A New Biotin Labeling and High-Molecular-Weight RNA Northern Method and Its Application in Viral RNA Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. RNA Isolation and Sample Denature
2.2. Probe Preparation
2.3. Gel Running, Transferring, and Staining
2.4. Crosslink and Hybridization
2.5. Chemiluminescence Detection and Stripping the Probes
3. Results
3.1. Design and Optimization of Probe Structure
3.2. Optimization of Gel Running and Transferring Procedure
3.3. Virus Detection Using Newly Optimized Methods
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hema, M.; Konakalla, N.C. Recent developments in detection and diagnosis of plant viruses. In Recent Developments in Applied Microbiology and Biochemistry; Viswanath, B., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; Volume 2, pp. 163–180. [Google Scholar]
- Wang, Z.; Gerstein, M.; Snyder, M. RNA-Seq: A revolutionary tool for transcriptomics. Nat. Rev. Genet. 2009, 10, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Gibson, U.E.; Heid, C.A.; Williams, P.M. A novel method for real time quantitative RT-PCR. Genome Res. 1996, 6, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Alwine, J.C.; Kemp, D.J.; Stark, G.R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc. Natl. Acad. Sci. USA 1977, 74, 5350–5354. [Google Scholar] [CrossRef]
- Rezaian, M.A.; Jackson, A.O. Low-molecular-weight RNAs associated with tobacco ringspot virus are satellites. Virology 1981, 114, 534–541. [Google Scholar] [CrossRef] [PubMed]
- He, S.L.; Green, R. Northern Blotting. In Laboratory Methods in Enzymology: RNA; Lorsch, J., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 530, pp. 75–87. [Google Scholar]
