Clinical Outcomes and Quantitative HBV Surface Antigen Levels in Diverse Chronic Hepatitis B Patients in Canada: A Retrospective Real-World Study of CHB in Canada (REVEAL-CANADA)
Abstract
:1. Introduction
2. Methods
2.1. Study Design
2.2. Data Elements
2.3. Statistical Analysis
2.4. Ethics
3. Results
3.1. Patients
3.2. Summary of Virological Outcomes
3.3. Summary of Antiviral Therapy and Outcomes
3.4. Comparison of Hepatic Outcomes between Groups
3.5. Other Factors Associated with HBsAg Loss
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Lay Summary/Key Points
References
- Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: A modelling study. Lancet Gastroenterol. Hepatol. 2018, 3, 383–403. [Google Scholar] [CrossRef]
- Global Burden of Disease Liver Cancer Collaboration. The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Lok, A.S.; McMahon, B.J.; Brown, R.S., Jr.; Wong, J.B.; Ahmed, A.T.; Farah, W.; Almasri, J.; Alahdab, F.; Benkhadra, K.; Mouchli, M.A.; et al. Antiviral therapy for chronic hepatitis B viral infection in adults: A systematic review and meta-analysis. Hepatology 2016, 63, 284–306. [Google Scholar] [CrossRef] [PubMed]
- Likhitsup, A.; Lok, A.S. Understanding the Natural History of Hepatitis B Virus Infection and the New Definitions of Cure and the Endpoints of Clinical Trials. Clin. Liver Dis. 2019, 23, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Liem, K.S.; Fung, S.; Wong, D.K.; Yim, C.; Noureldin, S.; Chen, J.; Feld, J.J.; Hansen, B.E.; Janssen, H.L.A. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: Results from a randomised controlled trial (Toronto STOP study). Gut 2019, 68, 2206–2213. [Google Scholar] [CrossRef] [PubMed]
- Coffin, C.S.; Rezaeeaval, M.; Pang, J.X.; Alcantara, L.; Klein, P.; Burak, K.W.; Myers, R.P. The incidence of hepatocellular carcinoma is reduced in patients with chronic hepatitis B on long-term nucleos(t)ide analogue therapy. Aliment. Pharmacol. Ther. 2014, 40, 1262–1269. [Google Scholar] [CrossRef] [PubMed]
- Cornberg, M.; Wong, V.W.-S.; Locarnini, S.; Brunetto, M.; Janssen, H.L.; Chan, H.L.-Y. The role of quantitative hepatitis B surface antigen revisited. J. Hepatol. 2017, 66, 398–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dusheiko, G.; Wang, B. Hepatitis B Surface Antigen Loss: Too Little, Too Late and the Challenge for the Future. Gastroenterology 2019, 156, 548–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, K.; Contag, C.; Whitaker, E.; Terrault, N. Spontaneous loss of surface antigen among adults living with chronic hepatitis B virus infection: A systematic review and pooled meta-analyses. Lancet Gastroenterol. Hepatol. 2019, 4, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Yeo, Y.H.; Ho, H.J.; Yang, H.-I.; Tseng, T.-C.; Hosaka, T.; Trinh, H.N.; Kwak, M.-S.; Park, Y.M.; Fung, J.Y.Y.; Buti, M.; et al. Factors Associated With Rates of HBsAg Seroclearance in Adults With Chronic HBV Infection: A Systematic Review and Meta-analysis. Gastroenterology 2019, 156, 635–646.e9. [Google Scholar] [CrossRef] [PubMed]
