Death Receptor DR5 as a Proviral Factor for Viral Entry and Replication of Coronavirus PEDV
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cells, Viruses, Reagents and Antibodies
2.2. Virus Titration
2.3. RNA Interference (RNAi) and Cell Viability Assay
2.4. Recombinant Plasmid and Transfection
2.5. Total RNA Extraction and Quantitative Real-Time PCR
2.6. Western Blot Analysis
2.7. Immunofluorescence Assay (IFA)
2.8. Annexin V and PI Staining Assay
2.9. PEDV Entry Assay
2.10. In Vivo Experiment
2.11. Statistical Analysis
3. Results
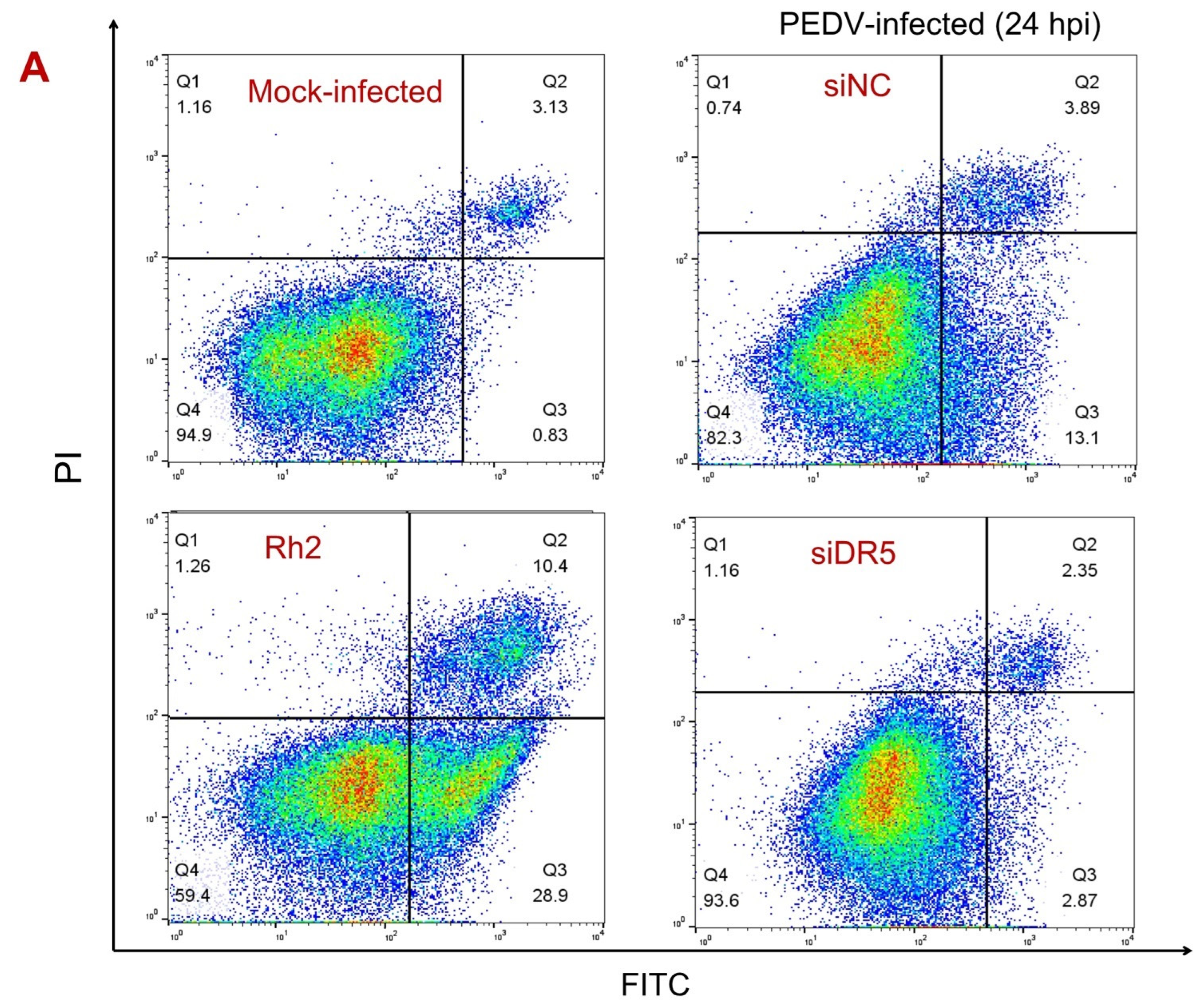
3.1. DR5 Facilitated Viral Infection of PEDV
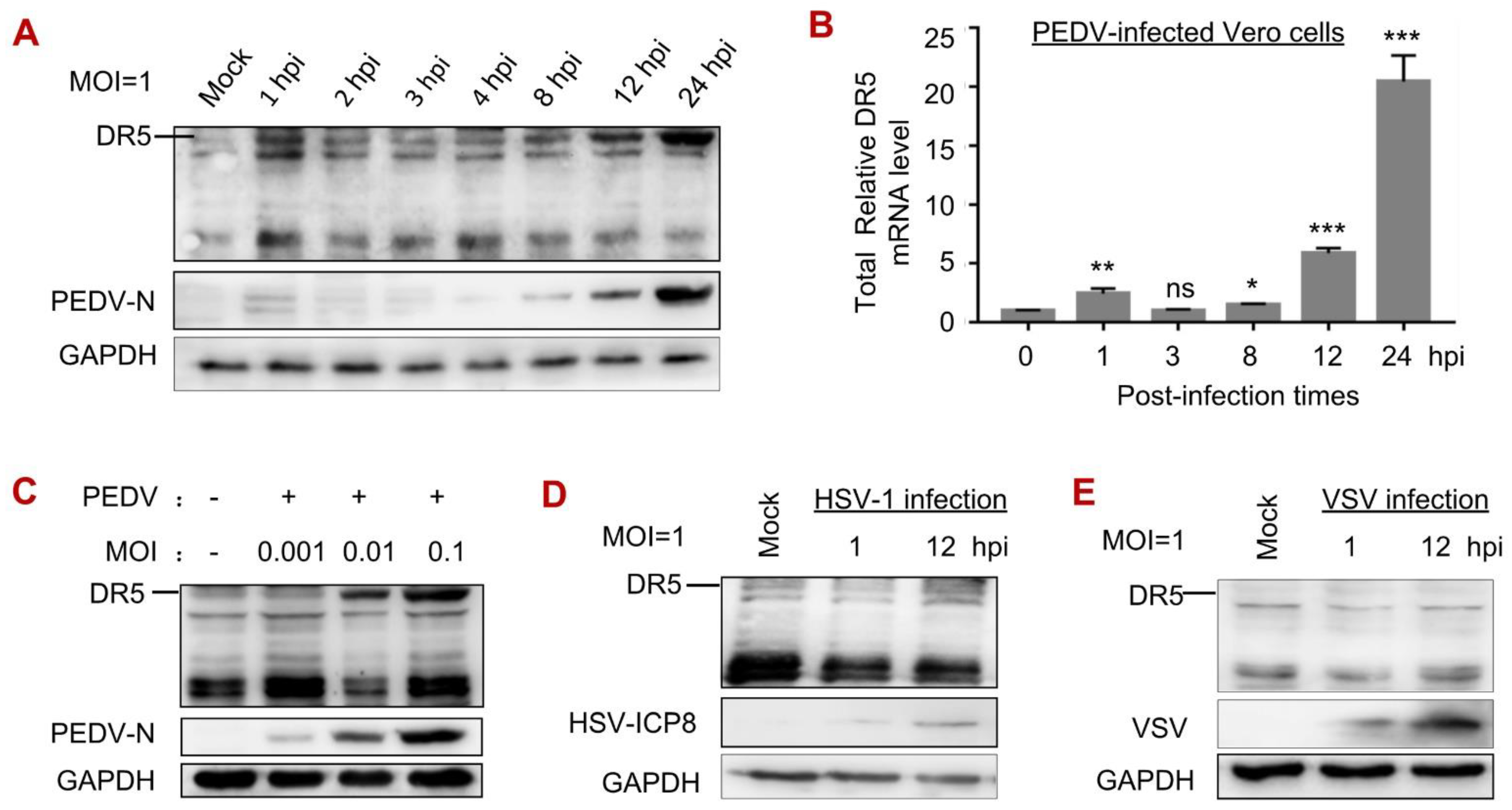
3.2. PEDV Infection Increased the Levels of DR5 mRNA and Protein
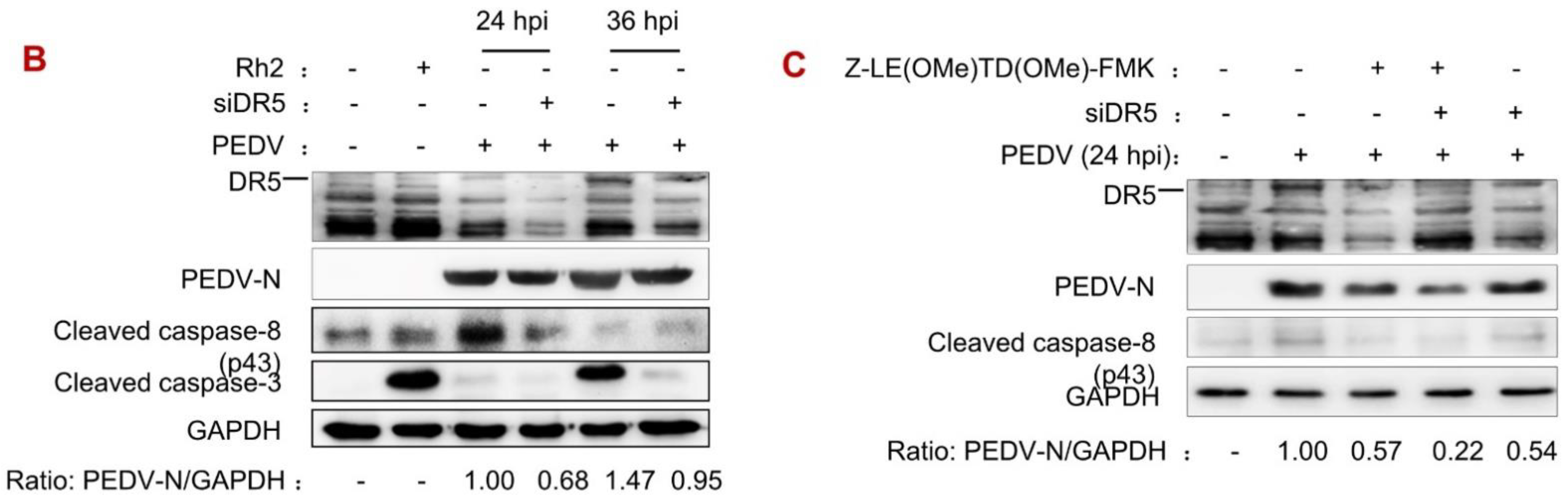
3.3. DR5 Activated Caspase-8-Dependent Apoptosis Induced by PEDV Infection
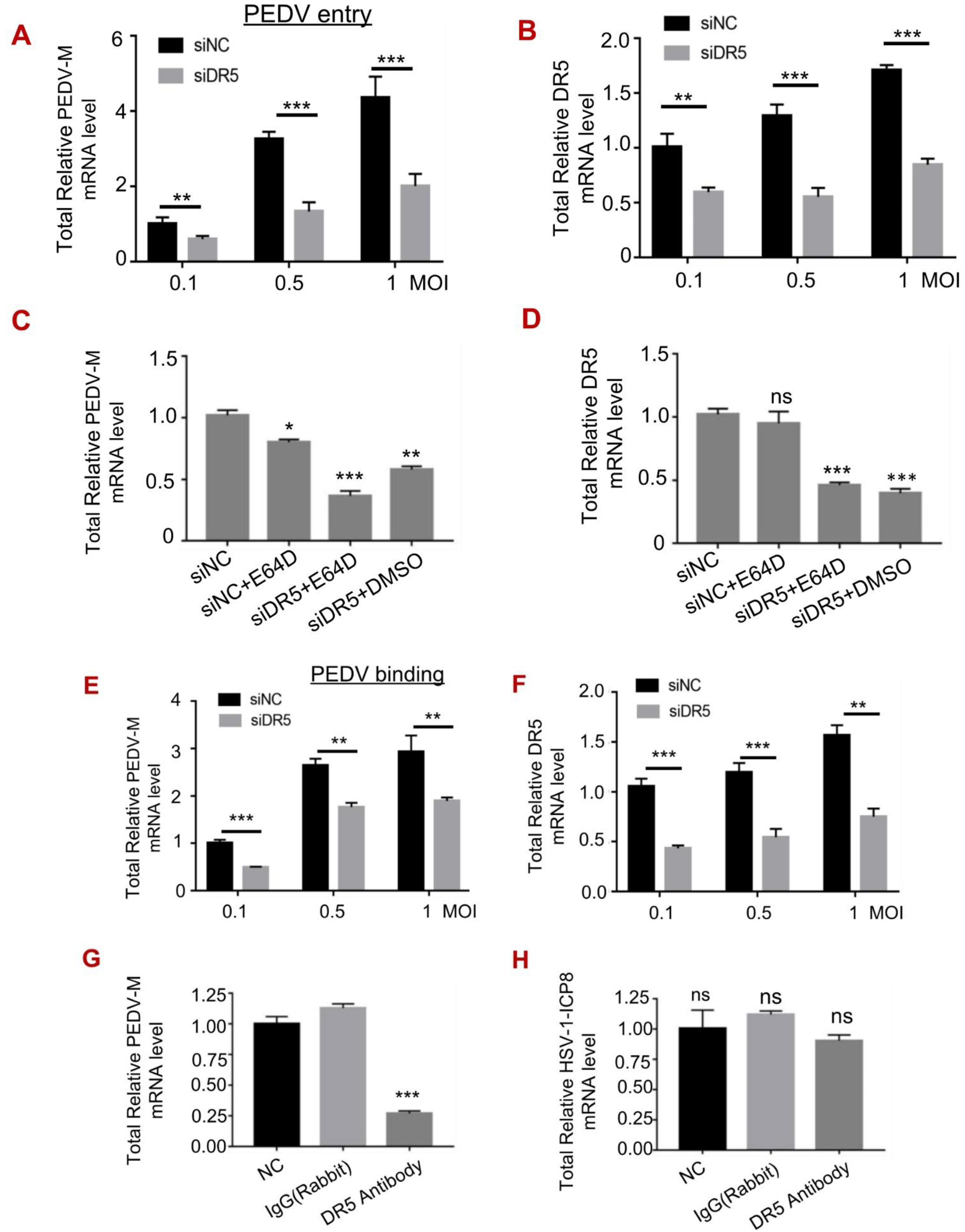
3.4. DR5 Promoted the Early Entry of PEDV
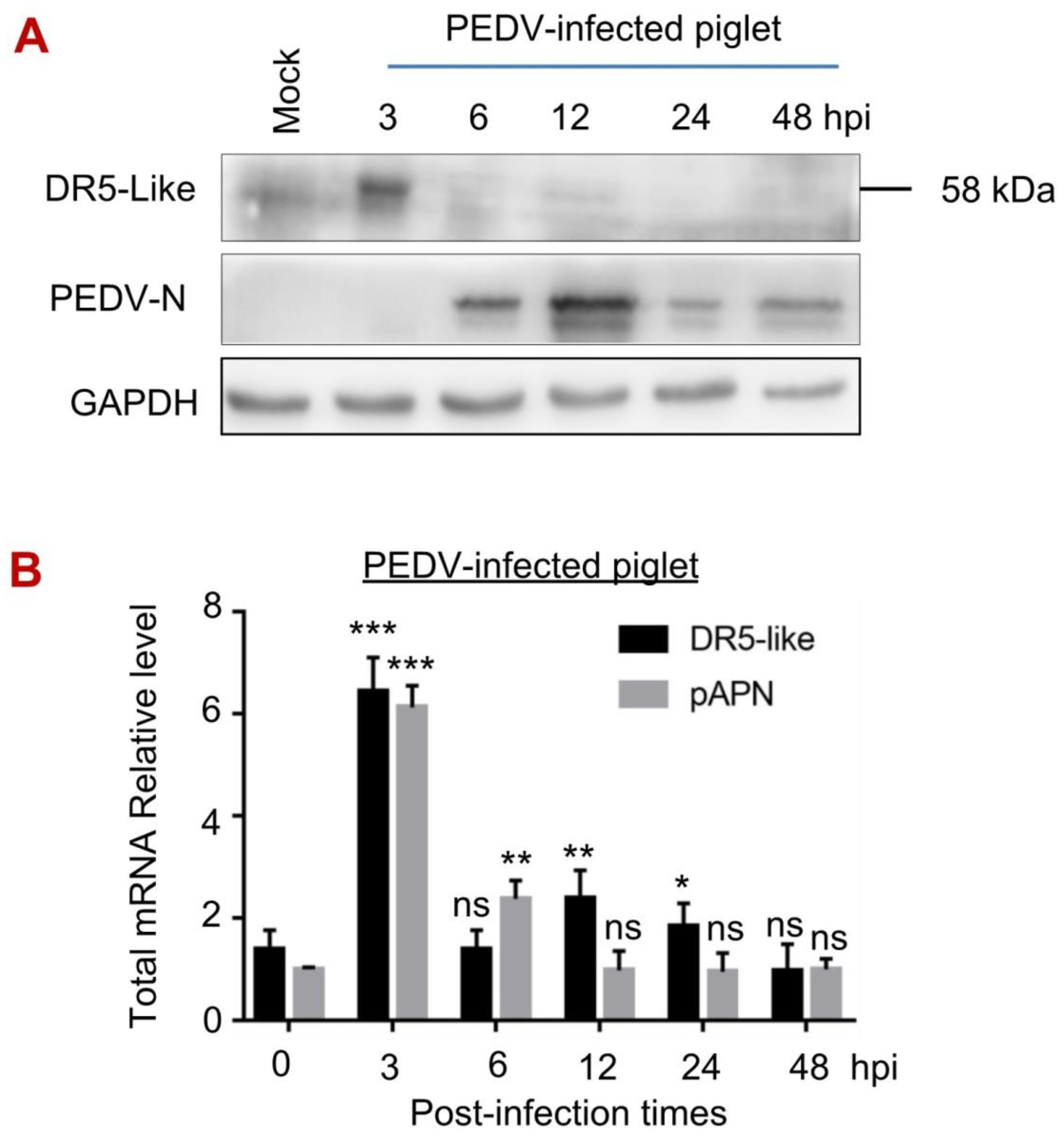
3.5. The Expression of DR5-like Gene in Piglets upon PEDV Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Pensaert, M.B.; de Bouck, P. A new coronavirus-like particle associated with diarrhea in swine. Arch. Virol. 1978, 58, 243–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Zhang, S.; Huang, Y.; Zhang, S.; Wang, H.; Bao, W. The aqueous aeaf extract of M. oleifera inhibits PEDV replication through suppressing oxidative stress-mediated apoptosis. Animals 2022, 12, 458. [Google Scholar] [CrossRef]
