Emergence of Salmon Gill Poxvirus
Abstract
:1. Introduction
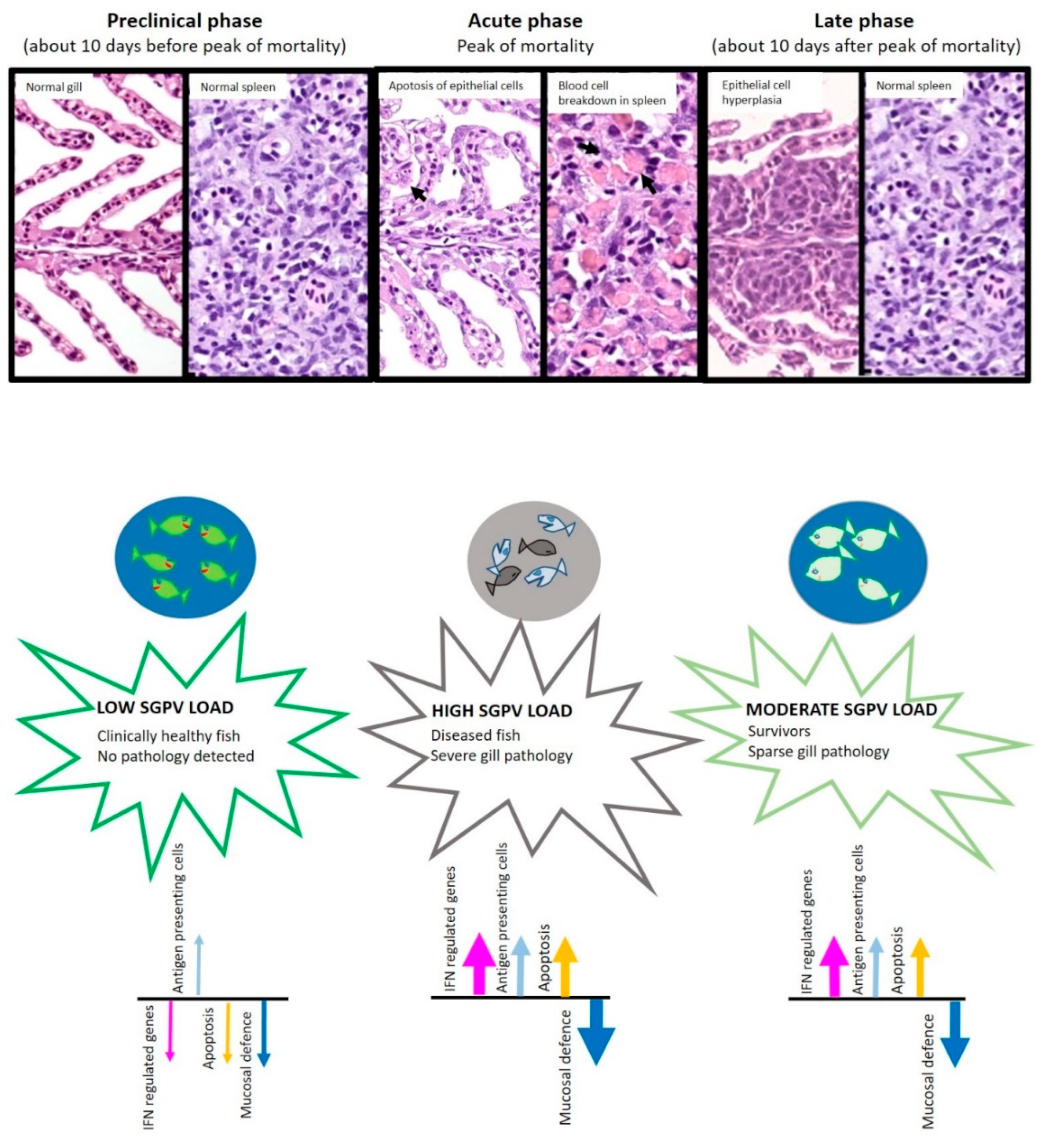
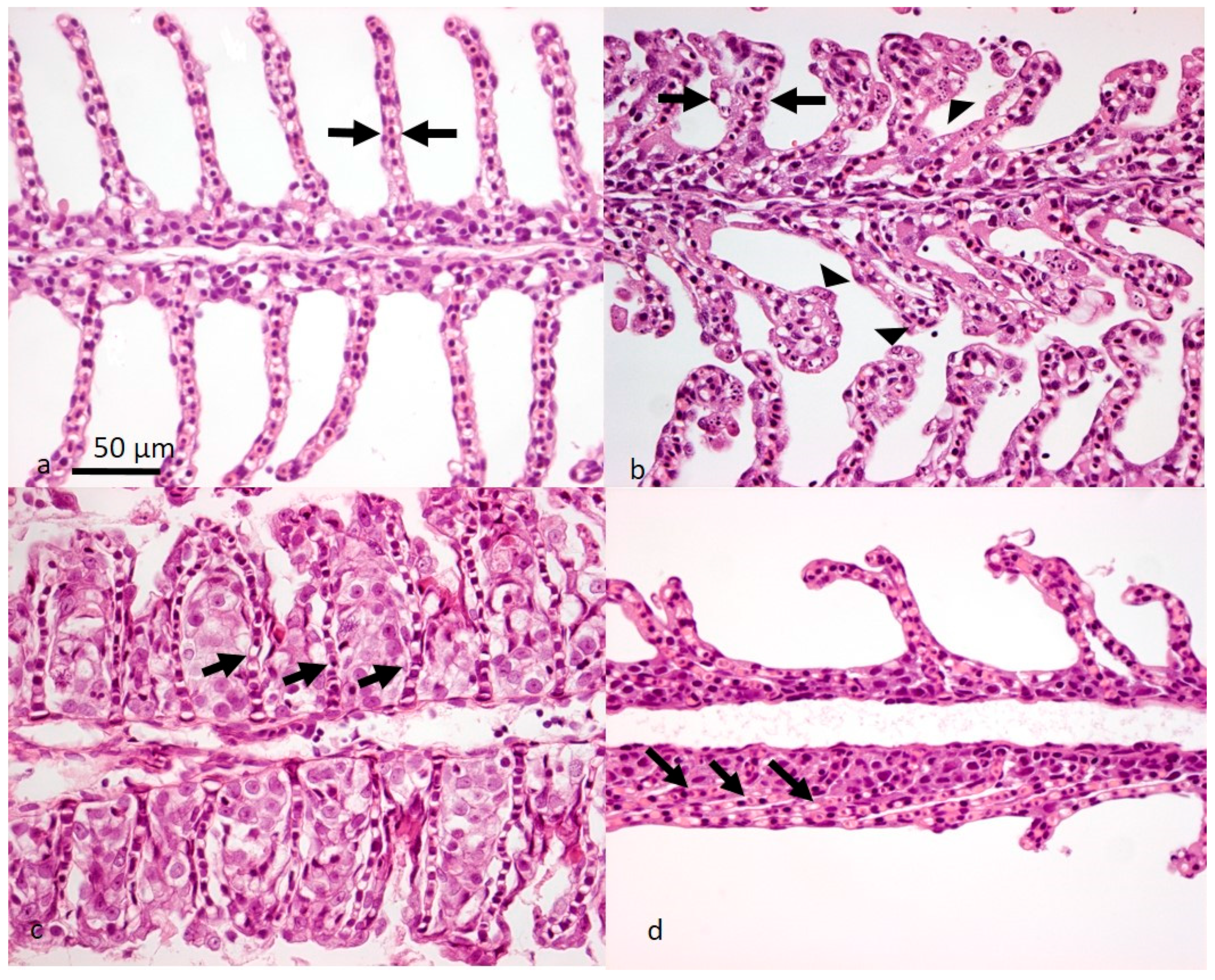
2. Clinical Manifestations—From Asymptomatic Infections to High Mortality Outbreaks
2.1. Is SGPVD a Manifestation of a Dysregulated Host Response in Combination with Respiratory Collapse?
2.2. SGPV as a Contributor in Complex Gill Disease
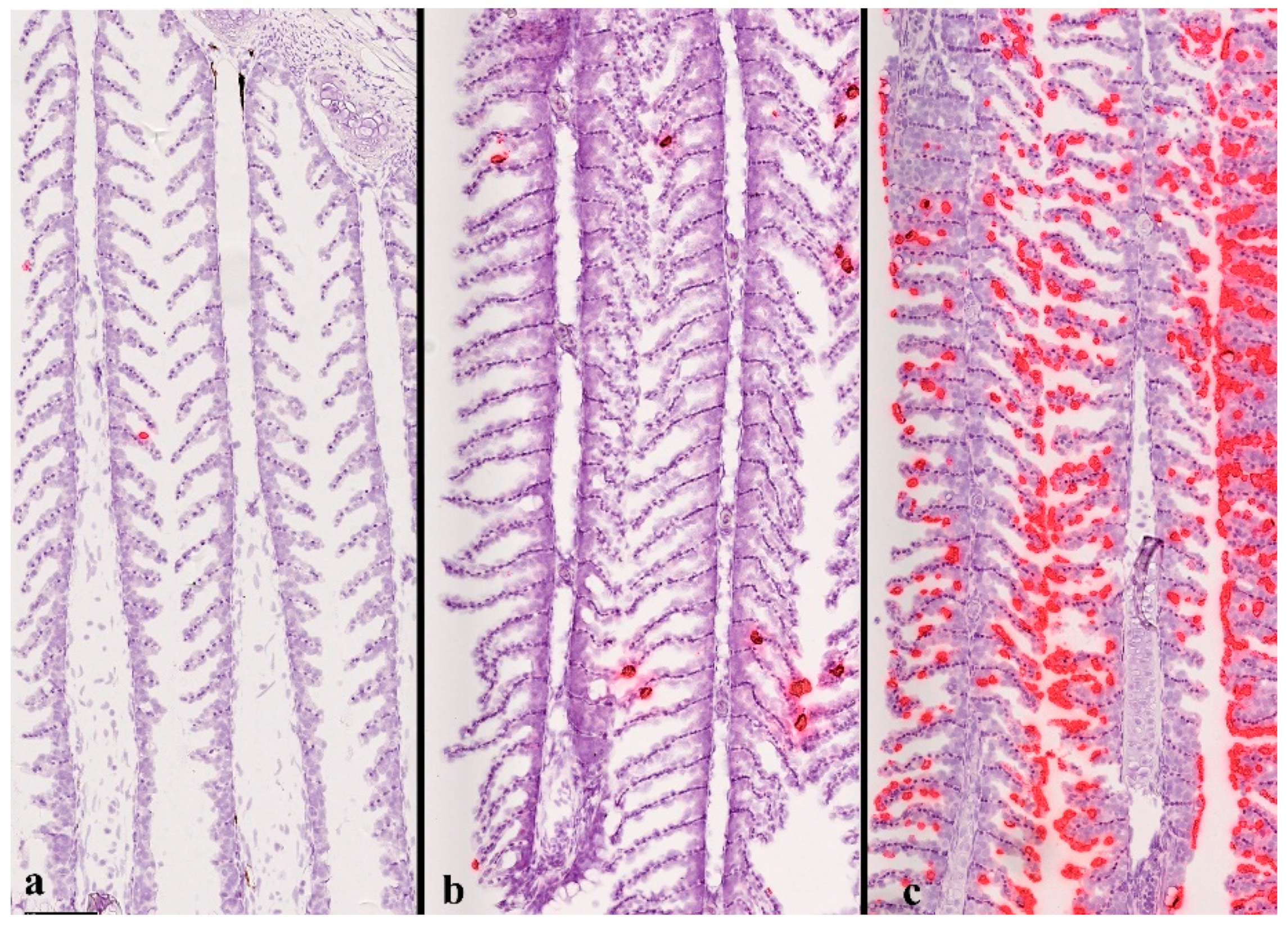
3. Diagnostics of SGPVD
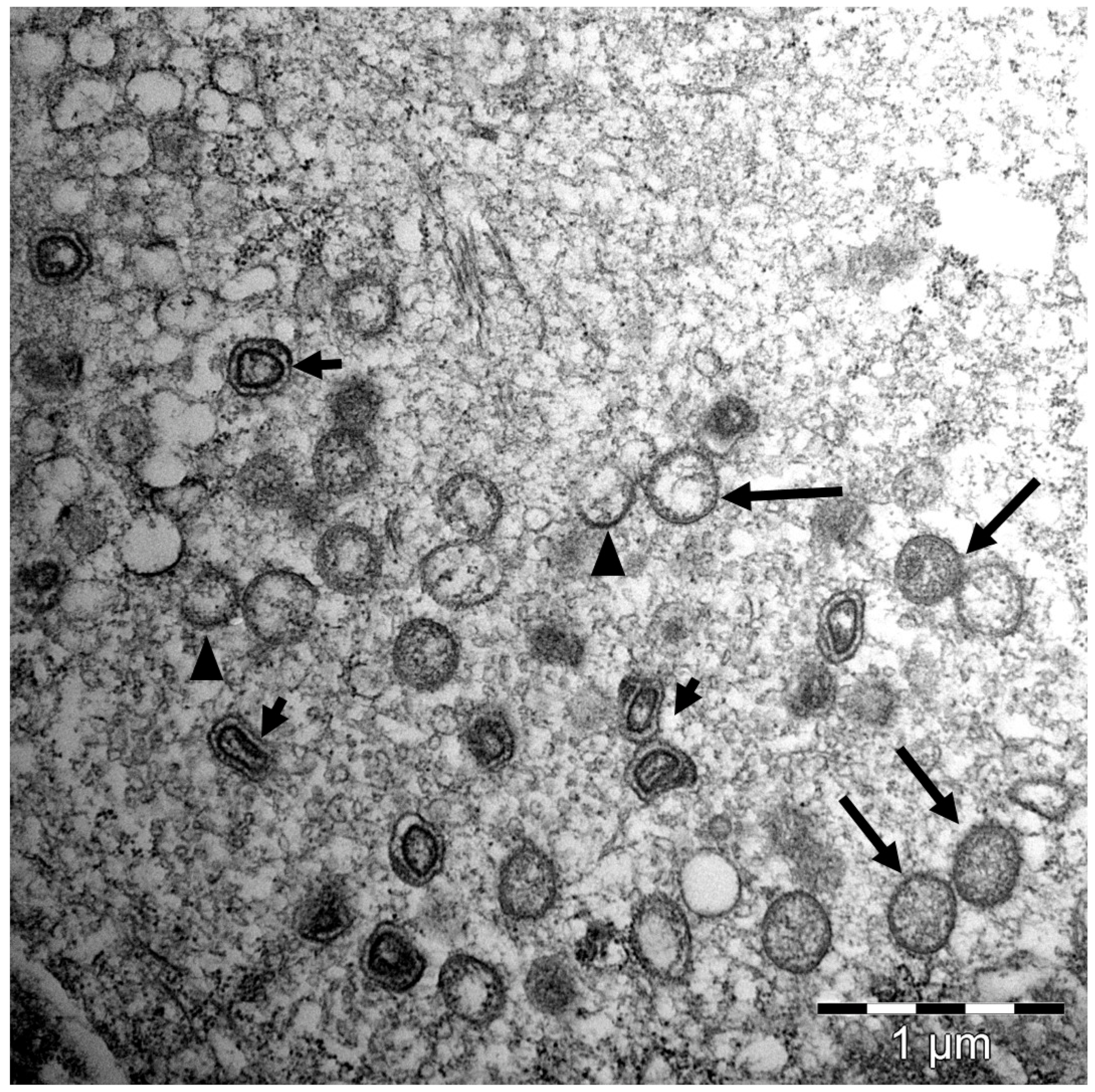
4. SGPV Is an Epitheliotropic Virus
5. Epidemiology
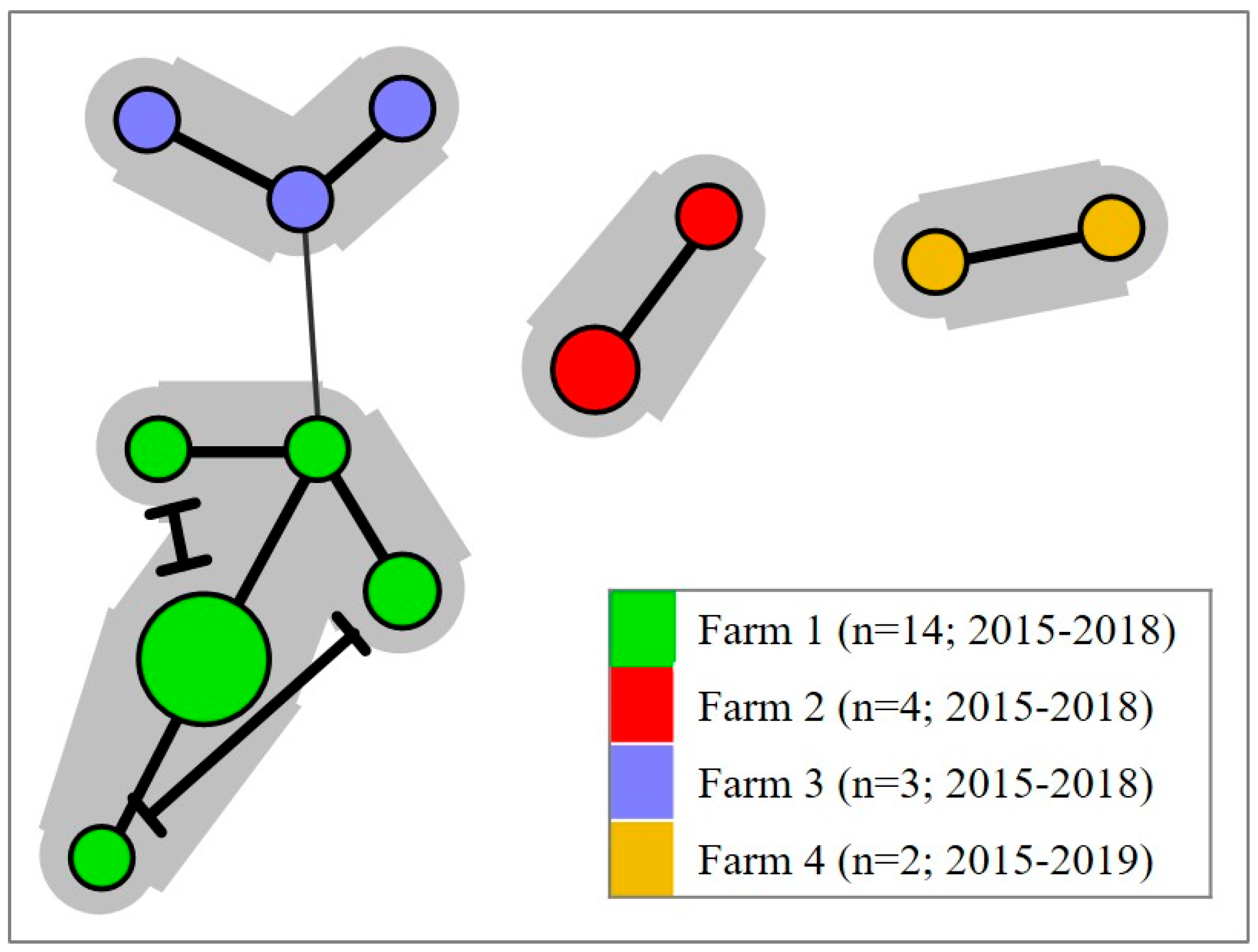
5.1. A Discrete Trans-Atlantic Divide within the SGPV Population
5.2. No Significant Genetic Distinction Was Found between SGPV Respectively Associated with Clinically Healthy Carriers and High-Mortality Outbreaks
5.3. Prevailing House Strains
5.4. Genetically Similar SGPV Strains Are Found in Geographically Linked Fjord Systems
5.5. Surveillance of SGPV by Detection in Water
6. Host Immune Responses to SGPV Infection
6.1. Interferon-Regulated Genes and Genes Involved in Antigen Presentation Are Upregulated in SGPV-Infected Gills
6.2. Adaptive Immune Responses—A Role of Cytotoxic T-cells in SGPV Defence?
7. Propagation of SGPV under In Vitro Conditions
8. Conclusions
9. Burning Questions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries, D.O. Statistics for Aquaculture. 2021. Available online: https://www.fiskeridir.no/English/Aquaculture/Statistics (accessed on 20 September 2022).
- Wilson, J.M.; Laurent, P. Fish gill morphology: Inside out. J. Exp. Zool. 2002, 293, 192–213. [Google Scholar] [CrossRef] [PubMed]