- Zhao, Y.; Du, L.; Zhang, N. Sensitivity of prestaining RNA with ethidium bromide before electrophoresis and performance of subsequent northern blots using heterologous DNA probes. Mol. Biotechnol. 2013, 54, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, W.; Gull, B.; Baby, J.; Mustafa, F. A Comprehensive Analysis of Northern versus Liquid Hybridization Assays for mRNAs, Small RNAs, and miRNAs Using a Non-Radiolabeled Approach. Curr. Issues Mol. Biol. 2021, 43, 457–484. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, M.; Zhang, N. Modified Northern blot protocol for easy detection of mRNAs in total RNA using radiolabeled probes. BMC Genom. 2022, 23, 66. [Google Scholar] [CrossRef]
- Holtke, H.J.; Ankenbauer, W.; Muhlegger, K.; Rein, R.; Sagner, G.; Seibl, R.; Walter, T. The digoxigenin (DIG) system for non-radioactive labelling and detection of nucleic acids—An overview. Cell Mol. Biol. 1995, 41, 883–905. [Google Scholar]
- Langer, P.R.; Waldrop, A.A.; Ward, D.C. Enzymatic synthesis of biotin-labeled polynucleotides: Novel nucleic acid affinity probes. Proc. Natl. Acad. Sci. USA 1981, 78, 6633–6637. [Google Scholar] [CrossRef]
- Sive, H.L.; Grainger, R.M.; Harland, R.M. Synthesis and purification of digoxigenin-labeled RNA probes for in situ hybridization. CSH Protoc. 2007, 2007, pdb.prot4778. [Google Scholar] [CrossRef]
- Miller, B.R.; Wei, T.; Fields, C.J.; Sheng, P.; Xie, M. Near-infrared fluorescent northern blot. RNA 2018, 24, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Chen, M.; Xiang, M.; Guo, Z. RNAi-Based Antiviral Innate Immunity in Plants. Viruses 2022, 14, 432. [Google Scholar] [CrossRef] [PubMed]
