Biology and Ultrastructural Characterization of Grapevine Badnavirus 1 and Grapevine Virus G
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus Transmission by the Vine Mealybug (Planoccocus ficus Sign.)
2.2. Mechanical Inoculation
2.3. Seed Transmission
2.4. Graft Transmission
2.5. Green Grafting Using T-Budding Technique
2.6. Electron Microscopy
2.7. Detection by Real-Time PCR Assays
3. Results
3.1. Vector Transmission
3.2. Mechanical Inoculation
3.3. Seed Transmission
3.4. Grafting
3.5. Green Grafting
3.6. Electron Microscopy
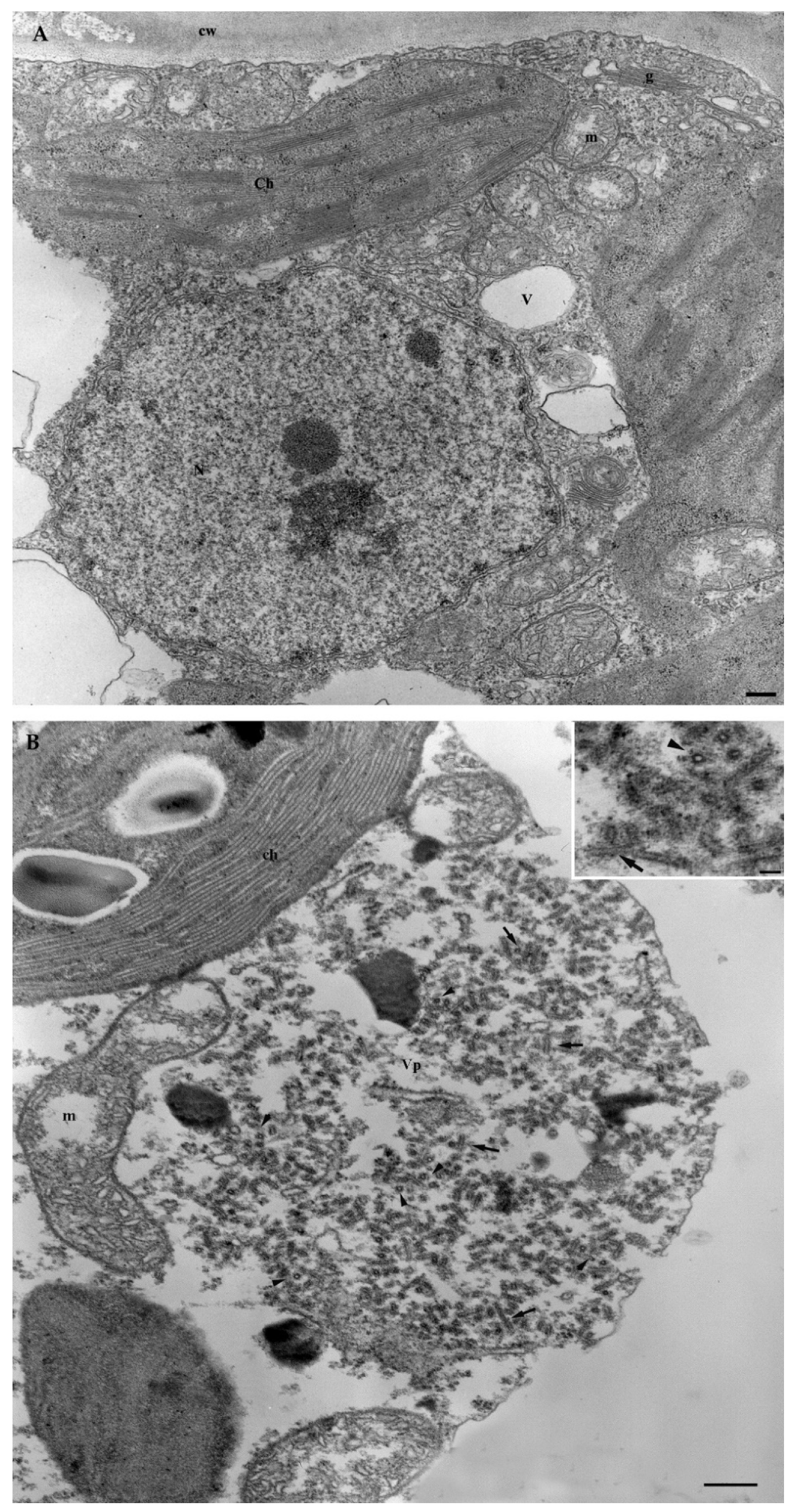
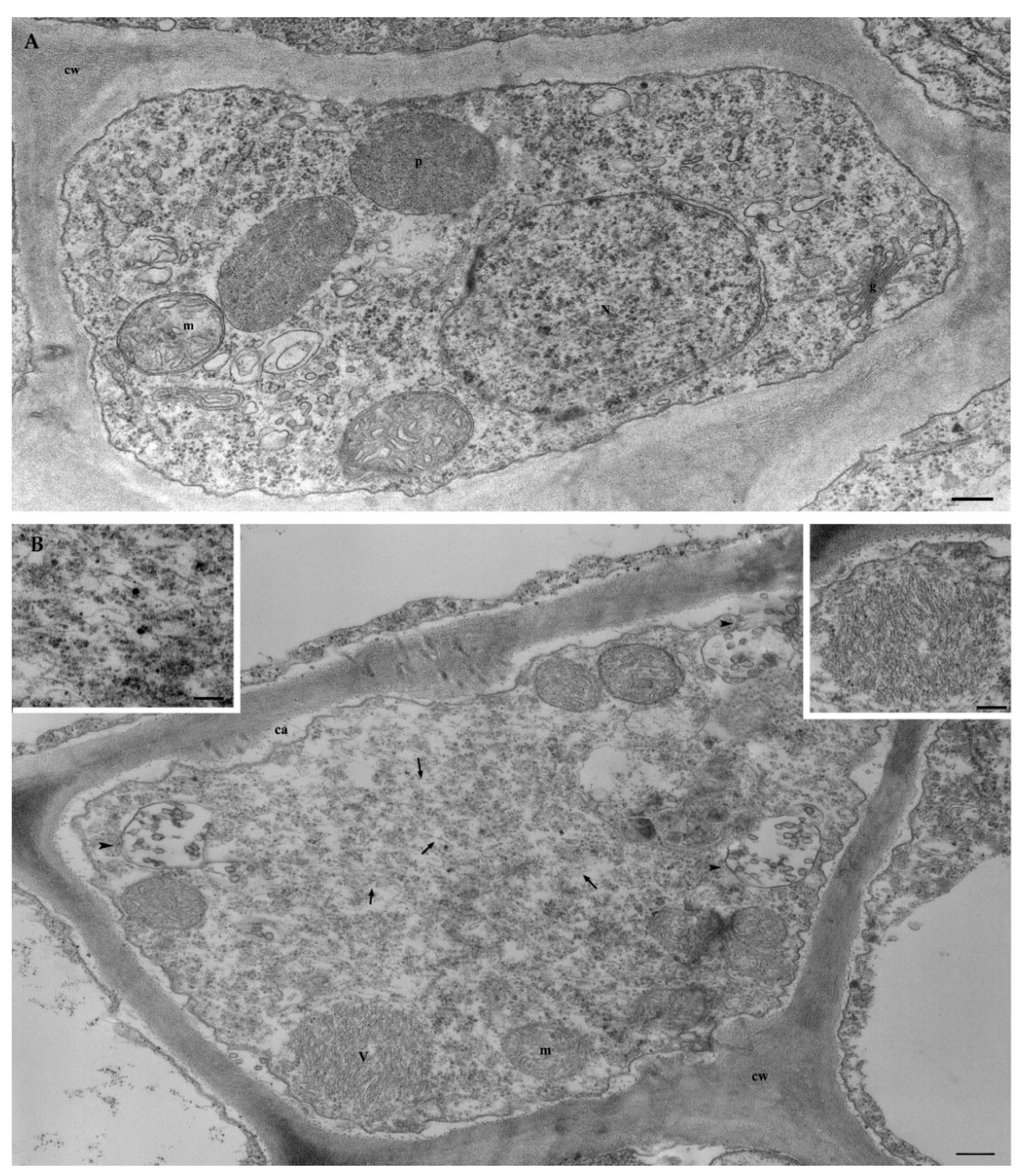
3.6.1. Ultrastructural Characterization of GBV-1-Infected Grapevine
3.6.2. Ultrastructural Characterization of GVG+GLRaV3-Infected Grapevine
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OIV. International Organisation of Vine and Wine. State of the World Vitivinicultural Sector in 2020. Available online: https://www.oiv.int/public/medias/8731/oiv-state-of-the-world-vitivinicultural-sector-in-2020.pdf (accessed on 21 September 2022).