- Haugarvoll, E.; Bjerkas, I.; Nowak, B.F.; Hordvik, I.; Koppang, E.O. Identification and characterization of a novel intraepithelial lymphoid tissue in the gills of Atlantic salmon. J. Anat. 2008, 213, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Resseguier, J.; Dalum, A.S.; Du Pasquier, L.; Zhang, Y.Q.; Koppang, E.O.; Boudinot, P.; Wiegertjes, G.F. Lymphoid Tissue in Teleost Gills: Variations on a Theme. Biology 2020, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Nylund, A.; Watanabe, K.; Nylund, S.; Karlsen, M.; Saether, P.A.; Arnesen, C.E.; Karlsbakk, E. Morphogenesis of salmonid gill poxvirus associated with proliferative gill disease in farmed Atlantic salmon (Salmo salar) in Norway. Arch. Virol. 2008, 153, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Gjessing, M.C.; Yutin, N.; Tengs, T.; Senkevich, T.; Koonin, E.; Ronning, H.P.; Alarcon, M.; Ylving, S.; Lie, K.I.; Saure, B.; et al. Salmon Gill Poxvirus, the Deepest Representative of the Chordopoxvirinae. J. Virol. 2015, 89, 9348–9367. [Google Scholar] [CrossRef] [Green Version]
- Gjessing, M.C.; Krasnov, A.; Timmerhaus, G.; Brun, S.; Afanasyev, S.; Dale, O.B.; Dahle, M.K. The Atlantic Salmon Gill Transcriptome Response in a Natural Outbreak of Salmon Gill Pox Virus Infection Reveals New Biomarkers of Gill Pathology and Suppression of Mucosal Defense. Front. Immunol. 2020, 11, 2154. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Thoen, E.; Tengs, T.; Skotheim, S.A.; Dale, O.B. Salmon gill poxvirus, a recently characterized infectious agent of multifactorial gill disease in freshwater- and seawater-reared Atlantic salmon. J. Fish Dis. 2017, 40, 1253–1265. [Google Scholar] [CrossRef]
