajbhandari, L.; Venkatesan, A.; Breuer, J.; Cohen, J.I. In vitro system using human neurons demonstrates that varicella-zoster vaccine virus is impaired for reactivation, but not latency. Proc. Natl. Acad. Sci. USA 2016, 113, E2403–E2412. [Google Scholar] [CrossRef] [Green Version]
- Koyuncu, O.O.; MacGibeny, M.A.; Hogue, I.B.; Enquist, L.W. Compartmented neuronal cultures reveal two distinct mechanisms for alpha herpesvirus escape from genome silencing. PLoS Pathog. 2017, 13, e1006608. [Google Scholar] [CrossRef] [Green Version]
- Van Breedam, E.; Nijak, A.; Buyle-Huybrecht, T.; Di Stefano, J.; Boeren, M.; Govaerts, J.; Quarta, A.; Swartenbroekx, T.; Jacobs, E.Z.; Menten, B.; et al. Luminescent Human iPSC-Derived Neurospheroids Enable Modeling of Neurotoxicity After Oxygen-glucose Deprivation. Neurotherapeutics 2022, 19, 550–569. [Google Scholar] [CrossRef]
- Sloutskin, A.; Goldstein, R. Infectious Focus Assays and Multiplicity of Infection (MOI) Calculations for Alpha-herpesviruses. Bio-protocol 2014, 4, e1295. [Google Scholar] [CrossRef]
- Sloutskin, A.; Goldstein, R.S. Laboratory preparation of Varicella-Zoster Virus: Concentration of virus-containing supernatant, use of a debris fraction and magnetofection for consistent cell-free VZV infections. J. Virol. Methods 2014, 206, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendling, M.; Pabinger, S.; Peters, K.; Wolff, N.; Conzemius, R.; Barišić, I. Oli2go: An automated multiplex oligonucleotide design tool. Nucleic Acids Res. 2018, 46, W252–W256. [Google Scholar] [CrossRef]
- Cohrs, R.J.; Gilden, D.H. Prevalence and Abundance of Latently Transcribed Varicella-Zoster Virus Genes in Human Ganglia. J. Virol. 2007, 81, 2950–2956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grose, C.; Yu, X.; Cohrs, R.J.; Carpenter, J.E.; Bowlin, J.L.; Gilden, D. Aberrant Virion Assembly and Limited Glycoprotein C Production in Varicella-Zoster Virus-Infected Neurons. J. Virol. 2013, 87, 9643–9648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Reder, A.T.; Feng, X. How Type I Interferons Work in Multiple Sclerosis and Other Diseases: Some Unexpected Mechanisms. J. Interf. Cytokine Res. 2014, 34, 589–599. [Google Scholar] [CrossRef] [Green Version]
- Talpaz, M.; Hehlmann, R.; Quintás-Cardama, A.; Mercer, J.; Cortes, J. Re-emergence of interferon-α in the treatment of chronic myeloid leukemia. Leukemia 2012, 27, 803–812. [Google Scholar] [CrossRef] [Green Version]
- Delhaye, S.; Paul, S.; Blakqori, G.; Minet, M.; Weber, F.; Staeheli, P.; Michiels, T. Neurons produce type I interferon during viral encephalitis. Proc. Natl. Acad. Sci. USA 2006, 103, 7835–7840. [Google Scholar] [CrossRef] [Green Version]
- Vandevenne, P.; Lebrun, M.; El Mjiyad, N.; Ote, I.; Di Valentin, E.; Habraken, Y.; Dortu, E.; Piette, J.; Sadzot-Delvaux, C. The Varicella-Zoster Virus ORF47 Kinase Interferes with Host Innate Immune Response by Inhibiting the Activation of IRF3. PLoS ONE 2011, 6, e16870. [Google Scholar] [CrossRef]
- Sen, N.; Sommer, M.; Che, X.; White, K.; Ruyechan, W.T.; Arvin, A.M. Varicella-Zoster Virus Immediate-Early Protein 62 Blocks Interferon Regulatory Factor 3 (IRF3) Phosphorylation at Key Serine Residues: A Novel Mechanism of IRF3 Inhibition among Herpesviruses. J. Virol. 2010, 84, 9240–9253. [Google Scholar] [CrossRef] [PubMed]