- Pall, G.S.; Hamilton, A.J. Improved northern blot method for enhanced detection of small RNA. Nat. Protoc. 2008, 3, 1077–1084. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pendon, J.A.; Li, F.; Li, W.X.; Ding, S.W. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in Arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell 2007, 19, 2053–2063. [Google Scholar] [CrossRef]
- Takahashi, T.; Mitsuda, T.; Okuda, K. An alternative nonradioactive method for labeling DNA using biotin. Anal. Biochem. 1989, 179, 77–85. [Google Scholar] [CrossRef]
- Green, M.R.; Sambrook, J. Random Priming: Labeling of Purified DNA Fragments by Extension of Random Oligonucleotides. Cold Spring Harb. Protoc. 2019, 2019, 233–240. [Google Scholar] [CrossRef]
- Flickinger, J.L.; Gebeyehu, G.; Buchman, G.; Haces, A.; Rashtchian, A. Differential incorporation of biotinylated nucleotides by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1992, 20, 2382. [Google Scholar] [CrossRef][Green Version]
- Freitag, S.; Le Trong, I.; Chilkoti, A.; Klumb, L.A.; Stayton, P.S.; Stenkamp, R.E. Structural studies of binding site tryptophan mutants in the high-affinity streptavidin-biotin complex. J. Mol. Biol. 1998, 279, 211–221. [Google Scholar] [CrossRef]
- Saito, I.; Sugiyama, H.; Furukawa, N.; Matsuura, T. Photochemical Ring-Opening of Thymidine and Thymine in the Presence of Primary Amines. Tetrahedron Lett. 1981, 22, 3265–3268. [Google Scholar] [CrossRef]
- Valoczi, A.; Hornyik, C.; Varga, N.; Burgyan, J.; Kauppinen, S.; Havelda, Z. Sensitive and specific detection of microRNAs by northern blot analysis using LNA-modified oligonucleotide probes. Nucleic Acids Res. 2004, 32, e175. [Google Scholar] [CrossRef]