- Tørud, B.; Gjessing, M.C. Resultater fra laksepox-workshop på Gardermoen. Norsk Fiskeoppdrett 2022, 5, 50–53. [Google Scholar]
- Gjessing, M.C.; Christensen, D.H.; Manji, F.; Mohammad, S.; Petersen, P.E.; Saure, B.; Skjengen, C.; Weli, S.C.; Dale, O.B. Salmon gill poxvirus disease in Atlantic salmon fry as recognized by improved immunohistochemistry also demonstrates infected cells in non-respiratory epithelial cells. J. Fish Dis. 2018, 41, 1103–1110. [Google Scholar] [CrossRef]
- Thoen, E.; Tartor, H.; Amundsen, M.; Dale, O.B.; Sveinsson, K.; Ronning, H.P.; Gronneberg, E.; Dahle, M.K.; Gjessing, M.C. First record of experimentally induced salmon gill poxvirus disease (SGPVD) in Atlantic salmon (Salmo salar L.). Vet. Res. 2020, 51, 63. [Google Scholar] [CrossRef]
- Pikula, J.; Pojezdal, L.; Papezikova, I.; Minarova, H.; Mikulikova, I.; Bandouchova, H.; Blahova, J.; Bednarska, M.; Mares, J.; Palikova, M. Carp Edema Virus Infection Is Associated with Severe Metabolic Disturbance in Fish. Front. Vet. Sci. 2021, 8, 679970. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Spilsberg, B.; Steinum, T.M.; Amundsen, M.; Austbø, L.; Hansen, H.; Colquhoun, D.; Olsen, A.B. Multi-agent in situ hybridization confirms Ca. Branchiomonas cysticola as a major contributor in complex gill disease in Atlantic salmon. Fish Shellfish. Immunol. Rep. 2021, 2, 100026. [Google Scholar] [CrossRef]
- Gjessing, M.C.; Steinum, T.; Olsen, A.B.; Lie, K.I.; Tavornpanich, S.; Colquhoun, D.J.; Gjevre, A.G. Histopathological investigation of complex gill disease in sea farmed Atlantic salmon. PLoS ONE 2019, 14, e0222926. [Google Scholar] [CrossRef] [Green Version]