- Nagel, M.A.; James, S.F.; Traktinskiy, I.; Wyborny, A.; Choe, A.; Rempel, A.; Baird, N.L.; Gilden, D. Inhibition of Phosphorylated-STAT1 Nuclear Translocation and Antiviral Protein Expression in Human Brain Vascular Adventitial Fibroblasts Infected with Varicella-Zoster Virus. J. Virol. 2014, 88, 11634–11637. [Google Scholar] [CrossRef] [Green Version]
- Ambagala, A.P.N.; Cohen, J.I. Varicella-Zoster Virus IE63, a Major Viral Latency Protein, Is Required To Inhibit the Alpha Interferon-Induced Antiviral Response. J. Virol. 2007, 81, 7844–7851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verweij, M.C.; Wellish, M.; Whitmer, T.; Malouli, D.; Lapel, M.; Jonjic, S.; Haas, J.G.; DeFilippis, V.R.; Mahalingam, R.; Früh, K. Varicella Viruses Inhibit Interferon-Stimulated JAK-STAT Signaling through Multiple Mechanisms. PLoS Pathog. 2015, 11, e1004901. [Google Scholar] [CrossRef] [PubMed]
- Como, C.N.; Pearce, C.M.; Cohrs, R.J.; Baird, N.L. Interleukin-6 and type 1 interferons inhibit varicella zoster virus replication in human neurons. Virology 2018, 522, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, S.; Kinchington, P.R.; Yang, I.H.; Selariu, A.; Zhu, H.; Yee, M.; Goldstein, R.S. Retrograde axonal transport of VZV: Kinetic studies in hESC-derived neurons. J. Neurovirol. 2012, 18, 462–470. [Google Scholar] [CrossRef] [Green Version]
- De Regge, N.; Van Opdenbosch, N.; Nauwynck, H.J.; Efstathiou, S.; Favoreel, H.W. Interferon alpha induces establishment of alphaherpesvirus latency in sensory neurons in vitro. PLoS ONE 2010, 5, e13076. [Google Scholar] [CrossRef]
- Lenschow, D.J.; Lai, C.; Frias-Staheli, N.; Giannakopoulos, N.V.; Lutz, A.; Wolff, T.; Osiak, A.; Levine, B.; Schmidt, R.E.; García-Sastre, A.; et al. IFN-stimulated gene 15 functions as a critical antiviral molecule against influenza, herpes, and Sindbis viruses. Proc. Natl. Acad. Sci. USA 2007, 104, 1371–1376. [Google Scholar] [CrossRef] [Green Version]
- Desloges, N.; Rahaus, M.; Wolff, M.H. Role of the protein kinase PKR in the inhibition of varicella-zoster virus replication by beta interferon and gamma interferon. J. Gen. Virol. 2005, 86, 1–6. [Google Scholar] [CrossRef]
- Ku, C.; Che, X.; Reichelt, M.; Rajamani, J.; Schaap-Nutt, A.; Huang, K.; Sommer, M.H.; Chen, Y.; Arvin, A.M. Herpes simplex virus-1 induces expression of a novel MxA isoform that enhances viral replication. Immunol. Cell Biol. 2010, 89, 173–182. [Google Scholar] [CrossRef] [Green Version]
- Thapa, M.; Welner, R.S.; Pelayo, R.; Carr, D.J. CXCL9 and CXCL10 Expression Are Critical for Control of Genital Herpes Simplex Virus Type 2 Infection through Mobilization of HSV-Specific CTL and NK Cells to the Nervous System. J. Immunol. 2008, 180, 1098–1106. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.; Khan, A.A.; Chilukuri, S.; Syed, S.A.; Tran, T.T.; Furness, J.; Bahraoui, E.; BenMohamed, L. CXCL10/CXCR3-Dependent Mobilization of Herpes Simplex Virus-Specific CD8(+) T(EM) and CD8(+) T(RM) Cells within Infected Tissues Allows Efficient Protection against Recurrent Herpesvirus Infection and Disease. J. Virol. 2017, 91, e00278-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steain, M.; Sutherland, J.P.; Rodriguez, M.; Cunningham, A.L.; Slobedman, B.; Abendroth, A. Analysis of T Cell Responses during Active Varicella-Zoster Virus Reactivation in Human Ganglia. J. Virol. 2014, 88, 2704–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, N.; Girke, T.; Sureshchandra, S.; Messaoudi, I. Acute Simian Varicella Virus Infection Causes Robust and Sustained Changes in Gene Expression in the Sensory Ganglia. J. Virol. 2016, 90, 10823–10843. [Google Scholar] [CrossRef] [PubMed]






