Epidemiological Aspects of Equid Herpesvirus-Associated Myeloencephalopathy (EHM) Outbreaks
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Operation Characteristics
3.2. EHM Outbreak Characteristics
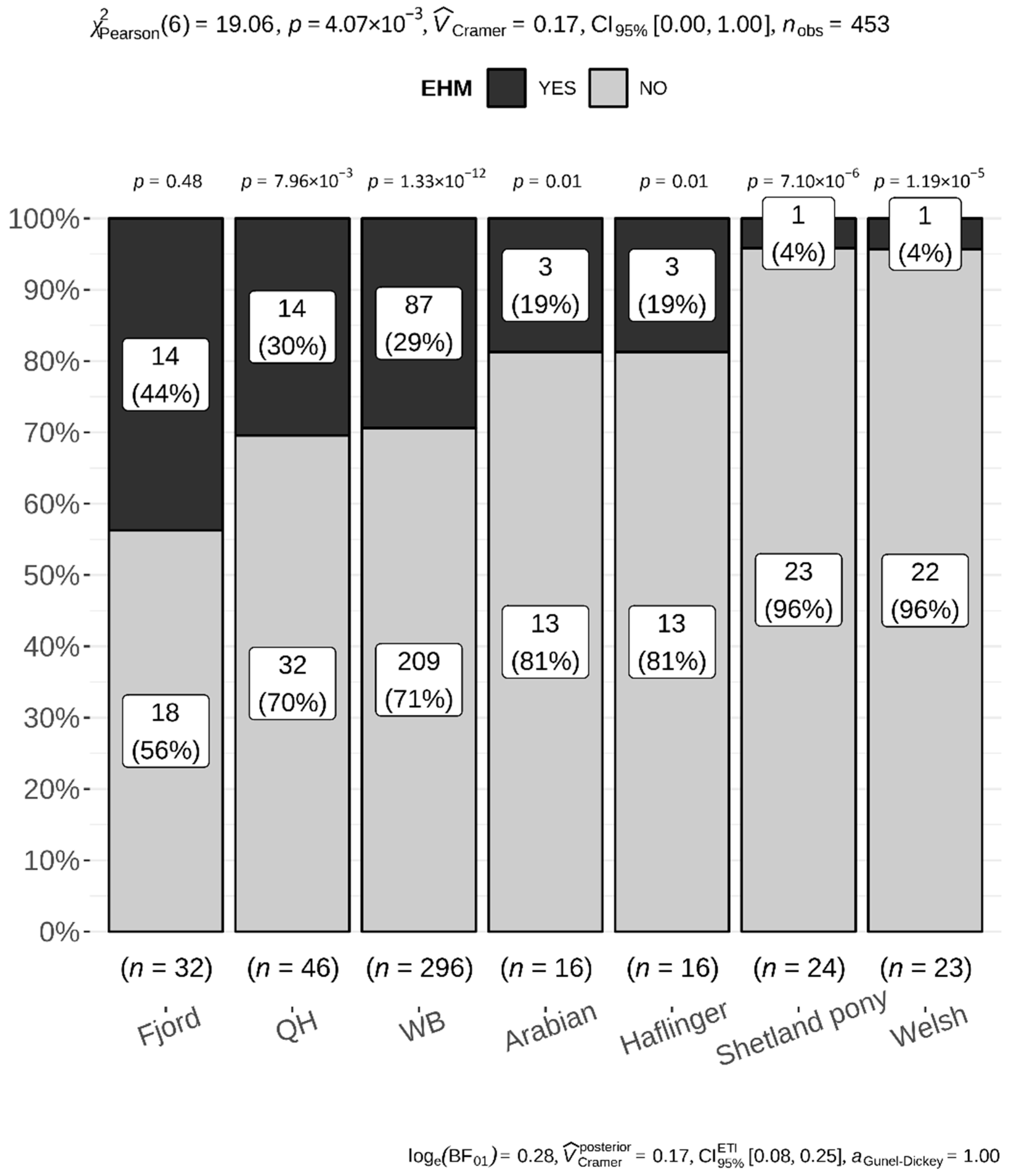
3.3. Animal Data and Risk Assessment
3.4. Vaccination
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lunn, D.; Davis-Poynter, N.; Flaminio, M.; Horohov, D.; Osterrieder, K.; Pusterla, N.; Townsend, H. Equine herpesvirus-1 consensus statement. J. Vet. Intern. Med. 2009, 23, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Wilsterman, S.; Soboll-Hussey, G.; Lunn, D.; Ashton, L.; Callan, R.; Hussey, S.; Rao, S.; Goehring, L. Equine herpesvirus-1 infected peripheral blood mononuclear cell subpopulations during viremia. Vet. Microbiol. 2011, 149, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Edington, N.; Welch, H.; Griffiths, L. The prevalence of latent equid herpesviruses in the tissues of 40 abattoir horses. Equine Vet. J. 1994, 26, 140–142. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.P.; Kydd, J.H.; Slater, J.D.; Smith, K.C. Advances in understanding of the pathogenesis, epidemiology and immunological control of equine herpesvirus abortion. In Equine Infectious Diseases, VIII ed.; Wernery, U., Wade, J., Mumford, J., Eds.; R&W Publications: Newmarket, Suffolk, UK, 1999; pp. 129–146. [Google Scholar]
- Maxwell, L.K.; Bentz, B.G.; Gilliam, L.L.; Ritchey, J.W.; Pusterla, N.; Eberle, R.; Holbrook, T.C.; McFarlane, D.; Rezabek, G.B.; Meinkoth, J.; et al. Efficacy of the early administration of valacyclovir hydrochloride for the treatment of neuropathogenic equine herpesvirus type-1 infection in horses. Am. J. Vet. Res. 2017, 78, 1126–1139. [Google Scholar] [CrossRef]
- Giessler, K.; Goehring, L.; Jacobs, S.; McCauley, A.; Esser, M.; Lee, Y.; Zarski, L.; Weber, P.; Soboll Hussey, G. Use of the old horse model to identify horst factors contributing to EHM pathogenesis. In Proceedings of the Conference of Research Workers in Animal Diseases, Chicago, IL, USA, 4–7 December 2020; p. 269. [Google Scholar]
- van Maanen, C.; van Oldruitenborgh-Oosterbaan, M.S.; Damen, E.; Derksen, A. Neurological disease associated with EHV-1-infection in a riding school: Clinical and virological characteristics. Equine Vet. J. 2001, 33, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Pusterla, N.; Hussey, G.S.; Goehring, L.S. Equine Herpesvirus-1 Myeloencephalopathy. Vet. Clin. N. Am. Equine Pract. 2022, 38, 339–362. [Google Scholar] [CrossRef]