- Tørud, B.; Sveinsson, K.; Gulla, S.; Dahle, M.K.; Gjessing, M.C.; Dale, O.B.; Thoen, E. Laksepox: Smittesporing i Fisk og Miljøprøver, Sanering av Anlegg og Mulig Vertikal Overføring; Norwegian Veterinary Institute: Oslo, Norway, 2020. [Google Scholar]
- Condit, R.C.; Moussatche, N.; Traktman, P. In a nutshell: Structure and assembly of the vaccinia virion. Adv. Virus Res. 2006, 66, 31–124. [Google Scholar] [CrossRef]
- Downie, A. A study of the lesions produced experimentally by cowpox virus. J. Pathol. Bacteriol. 1939, 48, 19. [Google Scholar] [CrossRef]
- Shisler, J.L.; Moss, B. Immunology 102 at poxvirus U: Avoiding apoptosis. Semin. Immunol. 2001, 13, 67–72. [Google Scholar] [CrossRef]
- Nichols, D.B.; De Martini, W.; Cottrell, J. Poxviruses Utilize Multiple Strategies to Inhibit Apoptosis. Viruses 2017, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Kruse, N.; Weber, O. Selective induction of apoptosis in antigen-presenting cells in mice by Parapoxvirus ovis. J. Virol. 2001, 75, 4699–4704. [Google Scholar] [CrossRef] [Green Version]
- Miyazaki, T.; Isshiki, T.; Katsuyuki, H. Histopathological and electron microscopy studies on sleepy disease of koi Cyprinus carpio koi in Japan. Dis. Aquat. Org. 2005, 65, 197–207. [Google Scholar] [CrossRef]
- Aamelfot, M.; Dale, O.B.; Weli, S.C.; Koppang, E.O.; Falk, K. Expression of the infectious salmon anemia virus receptor on atlantic salmon endothelial cells correlates with the cell tropism of the virus. J. Virol. 2012, 86, 10571–10578. [Google Scholar] [CrossRef] [Green Version]
- Brisse, E.; Wouters, C.H.; Andrei, G.; Matthys, P. How Viruses Contribute to the Pathogenesis of Hemophagocytic Lymphohistiocytosis. Front. Immunol. 2017, 8, 1102. [Google Scholar] [CrossRef]