- Jeng, W.-J.; Chen, Y.-C.; Chien, R.-N.; Sheen, I.-S.; Liaw, Y.-F. Incidence and predictors of hepatitis B surface antigen seroclearance after cessation of nucleos(t)ide analogue therapy in hepatitis B e antigen-negative chronic hepatitis B. Hepatology 2018, 68, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Coffin, C.S.; Ramji, A.; Cooper, C.L.; Miles, D.; Doucette, K.E.; Wong, P.; Tam, E.; Wong, D.K.; Wong, A.; Ukabam, S.; et al. Epidemiologic and clinical features of chronic hepatitis B virus infection in 8 Canadian provinces: A descriptive study by the Canadian HBV Network. CMAJ Open 2019, 7, E610–E617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coffin, C.S.; Fung, S.K.; Alvarez, F.; Cooper, C.L.; Doucette, K.E.; Fournier, C.; Kelly, E.; Ko, H.H.; Ma, M.M.; Martin, S.R.; et al. Management of Hepatitis B Virus Infection: 2018 Guidelines from the Canadian Association for the Study of Liver Disease and Association of Medical Microbiology and Infectious Disease Canada. Can. Liver J. 2018, 1, 156–217. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.H.; Patel, N.H.; Haylock-Jacobs, S.; Doucette, K.; Ma, M.M.; Cooper, C.; Kelly, E.; Elkhashab, M.; Tam, E.; Bailey, R.; et al. Severe Hepatic Steatosis Is Associated With Low-Level Viremia and Advanced Fibrosis in Patients With Chronic Hepatitis B in North America. Gastro Hep Adv. 2022, 1, 106–116. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.I.; Lee, M.H.; Lu, S.N.; Jen, C.L.; Batrla-Utermann, R.; Wang, L.Y.; You, S.L.; Hsiao, C.K.; Chen, P.J.; et al. Spontaneous seroclearance of hepatitis B seromarkers and subsequent risk of hepatocellular carcinoma. Gut 2014, 63, 1648–1657. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Yang, H.; Lee, M.; Jen, C.; Batrla-Utermann, R.; Lu, S.; Wang, L.; You, S.; Chen, C. Serum Levels of Hepatitis B Surface Antigen and DNA Can Predict Inactive Carriers With Low Risk of Disease Progression. Hepatology 2016, 64, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papaioannou, A.; Morin, S.; Cheung, A.M.; Atkinson, S.; Brown, J.P.; Feldman, S.; Hanley, D.A.; Hodsman, A.; Jamal, S.A.; Kaiser, S.M.; et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: Summary. Can. Med. Assoc. J. 2010, 182, 1864–1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habersetzer, F.; Moenne-Loccoz, R.; Meyer, N.; Schvoerer, E.; Simo-Noumbissie, P.; Dritsas, S.; Baumert, T.F.; Doffoël, M. Loss of hepatitis B surface antigen in a real-life clinical cohort of patients with chronic hepatitis B virus infection. Liver Int. 2015, 35, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.; Liu, C.; Su, T.; Wang, C.; Chen, C.; Chen, P.; Chen, D.; Kao, J. Serum Hepatitis B Surface Antigen Levels Predict Surface Antigen Loss in Hepatitis B e Antigen Seroconverters. Gastroenterology 2011, 141, 517–525.