- Croatian Bureau of Statisticks. Available online: https://web.dzs.hr/PXWeb/Table.aspx?layout=tableViewLayout1&px_tableid=PP28.px&px_path=Popis%20poljoprivrede%202020__Poljoprivredno%20zemlji%c5%a1te&px_language=hr&px_db=Popis%20poljoprivrede%202020&rxid=0732e953-1537-4a20-b0c6-02dce20d4c33 (accessed on 24 September 2022).
- Fuchs, M. Grapevine viruses: A multitude of diverse species with simple but overall poorly adopted management solutions in the vineyard. J. Plant Pathol. 2020, 102, 643–653. [Google Scholar] [CrossRef]
- Maliogka, V.I.; Martelli, G.P.; Fuchs, M.; Katis, N.I. Control of viruses infecting grapevine. Adv. Virus Res. 2015, 91, 175–227. [Google Scholar] [PubMed]
- Golino, D.A.; Fuchs, M.; Sim, S.; Farrar, K.; Martelli, G.P. Improvement of grapevine planting stock through sanitary selection and pathogen elimination. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 561–579. [Google Scholar] [CrossRef]
- EPPO. Pathogen-tested material of grapevine varieties and rootstocks (Certification scheme). Bull. OEPP 2008, 38, 422–429. [Google Scholar] [CrossRef]
- Blouin, A.G.; Keenan, S.; Napier, K.R.; Barrero, R.A.; MacDiarmid, R.M. Identification of a novel vitivirus from grapevines in New Zealand. Arch. Virol. 2017, 163, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Vončina, D.; Almeida, R.P.P. Screening of some Croatian autochthonous grapevine varieties reveals multitude of viruses including novel ones. Arch. Virol. 2018, 163, 2239–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diaz-Lara, A.; Brisbane, R.S.; Aram, K.; Golino, D.; Al Rwahnih, M. Detection of new vitiviruses infecting grapevine in California. Arch. Virol. 2019, 164, 2573–2580. [Google Scholar] [CrossRef] [PubMed]
- Jagunić, M.; Diaz-Lara, A.; Szőke, L.; Rwahnih, M.A.; Stevens, K.; Zdunić, G.; Vončina, D. Incidence and genetic diversity of grapevine virus G in Croatian vineyards. Plants 2022, 11, 2341. [Google Scholar] [CrossRef]
- Minafra, A.; Mawassi, M.; Goszczynski, D.; Saldarelli, P. Grapevine vitiviruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 229–256. [Google Scholar] [CrossRef]
- Zhang, Y.; Singh, K.; Kaur, R.; Qiu, W. Association of a novel DNA virus with the grapevine vein-clearing and vine decline syndrome. Phytopathology 2011, 101, 1081–1090. [Google Scholar] [CrossRef]
- Maliogka, V.I.; Olmos, A.; Pappi, P.G.; Lotos, L.; Efthimiou, K.; Grammatikaki, G.; Avgelis, A.D. A novel grapevine badnavirus is associated with the Roditis leaf discoloration disease. Virus Res. 2015, 203, 47–55. [Google Scholar] [CrossRef]
- Qiu, W.P.; Avery, J.D.; Lunden, S. Characterization of a severe virus-like disease in Chardonnay grapevines in Missouri. Plant Health Prog. 2007, 8, 39. [Google Scholar] [CrossRef] [Green Version]
- Cieniewicz, E.J.; Qiu, W.; Saldarelli, P.; Fuchs, M. Believing is seeing: Lessons from emerging viruses in grapevine. J. Plant Pathol. 2020, 102, 619–632. [Google Scholar] [CrossRef]
- Rumbos, I.C.; Avgelis, A.D. Roditis leaf discoloration—A new virus disease of grapevine: Symptomatology and transmission to indicators plants. J. Phytopathol. 1989, 152, 274–278. [Google Scholar] [CrossRef]
- Ekemen, M. Investigation of Etiology on Grapevine Roditis Leaf Discoloration-Associated Virus. Master’s Thesis, Niğde, Turkey, February 2021. Available online: http://acikerisim.ohu.edu.tr/xmlui/handle/11480/8548 (accessed on 5 October 2022).