| Gene | Primer/Probe Sequence | Reference |
|---|---|---|
| Human GAPDH_F | 5’-CACATGGCCTCCAAGGAGTAA-3’ | [46] |
| Human GAPDH_R | 5’-TGAGGGTCTCTCTCTTCCTCTTGT-3’ | |
| Human GAPDH_Probe | 5’-(Cy5)CTGGACCACCAGCCCCAGCAAG(IAbRQSp)-3′ | |
| VZV ORF23_F | 5′-CTTCTGGACAACAACCGCAA-3′ | n/a |
| VZV ORF23_R | 5′-CAGATTGTCCCGTGTGTGAC-3′ | |
| VZV ORF23_Probe | 5′-(TexRed-XN)-ACTGTCCAGCCAACAACCGG-(IabRQSp)-3′ | |
| VZV ORF62_F | 5′-CCTTGGAAACCACATGATCGT-3′ | [47] |
| VZV ORF62_R | 5′-AGCAGAAGCCTCCTCGACAA-3′ | |
| VZV ORF62_Probe | 5′-(HEX)-TGCAACCCGGGCGTCCG-(ZEN/IabRQSp)-3′ | |
| VZV ORF63_F | 5′-GATGGTGGTGAAGACGA-3′ | n/a |
| VZV ORF63_R | 5′-AATCGGTGCTCTCCTCT-3′ | |
| VZV ORF63_Probe | 5′-(FAM)-CGGAATCATCGGACGGGGAAG-(ZEN/IabRQSp)-3′ | |
| VLT 3-4_F | 5′-AATCGAGCCATACACCACCG-3′ | [24] |
| VLT 3-4_R | 5′-TGTATTCGGGCATGGACCTC-3′ | |
| VLT 3-4_Probe | 5′-(FAM)-GATCGAACAGCAGATGGATTGCA-(ZEN/IabRQSp)-3′ |
| Assay ID | Target |
|---|---|
| Hs00855471_g1 | IFNA1 |
| Hs00533748_s1 | IFNA14 |
| Hs03005057_sH | IFNA16 |
| Hs00819693_sH | IFNA17 |
| Hs02621172_s1 | IFNA2 |
| Hs01681284_sH | IFNA4 |
| Hs00818220_s1 | IFNA5 |
| Hs00819627_s1 | IFNA6 |
| Hs01652729_s1 | IFNA7 |
| Hs00932530_s1 | IFNA8 |
| Hs01066116_m1 | IFNAR1 |
| Hs00174198_m1 | IFNAR2 |
| Hs00277188_s1 | IFNB1 |
| Hs00989291_m1 | IFNG |
| Hs00166223_m1 | IFNGR1 |
| Hs00194264_m1 | IFNGR2 |
| Hs00737883_m1 | IFNK |
| Hs00357857_s1 | IFNW1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boeren, M.; Van Breedam, E.; Buyle-Huybrecht, T.; Lebrun, M.; Meysman, P.; Sadzot-Delvaux, C.; Van Tendeloo, V.F.; Mortier, G.; Laukens, K.; Ogunjimi, B.; et al. Activation of Interferon-Stimulated Genes following Varicella-Zoster Virus Infection in a Human iPSC-Derived Neuronal In Vitro Model Depends on Exogenous Interferon-α. Viruses 2022, 14, 2517. https://doi.org/10.3390/v14112517
Boeren M, Van Breedam E, Buyle-Huybrecht T, Lebrun M, Meysman P, Sadzot-Delvaux C, Van Tendeloo VF, Mortier G, Laukens K, Ogunjimi B, et al. Activation of Interferon-Stimulated Genes following Varicella-Zoster Virus Infection in a Human iPSC-Derived Neuronal In Vitro Model Depends on Exogenous Interferon-α. Viruses. 2022; 14(11):2517. https://doi.org/10.3390/v14112517
Chicago/Turabian StyleBoeren, Marlies, Elise Van Breedam, Tamariche Buyle-Huybrecht, Marielle Lebrun, Pieter Meysman, Catherine Sadzot-Delvaux, Viggo F. Van Tendeloo, Geert Mortier, Kris Laukens, Benson Ogunjimi, and et al. 2022. "Activation of Interferon-Stimulated Genes following Varicella-Zoster Virus Infection in a Human iPSC-Derived Neuronal In Vitro Model Depends on Exogenous Interferon-α" Viruses 14, no. 11: 2517. https://doi.org/10.3390/v14112517
APA StyleBoeren, M., Van Breedam, E., Buyle-Huybrecht, T., Lebrun, M., Meysman, P., Sadzot-Delvaux, C., Van Tendeloo, V. F., Mortier, G., Laukens, K., Ogunjimi, B., Ponsaerts, P., & Delputte, P. (2022). Activation of Interferon-Stimulated Genes following Varicella-Zoster Virus Infection in a Human iPSC-Derived Neuronal In Vitro Model Depends on Exogenous Interferon-α. Viruses, 14(11), 2517. https://doi.org/10.3390/v14112517