- Koshkin, A.A.; Singh, S.K.; Nielsen, P.; Rajwanshi, V.K.; Kumar, R.; Meldgaard, M.; Olsen, C.E.; Wengel, J. LNA (Locked Nucleic Acids): Synthesis of the adenine, cytosine, guanine, 5-methylcytosine, thymine and uracil bicyclonucleoside monomers, oligomerisation, and unprecedented nucleic acid recognition. Tetrahedron 1998, 54, 3607–3630. [Google Scholar] [CrossRef]
- Singh, S.K.; Nielsen, P.; Koshkin, A.A.; Wengel, J. LNA (locked nucleic acids): Synthesis and high-affinity nucleic acid recognition. Chem. Commun. 1998, 455–456. [Google Scholar] [CrossRef]

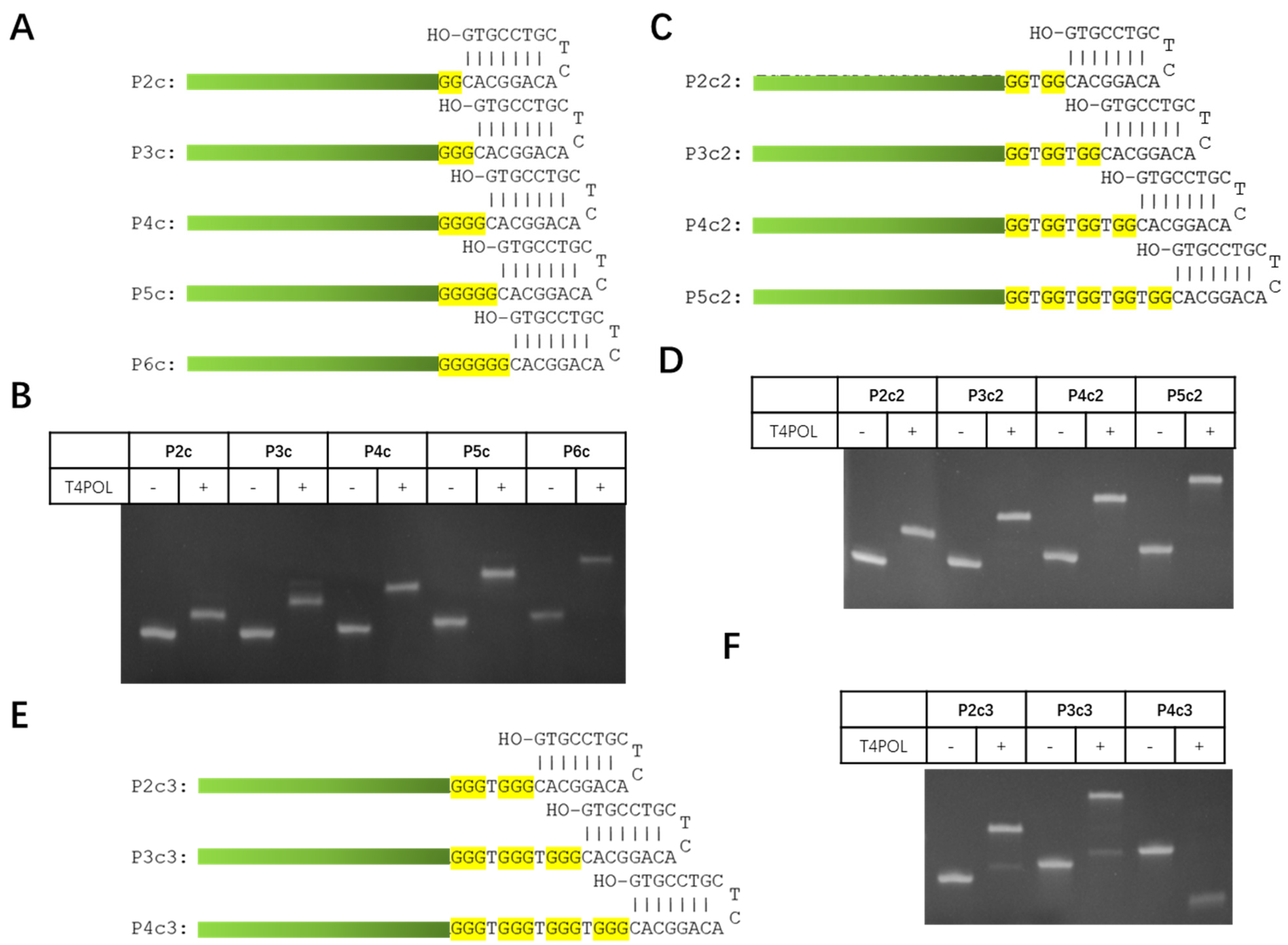
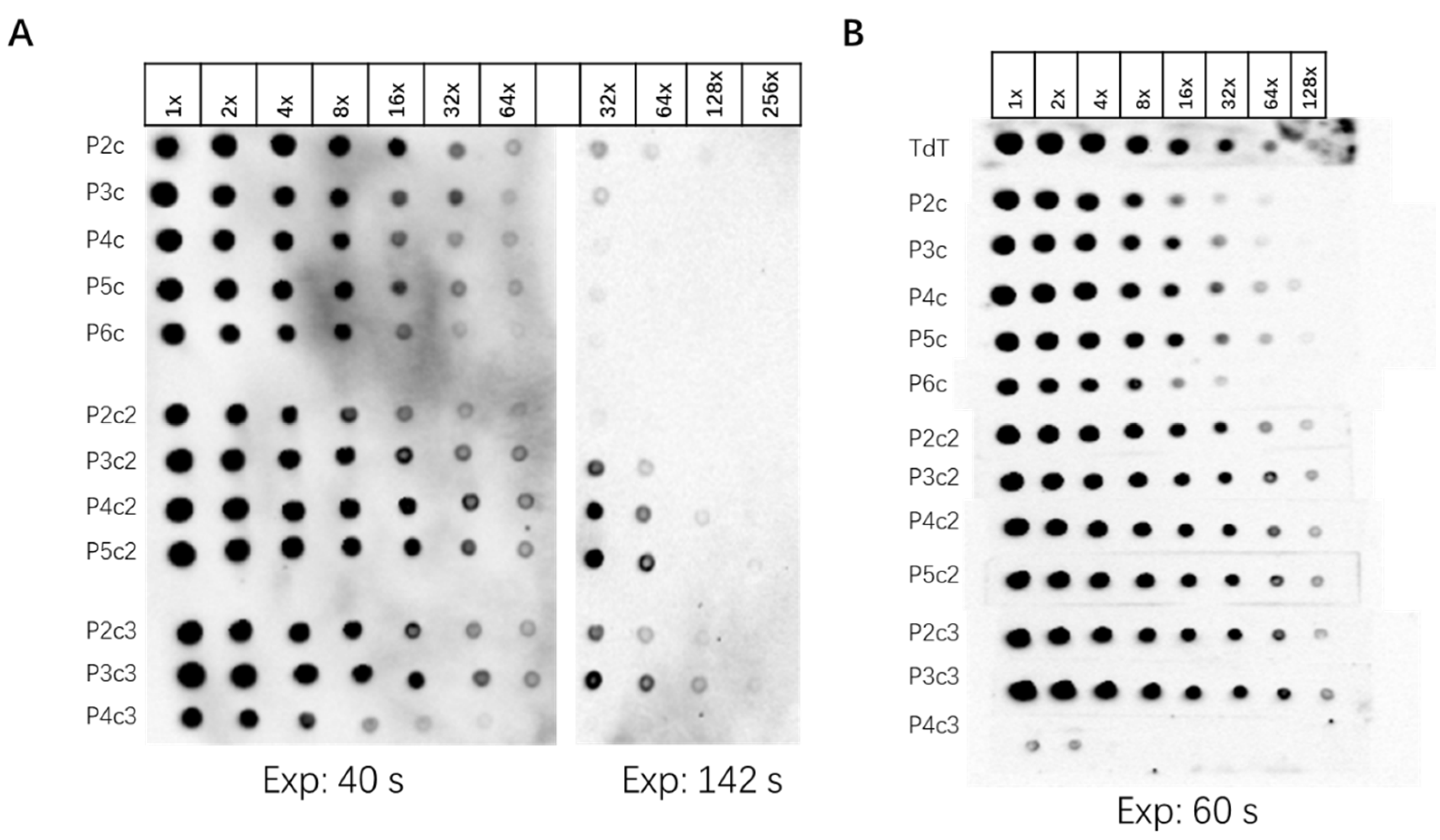

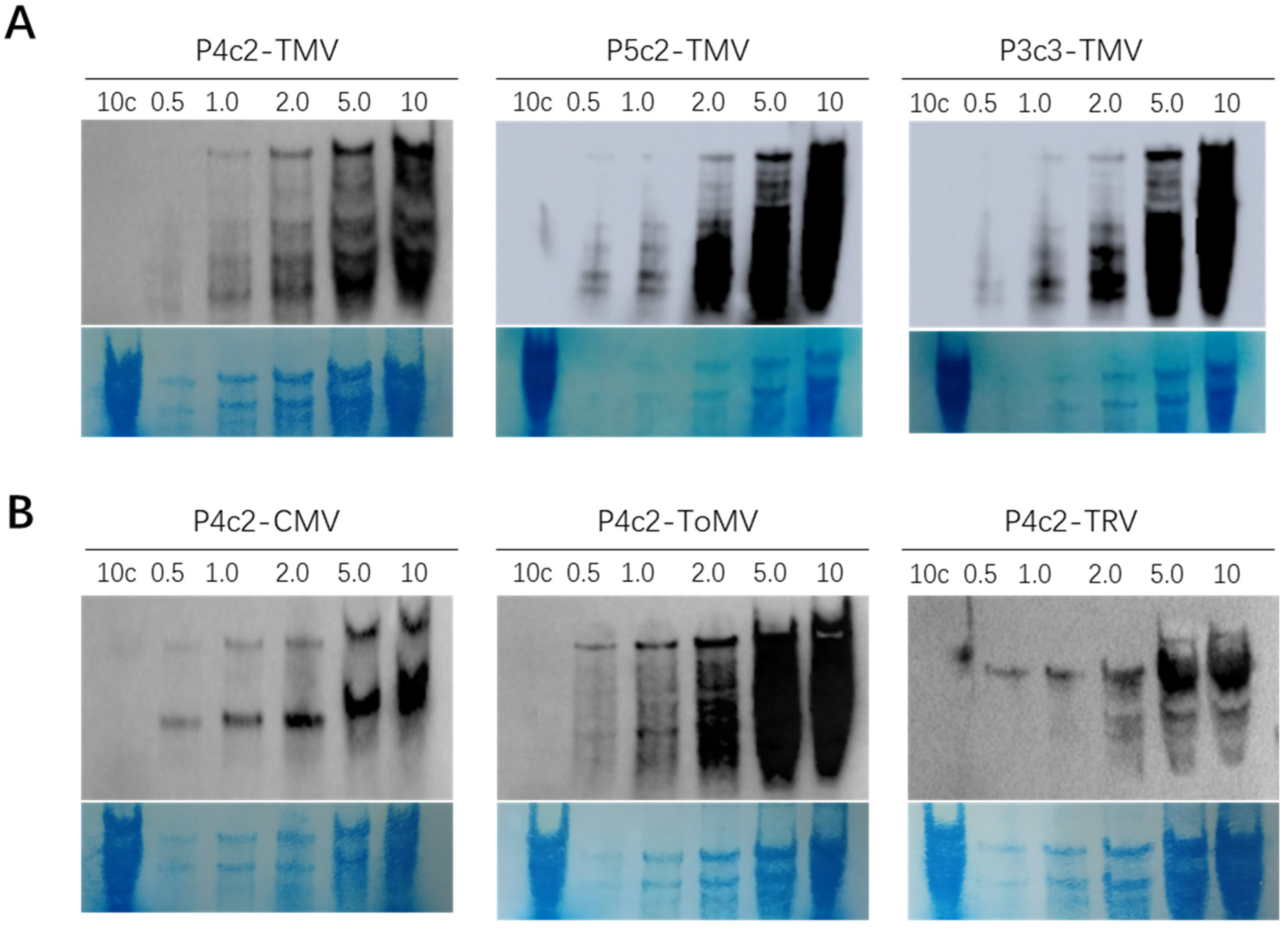
| Procedure | New Northern Protocol | Traditional mRNA Northern Blot |
|---|---|---|
| Step1, Gel preparation | ||
| Gel volume | Low 30–50 mL | High ~200 mL |
| Time | 30 min | 30 min |
| Caution | Pull the comb gently to avoid breaking wells | |
| Step2, Electrophoresis | ||
| Equipment | Vertical electrophoresis 120 V | Horizontal electrophoresis 100 V |
| Compatible with small RNA Northern blot | Not compatible | |
| Time | 45 min | 2 to 3 h |
| Step3, Gel blotting | ||
| Equipment | Semidry transfer | Capillary transfer |
| Time | 90 min | 12~16 h |
| Caution | Gel is thin and easy to break, handle with care, Rain-X can be applied to glass plates for easier disassembly | |
| Step4, Probe-labeling | ||
| Chemistry | T4 DNA pol reaction + biotin-dCTP | Random labeling kit + α-p32-dCTP |
| Time | 75 min | 30 min |
| Advantage | Accessible to all labs | |