- Teramura, T.; Tabata, Y.; Yagi, T.; Morimoto, A.; Hibi, S.; Imashuku, S. Quantitative analysis of cell-free Epstein-Barr virus genome copy number in patients with EBV-associated hemophagocytic lymphohistiocytosis. Leuk. Lymphoma 2002, 43, 173–179. [Google Scholar] [CrossRef]
- Ostevik, L.; Stormoen, M.; Hellberg, H.; Kraugerud, M.; Manji, F.; Lie, K.I.; Nodtvedt, A.; Rodger, H.; Alarcon, M. A cohort study of gill infections, gill pathology and gill-related mortality in sea-farmed Atlantic salmon (Salmo salar L.): A descriptive analysis. J. Fish Dis. 2022, 45, 1301–1321. [Google Scholar] [CrossRef] [PubMed]
- Rodger, H.D.; Richards, R.H. Haemorrhagic smolt syndrome: A severe anaemic condition in farmed salmon in Scotland. Vet. Rec. 1998, 142, 538–541. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.H.; Lee, K.H. Application of the polymerase chain reaction for the diagnosis of fowl poxvirus infection. J. Virol. Methods 1997, 63, 113–119. [Google Scholar] [CrossRef]
- Weli, S.C.; Traavik, T.; Tryland, M.; Coucheron, D.H.; Nilssen, O. Analysis and comparison of the 4b core protein gene of avipoxviruses from wild birds: Evidence for interspecies spatial phylogenetic variation. Arch. Virol. 2004, 149, 2035–2046. [Google Scholar] [CrossRef]
- Matras, M.; Borzym, E.; Stone, D.; Way, K.; Stachnik, M.; Maj-Paluch, J.; Palusinska, M.; Reichert, M. Carp edema virus in Polish aquaculture-evidence of significant sequence divergence and a new lineage in common carp Cyprinus carpio (L.). J. Fish Dis. 2017, 40, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.C.; Kamil, J.P. Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism. Viruses 2018, 10, 704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LeBlanc, F.; Ditlecadet, D.; Arseneau, J.R.; Steeves, R.; Boston, L.; Boudreau, P.; Gagne, N. Isolation and identification of a novel salmon gill poxvirus variant from Atlantic salmon in Eastern Canada. J. Fish Dis. 2019, 42, 315–318. [Google Scholar] [CrossRef]
- Garseth, A.H.; Gjessing, M.C.; Moldal, T.; Gjevre, A.G. A survey of salmon gill poxvirus (SGPV) in wild salmonids in Norway. J. Fish Dis. 2018, 41, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Gulla, S.; Tengs, T.; Mohammad, S.N.; Gjessing, M.; Garseth, A.H.; Sveinsson, K.; Moldal, T.; Petersen, P.E.; Torud, B.; Dale, O.B.; et al. Genotyping of Salmon Gill Poxvirus Reveals One Main Predominant Lineage in Europe, Featuring Fjord- and Fish Farm-Specific Sub-Lineages. Front. Microbiol. 2020, 11, 1071. [Google Scholar] [CrossRef]
- Costa, V.A.; Mifsud, J.C.O.; Gilligan, D.; Williamson, J.E.; Holmes, E.C.; Geoghegan, J.L. Metagenomic sequencing reveals a lack of virus exchange between native and invasive freshwater fish across the Murray-Darling Basin, Australia. Virus Evol. 2021, 7, veab034. [Google Scholar] [CrossRef]
- Tørud, B.S.K. Sanering av settefiskanlegg med laksepoxvirus. Norsk Fiskeoppdrett 2020, 8, 64–65. [Google Scholar]
- Bernhardt, L.V.; Myrmel, M.; Lillehaug, A.; Qviller, L.; Weli, S.C. Concentration and detection of salmonid alphavirus in seawater during a post-smolt salmon (Salmo salar) cohabitant challenge. Dis. Aquat. Org. 2021, 146, 65. [Google Scholar]