- Li, R.; Qiao, S.; Yang, Y.; Guo, J.; Xie, S.; Zhou, E.; Zhang, G. Genome sequencing and analysis of a novel recombinant porcine epidemic diarrhea virus strain from Henan, China. Virus Genes 2016, 52, 91–98. [Google Scholar] [CrossRef]
- Strizhakova, O.; Hanke, D.; Titov, I.; Blome, S.; Malogolovkin, A. Complete genome sequence of a porcine ppidemic diarrhea virus isolated in Belgorod, Russia, in 2008. Genome Announc. 2017, 5, e01026-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, C. Porcine epidemic diarrhea virus: An emerging and re-emerging epizootic swine virus. Virol. J. 2015, 12, 193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jung, K.; Wang, Q.; Scheuer, K.A.; Lu, Z.; Zhang, Y.; Saif, L.J. Pathology of US porcine epidemic diarrhea virus strain PC21A in gnotobiotic pigs. Emerg. Infect. Dis. 2014, 20, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Li, W.; Zhou, Q.; Li, Q.; Xu, Z.; Shen, H.; Chen, F. Characterization and pathogenicity of Vero cell-attenuated porcine epidemic diarrhea virus CT strain. Virol. J. 2019, 16, 121. [Google Scholar] [CrossRef] [Green Version]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef]
- Vui, D.T.; Tung, N.; Inui, K.; Slater, S.; Nilubol, D. Complete genome sequence of porcine epidemic diarrhea virus in Vietnam. Genome Announc. 2014, 2, e00753-14. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.Q.; Cai, R.J.; Chen, Y.Q.; Liang, P.S.; Chen, D.K.; Song, C.X. Outbreak of porcine epidemic diarrhea in suckling piglets, China. Emerg. Infect. Dis. 2012, 18, 161–163. [Google Scholar] [CrossRef]