- Goehring, L.S.; van Winden, S.C.; Van Maanen, C.; van Oldruitenborgh-Oosterbaan, M.M.S. Equine herpesvirus type 1-associated myeloencephalopathy in the Netherlands: A four-year retrospective study (1999–2003). J. Vet. Intern. Med. 2006, 20, 601–607. [Google Scholar]
- Goehring, L.; Wagner, B.; Bigbie, R.; Hussey, S.; Rao, S.; Morley, P.; Lunn, D. Control of EHV-1 viremia and nasal shedding by commercial vaccines. Vaccine 2010, 28, 5203–5211. [Google Scholar] [CrossRef]
- Nugent, J.; Birch-Machin, I.; Smith, K.; Mumford, J.; Swann, Z.; Newton, J.; Bowden, R.; Allen, G.; Davis-Poynter, N. Analysis of equid herpesvirus 1 strain variation reveals a point mutation of the DNA polymerase strongly associated with neuropathogenic versus nonneuropathogenic disease outbreaks. J. Virol. 2006, 80, 4047–4060. [Google Scholar] [CrossRef] [Green Version]
- Klouth, E.; Zablotski, Y.; Goehring, L.S. Apparent Breed Predilection for Equid Herpesvirus-1-Associated Myeloencephalopathy (EHM) in a Multiple-Breed Herd. Pathogens 2021, 10, 537. [Google Scholar] [CrossRef]
- Allen, G.P. Risk factors for development of neurologic disease after experimental exposure to equine herpesvirus-1 in horses. Am. J. Vet. Res. 2008, 69, 1595–1600. [Google Scholar] [CrossRef] [PubMed]
- Traub-Dargatz, J.L.; Pelzel-McCluskey, A.M.; Creekmore, L.; Geiser-Novotny, S.; Kasari, T.; Wiedenheft, A.; Bush, E.; Bjork, K. Case–control study of a multistate equine herpesvirus myeloencephalopathy outbreak. J. Vet. Intern. Med. 2013, 27, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Schnabel, C.L.; Babasyan, S.; Rollins, A.; Freer, H.; Wimer, C.L.; Perkins, G.A.; Raza, F.; Osterrieder, N.; Wagner, B. An equine herpesvirus type 1 (EHV-1) Ab4 open reading frame 2 deletion mutant provides immunity and protection from EHV-1 infection and disease. J. Virol. 2019, 93, e01011–e01019. [Google Scholar] [CrossRef]
- Henninger, R.W.; Reed, S.M.; Saville, W.J.; Allen, G.P.; Hass, G.F.; Kohn, C.W.; Sofaly, C. Outbreak of neurologic disease caused by equine herpesvirus-1 at a university equestrian center. J. Vet. Intern. Med. 2007, 21, 157–165. [Google Scholar] [CrossRef]
- Couroucé, A.; Tessier, C.; Pomares, R.; Thévenot, R.; Marcillaud-Pitel, C.; Pronost, S.; Legrand, L.; Pitel, P.H. EHV-1 neurological outbreak during a show jumping competition: A clinical and epidemiological study. In Proceedings of the 11th International Equine Infectious Diseases Conference, virtual conference, 27 September–1 October 2021; p. 52. [Google Scholar]