- Weli, S.C.; Bernhardt, L.V.; Qviller, L.; Myrmel, M.; Lillehaug, A. Development and evaluation of a method for concentration and detection of salmonid alphavirus from seawater. J. Virol. Methods 2021, 287, 113990. [Google Scholar] [CrossRef]
- Amundsen, M.M.; Tartor, H.; Andersen, K.; Sveinsson, K.; Thoen, E.; Gjessing, M.C.; Dahle, M.K. Mucosal and Systemic Immune Responses to Salmon Gill Poxvirus Infection in Atlantic Salmon Are Modulated Upon Hydrocortisone Injection. Front. Immunol. 2021, 12, 689302. [Google Scholar] [CrossRef]
- Sakala, I.G.; Chaudhri, G.; Buller, R.M.; Nuara, A.A.; Bai, H.; Chen, N.; Karupiah, G. Poxvirus-encoded gamma interferon binding protein dampens the host immune response to infection. J. Virol. 2007, 81, 3346–3353. [Google Scholar] [CrossRef] [Green Version]
- Baldanta, S.; Fernandez-Escobar, M.; Acin-Perez, R.; Albert, M.; Camafeita, E.; Jorge, I.; Vazquez, J.; Enriquez, J.A.; Guerra, S. ISG15 governs mitochondrial function in macrophages following vaccinia virus infection. PLoS Pathog. 2017, 13, e1006651. [Google Scholar] [CrossRef] [Green Version]
- Wiebe, M.S.; Traktman, P. Poxviral B1 kinase overcomes barrier to autointegration factor, a host defense against virus replication. Cell Host Microbe 2007, 1, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Zhang, F.; Yan, B.; Si, C.; Honda, H.; Nagamachi, A.; Sun, L.Z.; Xiang, Y. A paralogous pair of mammalian host restriction factors form a critical host barrier against poxvirus infection. PLoS Pathog. 2018, 14, e1006884. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, C.M.; Dabelic, R.; Waiboci, L.W.; Jager, L.D.; Heron, L.L.; Johnson, H.M. SOCS-1 mimetics protect mice against lethal poxvirus infection: Identification of a novel endogenous antiviral system. J. Virol. 