- Fan, H.; Zhang, J.; Ye, Y.; Tong, T.; Xie, K.; Liao, M. Complete genome sequence of a novel porcine epidemic diarrhea virus in south China. J. Virol. 2012, 86, 10248–10249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of porcine epidemic diarrhea virus in the United States: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, L.L.; Tonsor, G.T. Assessment of the economic impacts of porcine epidemic diarrhea virus in the United States. J. Anim. Sci. 2015, 93, 5111–5118. [Google Scholar] [CrossRef] [Green Version]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in development. Nature 2000, 407, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Letai, A.; Sarosiek, K. Regulation of apoptosis in health and disease: The balancing act of BCL-2 family proteins. Nat. Rev. Mol. Cell Biol. 2019, 20, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Trojel-Hansen, C.; Kroemer, G. Mitochondrial control of cellular life, stress, and death. Circ. Res. 2012, 111, 1198–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wyzewski, Z.; Mielcarska, M.B.; Gregorczyk-Zboroch, K.P.; Myszka, A. Virus-mediated inhibition of apoptosis in the context of EBV-associated diseases: Molecular mechanisms and therapeutic perspectives. Int. J. Mol. Sci. 2022, 23, 7265. [Google Scholar] [CrossRef]
- Fitzsimmons, L.; Cartlidge, R.; Chang, C.; Sejic, N.; Galbraith, L.C.A.; Suraweera, C.D.; Croom-Carter, D.; Dewson, G.; Tierney, R.J.; Bell, A.I.; et al. EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins. Cell Death Differ. 2020, 27, 1554–1568. [Google Scholar] [CrossRef]