- Walter, J.; Seeh, C.; Fey, K.; Bleul, U.; Osterrieder, N. Clinical observations and management of a severe equine herpesvirus type 1 outbreak with abortion and encephalomyelitis. Acta Vet. Scand. 2013, 55, 19. [Google Scholar] [CrossRef] [Green Version]
- Petersen, J.L.; Mickelson, J.R.; Cothran, E.G.; Andersson, L.S.; Axelsson, J.; Bailey, E.; Bannasch, D.; Binns, M.M.; Borges, A.S.; Brama, P. Genetic diversity in the modern horse illustrated from genome-wide SNP data. PLoS ONE 2013, 8, e54997. [Google Scholar] [CrossRef] [Green Version]
- Felicetti, M.; Lopes, M.; Verini-Supplizi, A.; da Câmara Machado, A.; Silvestrelli, M.; Mendonça, D.; Distl, O. Genetic diversity in the Maremmano horse and its relationship with other European horse breeds. Anim. Genet. 2010, 41, 53–55. [Google Scholar] [CrossRef] [PubMed]
- Funder, D.C.; Ozer, D.J. Evaluating effect size in psychological research: Sense and nonsense. Adv. Methods Pract. Psychol. Sci. 2019, 2, 156–168. [Google Scholar] [CrossRef]
- Jeffreys, H. The Theory of Probability; OUP: Oxford, UK, 1998. [Google Scholar]
- Vandenberghe, E.; Boshuizen, B.; Delesalle, C.J.; Goehring, L.S.; Groome, K.A.; van Maanen, K.; de Bruijn, C.M. New Insights into the Management of an EHV-1 (Equine Hospital) Outbreak. Viruses 2021, 13, 1429. [Google Scholar] [CrossRef]
- Pusterla, N.; Barnum, S.; Young, A.; Mendonsa, E.; Lee, S.; Hankin, S.; Brittner, S.; Finno, C.J. Molecular Monitoring of EHV-1 in Silently Infected Performance Horses through Nasal and Environmental Sample Testing. Pathogens 2022, 11, 720. [Google Scholar] [CrossRef] [PubMed]
- Friday, P.A.; Scarratt, W.K.; Elvinger, F.; Timoney, P.J.; Bonda, A. Ataxia and paresis with equine herpesvirus type 1 infection in a herd of riding school horses. J. Vet. Intern. Med. 2000, 14, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Bürki, F.; Nowotny, N.; Hinaidy, B.; Pallan, C. Die Ätiologie der Lipizzanerseuche in Piber 1983: Equine Herpesvirus 1. Wien Tierärztl Mschr 1984, 71, 312–320. [Google Scholar]
- Ahdy, A.M.; Ahmed, B.M.; Elgamal, M.A.; Shaalan, M.; Farag, I.M.; Mahfouz, E.R.; Darwish, H.R.; Sayed-Ahmed, M.Z.; Shalaby, M.A.; El-Sanousi, A.A. Detection of Equid Alphaherpesvirus 1 from Arabian Horses with different clinical presentations between 2016-2019 in Egypt. J. Equine Vet. Sci. 2022, 114, 103960. [Google Scholar] [CrossRef] [PubMed]