2009, 83, 1402–1415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, G.L.; Benfield, C.T.O.; Maluquer de Motes, C.; Mazzon, M.; Ember, S.W.J.; Ferguson, B.J.; Sumner, R.P. Vaccinia virus immune evasion: Mechanisms, virulence and immunogenicity. J. Gen. Virol. 2013, 94, 2367–2392. [Google Scholar] [CrossRef] [PubMed]
- Amara, R.R.; Nigam, P.; Sharma, S.; Liu, J.; Bostik, V. Long-lived poxvirus immunity, robust CD4 help, and better persistence of CD4 than CD8 T cells. J. Virol. 2004, 78, 3811–3816. [Google Scholar] [CrossRef] [PubMed]
- Alzhanova, D.; Hammarlund, E.; Reed, J.; Meermeier, E.; Rawlings, S.; Ray, C.A.; Edwards, D.M.; Bimber, B.; Legasse, A.; Planer, S.; et al. T cell inactivation by poxviral B22 family proteins increases viral virulence. PLoS Pathog. 2014, 10, e1004123. [Google Scholar] [CrossRef] [PubMed]
- Gjessing, M.C.; Aamelfot, M.; Batts, W.N.; Benestad, S.L.; Dale, O.B.; Thoen, E.; Weli, S.C.; Winton, J.R. Development and characterization of two cell lines from gills of Atlantic salmon. PLoS ONE 2018, 13, e0191792. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tartor, H.; Dahle, M.K.; Gulla, S.; Weli, S.C.; Gjessing, M.C. Emergence of Salmon Gill Poxvirus. Viruses 2022, 14, 2701. https://doi.org/10.3390/v14122701
Tartor H, Dahle MK, Gulla S, Weli SC, Gjessing MC. Emergence of Salmon Gill Poxvirus. Viruses. 2022; 14(12):2701. https://doi.org/10.3390/v14122701
Chicago/Turabian StyleTartor, Haitham, Maria K. Dahle, Snorre Gulla, Simon C. Weli, and Mona C. Gjessing. 2022. "Emergence of Salmon Gill Poxvirus" Viruses 14, no. 12: 2701. https://doi.org/10.3390/v14122701