- Chiumenti, M.; Morelli, M.; Giampetruzzi, A.; Palmisano, F.; Savino, V.N.; La Notte, P.; Martelli, G.P.; Saldarelli, P. First report of grapevine Roditis leaf discoloration-associated virus in Italy. J. Plant Pathol. 2015, 97, 551. [Google Scholar] [CrossRef]
- Ulubaş Serçe, Ç.; Altan, B.; Bolat, V.; Ayyaz, M.; Çifçi, O.; Önder, S.; Öztürk Gökçe, Z.N.; Maliogka, V.I. First Report of grapevine Roditis leaf discoloration-associated virus infecting grapevine (Vitis vinifera) in Turkey. Plant Dis. 2018, 2, 256. [Google Scholar] [CrossRef]
- Bester, R.; Lotos, L.; Vermeulen, A.; Pietersen, G.; Maliogka, V.I.; Maree, H.J. Genome sequence of a grapevine Roditis leaf discoloration-associated virus (GRLDaV) variant from South Africa. Arch Virol. 2021, 166, 2041–2044. [Google Scholar] [CrossRef] [PubMed]
- Jagunić, M.; Diaz-Lara, A.; Rwahnih, M.A.; Preiner, D.; Stevens, K.; Zdunić, G.; Hwang, M.; Vončina, D. Grapevine badnavirus 1: Detection, genetic diversity, and distribution in Croatia. Plants 2022, 11, 2135. [Google Scholar] [CrossRef]
- Daane, K.M.; Middleton, M.C.; Sforza, R.; Cooper, M.L.; Walton, V.M.; Walsh, D.B.; Zaviezo, T.; Almeida, R.P.P. Development of a multiplex PCR for identification of vineyard mealybugs. Environ. Entomol. 2011, 40, 1595–1603. [Google Scholar] [CrossRef]
- Osman, F.; Rowhani, A. Real-time RT-PCR (TaqMan®) assays for the detection of viruses associated with Rugose wood complex of grapevine. J. Virol. Methods 2008, 154, 69–75. [Google Scholar] [CrossRef]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Comparison of low-density arrays, RT-PCR and real-time TaqMan® RT-PCR in detection of grapevine viruses. J. Virol. Methods 2008, 149, 292–299. [Google Scholar] [CrossRef]
- Osman, F.; Leutenegger, C.; Golino, D.; Rowhani, A. Real-time RT-PCR (TaqMan®) assays for the detection of grapevine leafroll associated viruses 1–5 and 9. J. Virol. Methods 2007, 141, 22–29. [Google Scholar] [CrossRef]
- Diaz-Lara, A.; Klaassen, V.; Stevens, K.; Sudarshana, M.R.; Rowhani, A.; Maree, H.J.; Chooi, K.M.; Blouin, A.G.; Habili, N.; Song, Y.; et al. Characterization of grapevine leafroll-associated virus 3 genetic variants and application towards RT-qPCR assay design. PLoS ONE 2018, 13, e0208862. [Google Scholar] [CrossRef] [Green Version]
- Martelli, G.P. Facilities and tehniques for identification of diseases and their agents by biological methods. In Graft-Transmissible Diseases of Grapevines—Handbook for Detection and Diagnosis; ICVG, FAO: Viale Rome, Italy, 1993; pp. 157–158. [Google Scholar]
- Boscia, D.; Savino, V.; Minafra, A.; Namba, S.; Elicio, V.; Castellano, M.A.; Gonsalves, D.; Martelli, G.P. Properties of a filamentous virus isolated from grapevines affected by corky bark. Arch. Virol. 1993, 130, 109–120. [Google Scholar] [CrossRef]
- Martelli, G.P.; Russo, M. Use of thin sectioning for visualization and identification of plant viruses. Methods Virol. 1984, 8, 143–224. [Google Scholar]
- Rowhani, A.; Biardi, L.; Johnson, R.; Saldarelli, P.; Zhang, Y.P.; Chin, J.; Green, M. Simplified sample preparation method and one-tube RT-PCR for grapevine viruses. In Proceedings of the 13th Meeting of the ICVG, Adelaide, Australia, 12–18 March 2000; p. 148. [Google Scholar]