| Disadvantage | Special permit and facility required, material not easy to obtain. | |
| Step5, Hybridization | ||
| Equipment | Hybridization oven | Hybridization oven |
| Time | Overnight | Overnight (12~16 h) |
| Disadvantage | Special permit and facility required | |
| Step6, Signal detection | ||
| Equipment | Use the biotin-detection kit and ChemiDoc XRS System | Use a phosphoscreen and scanner |
| Time | about 1 h | Usually more than 8 h |
| Overall time frame |  | |
| New protocol | ||
 | ||
| Old protocol | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Zhang, Q.; Cheng, L.; Liu, D.; Wang, H.; Zhou, Y.; Ma, L.; Wang, J.; Li, F. A New Biotin Labeling and High-Molecular-Weight RNA Northern Method and Its Application in Viral RNA Detection. Viruses 2022, 14, 2664. https://doi.org/10.3390/v14122664
Zhang X, Zhang Q, Cheng L, Liu D, Wang H, Zhou Y, Ma L, Wang J, Li F. A New Biotin Labeling and High-Molecular-Weight RNA Northern Method and Its Application in Viral RNA Detection. Viruses. 2022; 14(12):2664. https://doi.org/10.3390/v14122664
Chicago/Turabian StyleZhang, Xi, Qingling Zhang, Long Cheng, Dan Liu, Hongzheng Wang, Yingjia Zhou, Liqun Ma, Jubin Wang, and Feng Li. 2022. "A New Biotin Labeling and High-Molecular-Weight RNA Northern Method and Its Application in Viral RNA Detection" Viruses 14, no. 12: 2664. https://doi.org/10.3390/v14122664
APA StyleZhang, X., Zhang, Q., Cheng, L., Liu, D., Wang, H., Zhou, Y., Ma, L., Wang, J., & Li, F. (2022). A New Biotin Labeling and High-Molecular-Weight RNA Northern Method and Its Application in Viral RNA Detection. Viruses, 14(12), 2664. https://doi.org/10.3390/v14122664