- Si, F.; Hu, X.; Wang, C.; Chen, B.; Wang, R.; Dong, S.; Yu, R.; Li, Z. Porcine epidemic diarrhea virus (PEDV) ORF3 enhances viral proliferation by inhibiting apoptosis of infected cells. Viruses 2020, 12, 214. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Lee, C. Porcine epidemic diarrhea virus induces caspase-independent apoptosis through activation of mitochondrial apoptosis-inducing factor. Virology 2014, 460–461, 180–193. [Google Scholar] [CrossRef]
- Xu, X.; Xu, Y.; Zhang, Q.; Yang, F.; Yin, Z.; Wang, L.; Li, Q. Porcine epidemic diarrhea virus infections induce apoptosis in Vero cells via a reactive oxygen species (ROS)/p53, but not p38 MAPK and SAPK/JNK signalling pathways. Vet. Microbiol. 2019, 232, 1–12. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Li, J.; Gao, Y.; Zhou, L.; Ge, X.; Han, J.; Guo, X.; Yang, H. Porcine epidemic diarrhea virus S1 protein is the critical inducer of apoptosis. Virol. J. 2018, 15, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Zhao, T.Q.; Xu, D.P.; Zhang, X.; Ji, C.J.; Zhang, D.L. The influence of porcine epidemic diarrhea virus on pig small intestine mucosal epithelial cell function. Arch. Virol. 2019, 164, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Li, B.; Chen, L.; Ma, Z.; He, K.; Fan, H. Differential protein analysis of IPEC-J2 cells infected with porcine epidemic diarrhea virus pandemic and classical strains elucidates the pathogenesis of infection. J. Proteome Res. 2017, 16, 2113–2120. [Google Scholar] [CrossRef]