- Maree, H.J.; Blouin, A.G.; Diaz-Lara, A.; Mostert, I.; Al Rwahnih, M.; Candresse, T. Status of the current vitivirus taxonomy. Arch. Virol. 2020, 165, 451–458. [Google Scholar] [CrossRef]
- Jagunić, M.; Lazarević, B.; Nikolić, K.; Stupić, D.; Preiner, D.; Vončina, D. Detection, transmission, and characterization of grapevine virus H in Croatia. Pathogens 2021, 10, 1578. [Google Scholar] [CrossRef]
- Masten Milek, T. Štitaste uši (Hemiptera: Coccoidea) na vinovoj lozi. Glas. Biljne Zašt. 2009, 5, 357–368. [Google Scholar]
- Bahder, B.W.; Zalom, F.G.; Sudarshana, M.R. An evaluation of the flora adjacent to wine grape vineyards for the presence of alternative host plants of grapevine red blotch-associated virus. Plant Dis. 2016, 100, 1571–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, S.; Keith, C.; Austin, K.; Howard, S.; Su, L.; Qiu, W. A natural reservoir and transmission vector of grapevine vein clearing virus. Plant Dis. 2018, 103, 571–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Digiaro, M.; Elbeaino, T.; Martelli, G.P. Grapevine fanleaf virus and other old world nepoviruses. In Grapevine Viruses: Molecular Biology, Diagnostics and Management, 1st ed.; Meng, B., Martelli, G.P., Golino, D.A., Fuchs, M., Eds.; Springer: Cham, Switzerland, 2017; pp. 47–82. [Google Scholar] [CrossRef]
- Horvath, J.; Tobias, I.; Hunyadi, K. New natural herbaceous hosts of grapevine fanleaf nepovirus. HortScience 1994, 26, 31–32. [Google Scholar]
- Izadpanah, K.; Zaki-Aghl, M.; Rowhani, A. Non-vitis hosts of grapevine fanleaf virus and their possible epidemiological significance. In Proceedings of the 14th Meeting of the ICVG, Locorotondo, Italy, 12–17 September 2003; p. 210. [Google Scholar]
- Izadpanah, K.; Zaki-Aghl, M.; Zhang, Y.P.; Daubert, S.D.; Rowhani, A. Bermuda grass as a potential reservoir host for grapevine fanleaf virus. Plant Dis. 2003, 87, 1179–1182. [Google Scholar] [CrossRef] [PubMed]
- Zakiaghl, M.; Izadpanah, K.; Gholampour, Z.; Kargar, M.; Mehrvar, M. Molecular characterization of grapevine fanleaf virus from non Vitis hosts. In Proceedings of the 18th meeting of ICVG, Ankara, Turkey, 7–11 September 2015; pp. 149–150. [Google Scholar]
- Demian, E.; Jaksa-Czotter, N.; Varallyay, E. Grapevine Pinot gris virus is present in different non-Vitis hosts. Plants 2022, 11, 1830. [Google Scholar] [CrossRef]
- Martelli, G.P. Where grapevine virology is heading to. In Proceedings of the 19th Meeting of the ICVG, Santiago, Chile, 9–12 April 2018; pp. 10–15. [Google Scholar]
- Conti, M.; Milne, R.G.; Luisoni, E.; Boccardo, G. A closterovirus from a stem pitting diseased grapevine. Phytopathology 1980, 70, 394–399. [Google Scholar] [CrossRef]
- Bonavia, M.; Digiaro, M.; Boscia, D.; Boari, A.; Bottalico, G.; Savino, V.; Martelli, G.P. Studies on “corky rugose wood” of grapevine and the diagnosis of grapevine virus B. Vitis 1996, 35, 53–58. [Google Scholar]
- Abou Ghanem, N.; Saldarelli, P.; Minafra, A.; Buzkan, N.; Castellano, M.A.; Martelli, G.P. Properties of grapevine virus D, a novel putative trichovirus. J. Plant Pathol. 1997, 79, 15–25. [Google Scholar]
- La Notte, P.; Buzkan, N.; Choueiri, E.; Minafra, A.; Martelli, G.P. Acquisition and transmission of grapevine virus A by the mealybug Pseudococcus longispinus. J. Plant Pathol. 1997, 79, 79–85. [Google Scholar]