- Minke, J.; Fischer, L.; Baudu, P.; Guigal, P.; Sindle, T.; Mumford, J.; Audonnet, J. Use of DNA and recombinant canarypox viral (ALVAC) vectors for equine herpes virus vaccination. Vet. Immunol. Immunopathol. 2006, 111, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Allen, G.; Breathnach, C. Quantification by real-time PCR of the magnitude and duration of leucocyte-associated viraemia in horses infected with neuropathogenic vs. non-neuropathogenic strains of EHV-1. Equine Vet. J. 2006, 38, 252–257. [Google Scholar] [CrossRef] [PubMed]


| Breed Denomination | n | n with EHM | Cluster Assignment |
|---|---|---|---|
| American Paint Horse | 1 | 1 | 1 |
| American Quarter Horse (AQH) | 46 | 14 | 1 |
| Appaloosa | 1 | 0 | 1 |
| Standardbred | 2 | 1 | 1 |
| Thoroughbred | 3 | 2 | 1 |
| Warmblood (all studbooks) (WB) | 296 | 87 | 1 |
| F1 crossbred within cluster | 1 | 0 | 1 |
| Arabian | 16 | 3 | 2 |
| Criollo | 1 | 0 | 3 |
| Lusitano | 2 | 1 | 3 |
| Mestizio | 1 | 0 | 3 |
| Pura raza espanola (PRE) | 10 | 1 | 3 |
| Draft horse | 5 | 0 | 4 |
| Friesian | 3 | 1 | 4 |
| Haflinger | 16 | 3 | 4 |
| F1 crossbred within cluster | 1 | 0 | 4 |
| Connemara | 5 | 1 | 5 |
| New Forest | 1 | 0 | 5 |
| Welsh pony (classes A–C) | 23 | 1 | 5 |
| Fjord | 32 | 14 | 6 |
| Gotland pony | 1 | 0 | 6 |
| Huzul (Carpathian) | 2 | 0 | 6 |
| Icelandic | 8 | 2 | 6 |
| Konik | 1 | 0 | 6 |
| Shetland pony | 24 | 1 | 6 |
| Total assigned to clusters | 502 | 133 | |
| Duelmener | 1 | 1 | unassigned |
| Lewitzer | 1 | 1 | unassigned |
| Lipizzaner | 1 | 0 | unassigned |
| Pinto | 1 | 0 | unassigned |
| Robust pony (unknown pedigree; combined based on size, built & appearance) | 20 | 0 | unassigned |
| Sport pony (unknown pedigree; combined based on built & use (dressage, show jumping) | 40 | 9 | unassigned |
| F1 crossbreeds between different cluster | 15 | 6 | unassigned |
| Pedigree unknown horses | 8 | 0 | unassigned |
| Total | 589 | 150 |
| Country Denmark (DK) Germany (GE) Sweden (SE) | Farm ID | Stable Units with Infection, Out of Total Units | EHV-1 Genotype | Horses in Affected Units | Horses with Fever | Horses with Neurological Signs (EHM) | EHV-1 Vaccinated Horses | Questionnaire Return Rate (1) | |
|---|---|---|---|---|---|---|---|---|---|
| Total | with EHM | ||||||||
| GE | A | 2 of 4 | D | 51 | 46 (90%) | 22 (43%) | - | - | 100% |
| GE | B | 2 of 2 | D | 45 | 30 (67%) | 25 (56%) | 0 | 0 | 49% |