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Lee, M.-H.; Batrla-Utermann, R.; Jen, C.-L.; Iloeje, U.H.; Lu, S.-N.; Wang, L.-Y.; You, S.-L.; Hsiao, C.K.; Yang, H.-I.; et al. A predictive scoring system for the seroclearance of HBsAg in HBeAg-seronegative chronic hepatitis B patients with genotype B or C infection. J. Hepatol. 2013, 58, 853–860. [Google Scholar] [CrossRef] [PubMed]
- de Franchis, R.; Meucci, G.; Vecchi, M.; Tatarella, M.; Colombo, M.; del Ninno, E.; Rumi, M.G.; Donato, M.F.; Ronchi, G. The natural history of asymptomatic hepatitis B surface antigen carriers. Ann. Intern. Med. 1993, 118, 191–194. [Google Scholar] [CrossRef]
- Striki, A.; Manolakopoulos, S.; Deutsch, M.; Kourikou, A.; Kontos, G.; Kranidioti, H.; Hadziyannis, E.; Papatheodoridis, G. Hepatitis B s antigen kinetics during treatment with nucleos(t)ides analogues in patients with hepatitis B e antigen-negative chronic hepatitis B. Liver Int. 2017, 37, 1642–1650. [Google Scholar] [CrossRef]
- Van Hees, S.; Chi, H.; Hansen, B.; Bourgeois, S.; Van Vlierberghe, H.; Sersté, T.; Francque, S.; Wong, D.; Sprengers, D.; Moreno, C.; et al. Caucasian Ethnicity, but Not Treatment Cessation is Associated with HBsAg Loss Following Nucleos(t)ide Analogue-Induced HBeAg Seroconversion. Viruses 2019, 11, 687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.H.; Hoang, J.; Vu, V.D.; Wang, C.; Trinh, H.N.; Li, J.; Zhang, J.Q. Ethnic differences in incidence of hepatitis B surface antigen seroclearance in a real-life multicenter clinical cohort of 4737 patients with chronic hepatitis B infection. Aliment. Pharmacol. Ther. 2016, 44, 390–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrault, N.A.; Wahed, A.S.; Feld, J.J.; Cooper, S.L.; Ghany, M.G.; Lisker-Melman, M.; Perrillo, R.; Sterling, R.K.; Khalili, M.; Chung, R.T.; et al. Incidence and prediction of HBsAg seroclearance in a prospective multi-ethnic HBeAg-negative chronic hepatitis B cohort. Hepatology 2021, 75, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Lauret, E.; González-Diéguez, M.L.; Rodríguez, M.; González, M.; Melón, S.; Rodrigo, L.; Rodríguez, M. Long-term outcome in Caucasian patients with chronic hepatitis B virus infection after HBsAg seroclearance. Liver Int. 2015, 35, 140–147. [Google Scholar] [CrossRef]
- Yip, T.C.-F.; Wong, V.W.-S.; Tse, Y.-K.; Liang, L.Y.; Hui, V.W.-K.; Zhang, X.; Li, G.-L.; Lui, G.C.-Y.; Chan, H.L.-Y.; Wong, G.L.-H. Similarly low risk of hepatocellular carcinoma after either spontaneous or nucleos(t)ide analogue-induced hepatitis B surface antigen loss. Aliment. Pharmacol. Ther. 2021, 53, 321–331. [Google Scholar]