- Goszczynski, D.E.; Jooste, A.E.C. Shiraz disease (SD) is transmitted by the mealybug Planococcus ficus and associated with grapevine virus A. In Proceedings of the 14th Meeting of the ICVG, Locorotondo, Italy, 12–17 September 2003; p. 219. [Google Scholar]
- Garau, R.; Prota, V.A.; Boscia, D.; Fiori, M.; Prota, U. Pseudococcus affinis new vector of grapevine trichoviruses A and B. Vitis 1995, 34, 67–68. [Google Scholar]
- Hommay, G.; Komar, V.; Lemaire, O.; Herrbach, E. Grapevine virus A transmission by larvae of Parthenolecanium corni. Eur. J. Plant Pathol. 2007, 121, 185–188. [Google Scholar] [CrossRef]
- Gasparro, M.; Caputo, A.R.; Forleo, L.R.; Perniola, R.; Alba, V.; Milella, R.A.; Antonacci, D. Study of main grapevine viruses transmission in breeding programs. BIO Web Conf. 2016, 7, 01039. [Google Scholar] [CrossRef] [Green Version]
- Laimer, M.; Lemaire, O.; Herrbach, E.; Goldschmidt, V.; Minafra, A.; Wetzel, P.B. Resistance to viruses, phytoplasmas and their vectors in the grapevine in Europe: A review. J. Plant Pathol. 2009, 91, 7–23. [Google Scholar]
- Lazar, J.; Kolber, M.; Lehoczky, J. Detection of some nepoviruses (GFV, GFV-YM, GCMV, ArMV) in the seeds and seedlings of grapevine by ELISA. Kertgazdasag 1990, 22, 58–72. [Google Scholar]
- Zhang, C.W.; Huang, H.Q.; Huang, W.T.; Li, H.W.; Chi, H.; Cheng, Y.Q. Grapevine leafroll-associated virus 2 and grapevine ‘Pinot gris’ virus are present in seedlings developed from seeds of infected grapevine plants. Vitis 2022, 61, 21–25. [Google Scholar]
- Lima, M.F.; Rosa, C.; Golino, D.A.; Rowhani, A. Detection of Rupestris stem pitting associated virus in seedlings of virus-infected maternal grapevine plants. In Proceedings of the 15th Meeting of ICVG, Stellenbosch, South Africa, 3–7 April 2006; pp. 244–245. [Google Scholar]
- Chirkov, S.; Sheveleva, A.; Tsygankova, S.; Sharko, F.; Mitrofanova, I. Characterization of divergent grapevine badnavirus 1 isolates found on different fig species (Ficus spp.). Plants 2022, 11, 2532. [Google Scholar] [CrossRef] [PubMed]
- Pathirana, R.; McKenzie, M.J. A modified green-grafting technique for large-scale virus indexing of grapevine (Vitis vinifera L.). Sci. Hortic. 2005, 107, 97–102. [Google Scholar] [CrossRef]
- Wu, Q.; Habili, N.; Constable, F.; Al Rwahnih, M.; Goszczynski, D.E.; Wang, Y.; Pagay, V. Virus pathogens in Australian vineyards with an emphasis on Shiraz disease. Viruses 2020, 12, 818. [Google Scholar] [CrossRef]
- Rowhani, A.; Daubert, S.; Arnold, K.; Al Rwahnih, M.; Klaassen, V.; Golino, D.; Uyemoto, J.K. Synergy between grapevine vitiviruses and grapevine leafroll viruses. Eur. J. Plant Pathol. 2018, 151, 919–925. [Google Scholar] [CrossRef]
- Bertin, S.; Pacifico, D.; Cavalieri, V.; Marzachì, C.; Bosco, D. Transmission of grapevine virus A and grapevine leafroll-associated viruses 1 and 3 by Planococcus ficus and Planococcus citri fed on mixed-infected plants. Ann. Appl. Biol. 2016, 169, 53–63. [Google Scholar] [CrossRef]
- Goszczynski, D.E.; Habili, N. Grapevine virus A variants of group II associated with Shiraz disease in South Africa are present in plants affected by Australian Shiraz disease, and have also been detected in the USA. Plant Pathol. 2012, 61, 205–214. [Google Scholar] [CrossRef]
- Ngo, T.H.; Webb, R.; Crew, K.S.; Vance, M.E.; Thomas, J.E.; Geering, A.D.W. Identification of putative viroplasms within banana cells infected by banana streak MY virus. J. Gen. Virol. 2020, 101, 1305–1312. [Google Scholar] [CrossRef]