- Gibson, C.J.; Davids, M.S. BCL-2 Antagonism to target the intrinsic mitochondrial pathway of apoptosis. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 5021–5029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, R.; Emi, M.; Tanabe, K. Caspase-dependent and -independent cell death pathways after DNA damage (Review). Oncol. Rep. 2005, 14, 595–599. [Google Scholar] [CrossRef]
- Wang, S.; El-Deiry, W.S. TRAIL and apoptosis induction by TNF-family death receptors. Oncogene 2003, 22, 8628–8633. [Google Scholar] [CrossRef] [Green Version]
- Sola-Riera, C.; Gupta, S.; Maleki, K.T.; Gonzalez-Rodriguez, P.; Saidi, D.; Zimmer, C.L.; Vangeti, S.; Rivino, L.; Leo, Y.S.; Lye, D.C.; et al. Hantavirus inhibits TRAIL-mediated killing of infected cells by downregulating death receptor 5. Cell Rep. 2019, 28, 2124–2139.e2126. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.M.; Kim, Y.S.; Kim, K.H.; Hur, D.Y. Ampelopsin induces DR5-mediated apoptotic cell death in EBV-infected cells through the p38 Pathway. Nutr. Cancer 2020, 72, 489–494. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.M. Modulation of TRAIL signaling for cancer therapy. Vitam. Horm. 2004, 67, 275–290. [Google Scholar] [CrossRef]