| GE | C * | 7 of 11 | D | 79 | 55 (70%) | 8 (10%) | 32 | 4 | 100% (2) |
| GE | D | 4 of 4 | - | 45 | 4 (9%) | 1 (2%) | - | 0 | 38% |
| GE | E ** | 3 of 3 | - | 141 | est. 40% | 33 (23%) | 1 | 0 | 100% (2) |
| GE | F | 2 of 2 | - | 70 | 11 (16%) | 2 (3%) | - | - | 20% |
| GE | G | 4 of 4 | D | 54 | 23 (43%) | 9 (17%) | 29 | 5 | 100% |
| GE | H | 1 of 6 | - | 23 | 19 (83%) | 18 (78%) | 5 | 5 | 100% |
| DK | I | 4 of 5 | D | 70 | 68 (97%) | 7 (10%) | 0 | 0 | 9% |
| SE | J | 1 of 1 | D | 59 | 35 (59%) | 20 (34%) | 1 | 0 | 100% |
| SE | K | 2 of 2 | D | 40 | 22 (55%) | 4 (10%) | 1 | 0 | 100% |
| GE | L | 3 of 3 | N | 41 | 15 (37%) | 1 (2%) | 8 | 0 | 100% |
| DK | M | 3 of 3 | - | 54 | 42 (78%) | 12 (22%) | 0 | 0 | 100% |
| Min | 23 | 9% | 2% | 9% | |||||
| Max | 141 | 97% | 78% | 100% | |||||
| Mean | 594 | 59% | 24% | 76% | |||||
| EHM | |||
|---|---|---|---|
| Predictors | Odds Ratios | 95% CI | p |
| (Intercept) | 0.02 ** | 0.01–0.11 | <0.001 |
| Age | 1.06 * | 1.01–1.12 | 0.032 |
| Sex (female) | 1.94 * | 1.02–3.67 | 0.042 |
| Vaccination (yes) | 1.02 | 0.35–2.98 | 0.965 |
| Fever (yes) | 13.95 ** | 5.75–33.81 | <0.001 |
| Random Effects | |||
| σ2 | 3.29 | ||
| τ00 farm | 2.62 | ||
| ICC | 0.44 | ||
| Nfarm | 12 | ||
| Observations | 339 | ||
| Marginal R²/Conditional R² | 0.249/0.582 | ||
| AIC | 292.679 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klouth, E.; Zablotski, Y.; Petersen, J.L.; de Bruijn, M.; Gröndahl, G.; Müller, S.; Goehring, L.S. Epidemiological Aspects of Equid Herpesvirus-Associated Myeloencephalopathy (EHM) Outbreaks. Viruses 2022, 14, 2576. https://doi.org/10.3390/v14112576
Klouth E, Zablotski Y, Petersen JL, de Bruijn M, Gröndahl G, Müller S, Goehring LS. Epidemiological Aspects of Equid Herpesvirus-Associated Myeloencephalopathy (EHM) Outbreaks. Viruses. 2022; 14(11):2576. https://doi.org/10.3390/v14112576
Chicago/Turabian StyleKlouth, Eva, Yury Zablotski, Jessica L. Petersen, Marco de Bruijn, Gittan Gröndahl, Susanne Müller, and Lutz S. Goehring. 2022. "Epidemiological Aspects of Equid Herpesvirus-Associated Myeloencephalopathy (EHM) Outbreaks" Viruses 14, no. 11: 2576. https://doi.org/10.3390/v14112576
APA StyleKlouth, E., Zablotski, Y., Petersen, J. L., de Bruijn, M., Gröndahl, G., Müller, S., & Goehring, L. S. (2022). Epidemiological Aspects of Equid Herpesvirus-Associated Myeloencephalopathy (EHM) Outbreaks. Viruses, 14(11), 2576. https://doi.org/10.3390/v14112576