- Monette, P.L.; Godkin, S.E. Ultrastructure of grapevine virus A-infected Nicotiana benthamiana leaves. Can. J. Plant Pathol. 1992, 14, 1–9. [Google Scholar] [CrossRef]
- Rosciglione, B.; Castellano, M.A.; Martelli, G.P.; Savino, V.; Cannizzaro, G. Mealybug transmission of grapevine virus A. Vitis 1983, 22, 331–347. [Google Scholar]
- Faoro, F.; Carzaniga, R. Citochemistry and immunocytochemistry of the inclusion bodies induced by grapevine leafroll-associated closteroviruses GLRaV-1 and GLRaV-3. Riv. Patol. Veg. 1995, 5, 85–94. [Google Scholar]

| Virus | Year | Recipient Plants | Species | No. of Infected Plants/No. of Used Plants (%) |
|---|---|---|---|---|
| GBV-1 | 2020 | Herbaceous test plants | Chenopodium murale | 0/10 |
| Nicotiana benthamiana | 0/20 | |||
| Weeds | Amaranthus retroflexus | 0/10 | ||
| Ambrosia artemisifolia | 0/20 | |||
| Chenopodium album | 0/20 | |||
| Galinsoga parviflora | 0/10 | |||
| Grapevine seedlings | Vitis vinifera | 9/20 (45%) | ||
| 2021 | Grapevine seedling | Vitis vinifera | 16/21 (76.2%) | |
| GVG | 2020 | Herbaceous test plants | Chenopodium murale | 0/10 |
| Nicotiana benthamiana | 0/20 | |||
| Weeds | Amaranthus retroflexus | 0/10 | ||
| Ambrosia artemisifolia | 0/20 | |||
| Chenopodium album | 0/20 | |||
| Galinsoga parviflora | 0/10 | |||
| Grapevine seedlings | Vitis vinifera | 4/20 (20%) | ||
| 2021 | Grapevine seedlings | Vitis vinifera | 2/21 (9.5%) |
| Virus | Indicator | No. of Positive/No. of Grafted Plants (%) | |
|---|---|---|---|
| GBV-1 | Kober 5BB | 4/9 (44.4%) | |
| Vitis rupestris | 1/1 (100%) | ||
| LN 33 | 1/2 (50%) | ||
| TOTAL | 6/12 (50%) | ||
| GVG | Kober 5BB | 36/36 (100%) | |
| Vitis rupestris | 3/3 (100%) | ||
| LN 33 | 4/4 (100%) | ||
| Vitis riparia | 6/6 (100%) | ||
| TOTAL | 49/49 (100%) | ||
| GBV-1+GVG | Kober 5BB | GBV-1 | 4/7 (57%) |
| GVG | 7/7 (100%) | ||
| Vitis rupestris | GBV-1 | 1/2 (50%) | |
| GVG | 2/2 (100%) | ||
| LN 33 | GBV-1 | 2/3 (66.7%) | |
| GVG | 3/3 (100%) | ||
| Vitis riparia | GBV-1 | 2/2 (100%) | |
| GVG | 2/2 (100%) | ||
| TOTAL | GBV-1 | 9/14 (64.3%) | |
| GVG | 14/14 (100%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jagunić, M.; De Stradis, A.; Preiner, D.; La Notte, P.; Al Rwahnih, M.; Almeida, R.P.P.; Vončina, D. Biology and Ultrastructural Characterization of Grapevine Badnavirus 1 and Grapevine Virus G. Viruses 2022, 14, 2695. https://doi.org/10.3390/v14122695
Jagunić M, De Stradis A, Preiner D, La Notte P, Al Rwahnih M, Almeida RPP, Vončina D. Biology and Ultrastructural Characterization of Grapevine Badnavirus 1 and Grapevine Virus G. Viruses. 2022; 14(12):2695. https://doi.org/10.3390/v14122695
Chicago/Turabian StyleJagunić, Martin, Angelo De Stradis, Darko Preiner, Pierfederico La Notte, Maher Al Rwahnih, Rodrigo P. P. Almeida, and Darko Vončina. 2022. "Biology and Ultrastructural Characterization of Grapevine Badnavirus 1 and Grapevine Virus G" Viruses 14, no. 12: 2695. https://doi.org/10.3390/v14122695
APA StyleJagunić, M., De Stradis, A., Preiner, D., La Notte, P., Al Rwahnih, M., Almeida, R. P. P., & Vončina, D. (2022). Biology and Ultrastructural Characterization of Grapevine Badnavirus 1 and Grapevine Virus G. Viruses, 14(12), 2695. https://doi.org/10.3390/v14122695