- Dong, H.J.; Wang, Z.H.; Meng, W.; Li, C.C.; Hu, Y.X.; Zhou, L.; Wang, X.J. The natural compound homoharringtonine presents broad antiviral activity in vitro and in vivo. Viruses 2018, 10, 601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.X.; Li, Y.; Sun, C.; Jiang, D.; Lin, Y.J.; Jin, F.X.; Lee, S.K.; Jin, Y.H. p53-dependent Fas expression is critical for Ginsenoside Rh2 triggered caspase-8 activation in HeLa cells. Protein Cell 2014, 5, 224–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.M.; Burrough, E. The effects of swine coronaviruses on ER stress, autophagy, apoptosis, and alterations in cell morphology. Pathogens 2022, 11, 940. [Google Scholar] [CrossRef] [PubMed]
- Thomadaki, H.; Scorilas, A.; Tsiapalis, C.M.; Havredaki, M. The role of cordycepin in cancer treatment via induction or inhibition of apoptosis: Implication of polyadenylation in a cell type specific manner. Cancer Chemother. Pharmacol. 2008, 61, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Huang, J.; Yao, Y.; Zhao, W.; Zhang, Y.; Qing, J.; Ren, J.; Yan, Z.; Wang, Z.; et al. Isolation and oral immunogenicity assessment of porcine epidemic diarrhea virus NH-TA2020 strain: One of the predominant strains circulating in China from 2017 to 2021. Virol. Sin. 2022, 37, 646–655. [Google Scholar] [CrossRef]
- Garcia-Hernandez, M.E.; Trujillo-Ortega, M.E.; Alcaraz-Estrada, S.L.; Lozano-Aguirre-Beltran, L.; Sandoval-Jaime, C.; Taboada-Ramirez, B.I.; Sarmiento-Silva, R.E. Molecular detection and characterization of porcine epidemic diarrhea virus and porcine aichivirus C coinfection in Mexico. Viruses 2021, 13, 738. [Google Scholar] [CrossRef]