- Hsu, Y.-C.; Yeh, M.-L.; Wong, G.L.-H.; Chen, C.-H.; Peng, C.-Y.; Buti, M.; Enomoto, M.; Xie, Q.; Trinh, H.; Preda, C.; et al. Incidences and Determinants of Functional Cure During Entecavir or Tenofovir Disoproxil Fumarate for Chronic Hepatitis B. J. Infect. Dis. 2021, 224, 1890–1899. [Google Scholar] [CrossRef]
- Mak, L.-Y.; Hui, R.W.-H.; Fung, J.; Liu, F.; Wong, D.K.-H.; Cheung, K.-S.; Yuen, M.-F.; Seto, W.-K. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J. Hepatol. 2020, 73, 800–806. [Google Scholar] [CrossRef]
- Congly, S.E.; Wong, P.; Al-Busafi, S.A.; Doucette, K.; Fung, S.K.; Ghali, P.; Fonseca, K.; Myers, R.P.; Osiowy, C.; Coffin, C.S. Characterization of hepatitis B virus genotypes and quantitative hepatitis B surface antigen titres in North American tertiary referral liver centres. Liver Int. 2013, 33, 1363–1369. [Google Scholar] [CrossRef]
- O’Neil, C.R.; Congly, S.E.; Rose, M.S.; Lee, S.S.; Borman, M.A.; Charlton, C.L.; Osiowy, C.; Swain, M.G.; Burak, K.W.; Coffin, C.S. Long-Term Follow-up and Quantitative Hepatitis B Surface Antigen Monitoring in North American Chronic HBV Carriers. Ann. Hepatol. 2018, 17, 232–241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.Q.; Shi, B.S.; Lu, W.; Liu, D.P.; Huang, D.; Feng, Y.L. Quantitative HBcrAg and HBcAb versus HBsAg and HBV DNA in predicting liver fibrosis levels of chronic hepatitis B patients. Gastroenterol. Hepatol. 2020, 43, 526–536. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.-N.; Tsai, H.-W.; Chiu, Y.-C.; Ho, C.-H.; Wu, I.-C.; Chang, T.-T. Clinical significance of serum HBsAg levels and association with liver histology in HBeAg positive chronic hepatitis B. J. Clin. Virol. 2013, 57, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Mathurin, P.; Lai, C.-L.; Guyader, D.; Poupon, R.; Tainturier, M.-H.; Myers, R.P.; Muntenau, M.; Ratziu, V.; Manns, M.; et al. A comparison of fibrosis progression in chronic liver diseases. J. Hepatol. 2003, 38, 257–265. [Google Scholar] [CrossRef]
- Liaw, Y.-F. Clinical utility of HBV surface antigen quantification in HBV e antigen-negative chronic HBV infection. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 631–641. [Google Scholar] [CrossRef]

| Quantitative HBsAg Levels IU/mL (Sample Size) n = 844 | HBsAg Neg (n = 237) | 1–100 (n = 190) | 100–500 (n = 91) | 500–1000 (n = 54) | >1000 (n = 272) | p-Value HBsAg Neg vs. Pos. |
|---|---|---|---|---|---|---|
| Age, median (95% CI, n known) | 53 (51.5, 54.6, 235/237) | 55 (53.1, 56.8, 190) | 55.4 (52.8, 58.1, 91) | 52.5 (48.7, 56.2, 54) | 44.1 (42.6, 45.6, 272) | <0.001 * |
| Female, % (95% CI, n) | 37.6 (31.4, 44.1, 89/237) | 34.7 (28.1, 42, 66/190) | 34.1 (24.7, 44.8, 31/91) | 33.3 (21.5, 47.6, 18/54) | 41.2 (35.3, 47.3, 112/272) | 0.701 |
| Born in Canada, % (95% CI, n known) | 3.4 (1.4, 7.6, 6/176) | 2.1 (0.5, 6.4, 3/146) | 1.5 (0.1, 9.4, 1/65) | 6 (1.6, 17.5, 3/50) | 5.4 (3, 9.5, 12/221) | 0.442 |
| Asian, % (95% CI, n known) | 74.9 (68.2, 80.6, 149/199) | 85.5 (78.9, 90.4, 136/159) | 90.2 (81.2, 95.4, 74/82) | 86.8 (74, 94.1, 46/53) | 72 (66, 77.3, 185/257) | 0.198 |
| Black/African/ Caribbean (95% CI, n known) | 13.1 (8.9, 18.7, 26/199) | 7.5 (4.1, 13.1, 12/159) | 7.3 (3, 15.8, 6/82) | 9.4 (3.5, 21.4, 5/53) | 20.2 (15.6, 25.8, 52/257) | 0.071 |
| White (95% CI, n known) | 7 (4, 11.8, 14/199) | 4.4 (1.9, 9.2, 7/159) | 0 (0, 5.6, 0/82) | 1.9 (0.1, 11.4, 1/53) | 4.7 (2.5, 8.2, 12/257) | 0.171 |
| Other/ unknown ethnicity % (95% CI, n known) | 5 (2.6, 9.3, 10/199) | 2.5 (0.8, 6.7, 4/159) | 2.4 (0.4, 9.4, 2/82) | 1.9 (0.1, 11.4, 1/53) | 3.1 (1.5, 6.3, 8/257) | 0.536 |
| Antiviral treatment for HBV at any time, % (95% CI, n known) | 38.3 (32.2, 44.9, 91/237) | 51.6 (44.3, 58.8, 98/190) | 76.9 (66.7, 84.8, 70/91) | 66.7 (52.4, 78.5, 36/54) | 61 (54.9, 66.8, 166/272) | <0.001 * |
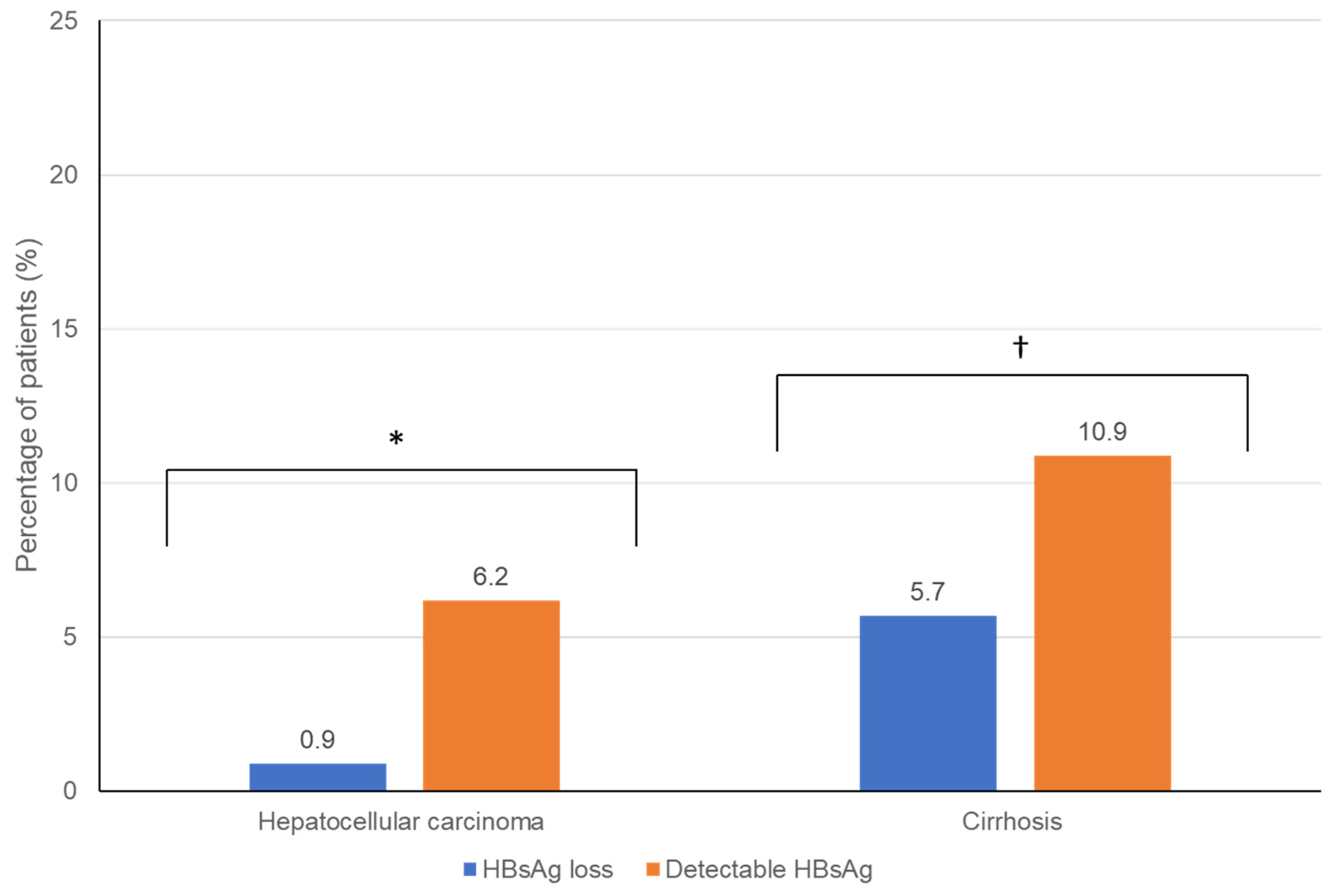
| Cirrhosis, % (95% CI, n known) | 4.8 (2.4, 8.9, 10/209) | 20.4 (14.6, 27.6, 33/162) | 14.3 (8.1, 23.6, 13/91) | 7.4 (2.4, 18.7, 4/54) | 5.1 (3, 8.7, 14/272) | 0.021 * |
| HCC, % (95% CI, n known) | 1 (0.2, 3.8, 2/210) | 9.2 (5.3, 15.2, 14/153) | 7.7 (3.4, 15.7, 7/91) | 9.3 (3.5, 21.1, 5/54) | 2.9 (1.4, 5.9, 8/272) | 0.001 * |
| NAFLD, % (95% CI, n known) | 17.6 (12.9, 23.6, 37/210) | 26.5 (20.1, 34.2, 43/162) | 26.4 (17.9, 36.8, 24/91) | 11.1 (4.6, 23.3, 6/54) | 18.8 (14.4, 24, 51/272) | 0.019 * |
| Clinical Data (% (95% CI, n Known)) | HBsAg Negative (n = 237) | HBsAg 1–100 IU/mL (n = 190) | HBsAg ≥100 IU/mL (n = 417) | p-Value (HBsAg Loss vs. qHBsAg <100 IU/mL) † |
|---|---|---|---|---|
| Laboratory | ||||
| qHBsAg level % (95% CI, n known) | 0 (237) | 20.1 (16.0, 24.1, 190) | 8088 (5286.1–10891.8, 417) | N/A N/A |
| % HBeAg negative, (95% CI, n known) | 98.9% (95.8, 99.8, 184/186) | 94.1% (88.8, 97.1, 144/153) | 78.9% (74.2, 83.1, 270/342) | 0.004 * |
| HBV DNA, Log IU/mL (95% CI, n known) | 0.21 (0.13–0.3, 182) | 0.97 (0.76–1.17, 153) | 2.15 (1.9–2.4, 378) | <0.001 * |
| ALT (U/L), (95% CI, n known) | 25.9 (23.9–27.9, 224) | 30.0 (26.1–33.9, 174) | 42.8 (35.5–50.1, 373) | 0.023 * |
| Liver Stiffness Measurement Using Transient Elastography (TE) (kPa) | ||||
| TE kpA (95% CI, n known) | 5.7 (5.1-6.3, 109) | 8.2 (6.5–9.9, 111) | 6.0 (5.5–6.5, 302) | 0.005 * |
| % >10.7 kpA (possible fibrosis stage 3) | 6.7% (8/120) | 17.5% (17/97) | 4.3% (13/302) | 0.013 * |
| Treatment 54.6% (n = 461/844) | ||||
| On treatment at any time, % | 46.4% (110/237) | 41.6% (79/190) | 65.2% (272/417) | 0.002 * |
| Tenofovir-based ††, % | 27.8% (66/237) | 25.3% (48/190) | 41.7% (174/417) | 0.027 * |
| Lamivudine, % | 13.1% (31/237) | 12.1% (23/190) | 9.8% (41/417) | 0.140 |
| Entecavir, % | 9.7% (23/237) | 18.9% (36/190) | 23.3% (97/417) | <0.001 * |
| Interferon, % | 3.0% (7/237) | 3.2% (6/190) | 12.7% (53/417) | 0.336 |
| Months on treatment, mean (95% CI, n known) | 84 (73.6–94.4, 87) | 104.3 (90–118.5, 72) | 87.6 (75.4–99.7, 260) | 0.020 * |
| Variable | No Treatment (n = 383) | Treatment (n = 461) |
|---|---|---|
| Age | 48.1 (46.8, 49.3) missing: 1/383 | 53.1 (51.9, 54.3) missing: 1/461 |
| % Female | 45.4 (40.4, 50.6, n = 174) | 30.8 (26.7, 35.3, n = 142) |
| % Born in Canada | 2.7 (1.3, 5.2, 9/333) | 4.9 (2.9, 8, 16/325) |
| % Country of birth-Endemic * | 54.4 (48.8, 59.8) 181/333 | 59.7 (54.1, 65) 194/325 |
| % Asian | 69 (63.9, 73.8) 243/352 | 87.2 (83.4, 90.2) 47/398 |
| % Black | 21.9 (17.7, 26.6) 77/352 | 6 (4, 9) 24/398 |
| % White | 4 (2.3, 6.7) 14/352 | 5 (3.2, 7.8) 20/398 |
| % Other/not reported | 5.1 (3.1, 8.1) 18/352 | 1.8 (0.8, 3.8) 7/398 |
| % >2 Comorbidities ** | 9.4 (6.8, 12.9) 36/383 | 18 (14.7, 21.9) 83/461 |
| % NAFLD | 25.1 (20.7, 30) 87/347 | 16.7 (13.4, 20.6) 74/442 |
| % Other cancer (not HCC) | 2.1 (0.9, 4.4) 7/341 | 7.1 (4.9, 10) 31/439 |
| % HBsAg negative | 38.1 (33.3, 43.2) 146/383 | 19.7 (16.3, 23.7) 91/461 |
| % HBsAg 1–100 IU/mL | 24 (19.9, 28.7) 92/383 | 21.3 (17.7, 25.3) 98/461 |
| % HBsAg >100 IU/mL | 37.9 (33, 42.9) 145/383 | 59.0 (54.3, 63.5) 272/461 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coffin, C.S.; Haylock-Jacobs, S.; Doucette, K.; Ramji, A.; Ko, H.H.; Wong, D.K.; Elkhashab, M.; Bailey, R.; Uhanova, J.; Minuk, G.; et al. Clinical Outcomes and Quantitative HBV Surface Antigen Levels in Diverse Chronic Hepatitis B Patients in Canada: A Retrospective Real-World Study of CHB in Canada (REVEAL-CANADA). Viruses 2022, 14, 2668. https://doi.org/10.3390/v14122668
Coffin CS, Haylock-Jacobs S, Doucette K, Ramji A, Ko HH, Wong DK, Elkhashab M, Bailey R, Uhanova J, Minuk G, et al. Clinical Outcomes and Quantitative HBV Surface Antigen Levels in Diverse Chronic Hepatitis B Patients in Canada: A Retrospective Real-World Study of CHB in Canada (REVEAL-CANADA). Viruses. 2022; 14(12):2668. https://doi.org/10.3390/v14122668
Chicago/Turabian StyleCoffin, Carla S., Sarah Haylock-Jacobs, Karen Doucette, Alnoor Ramji, Hin Hin Ko, David K. Wong, Magdy Elkhashab, Robert Bailey, Julia Uhanova, Gerald Minuk, and et al. 2022. "Clinical Outcomes and Quantitative HBV Surface Antigen Levels in Diverse Chronic Hepatitis B Patients in Canada: A Retrospective Real-World Study of CHB in Canada (REVEAL-CANADA)" Viruses 14, no. 12: 2668. https://doi.org/10.3390/v14122668