- Puente, H.; Arguello, H.; Mencia-Ares, O.; Gomez-Garcia, M.; Rubio, P.; Carvajal, A. Detection and genetic diversity of porcine coronavirus involved in diarrhea outbreaks in Spain. Front. Vet. Sci. 2021, 8, 651999. [Google Scholar] [CrossRef]
- Li, Z.; Ma, Z.; Li, Y.; Gao, S.; Xiao, S. Porcine epidemic diarrhea virus: Molecular mechanisms of attenuation and vaccines. Microb. Pathog. 2020, 149, 104553. [Google Scholar] [CrossRef]
- Shin, G.C.; Kang, H.S.; Lee, A.R.; Kim, K.H. Hepatitis B virus-triggered autophagy targets TNFRSF10B/death receptor 5 for degradation to limit TNFSF10/TRAIL response. Autophagy 2016, 12, 2451–2466. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.Y.; Kim, S.J.; Cho, E.K.; Jeong, S.W.; Park, E.J.; Lee, W.C.; Lee, S.H.; Kim, S.G.; Kim, Y.S.; Kim, H.S.; et al. TRAIL enhances apoptosis of human hepatocellular carcinoma cells sensitized by hepatitis C virus infection: Therapeutic implications. PLoS ONE 2014, 9, e98171. [Google Scholar] [CrossRef]
- Ding, L.; Li, J.; Li, W.; Fang, Z.; Li, N.; Wu, S.; Li, J.; Hong, M. p53- and ROS-mediated AIF pathway involved in TGEV-induced apoptosis. J. Vet. Med. Sci. 2018, 80, 1775–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zhang, Y.; Cao, Y. The roles of apoptosis in swine response to viral infection and pathogenesis of swine enteropathogenic coronaviruses. Front. Vet. Sci. 2020, 7, 572425. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lee, C. Porcine deltacoronavirus induces caspase-dependent apoptosis through activation of the cytochrome c-mediated intrinsic mitochondrial pathway. Virus Res. 2018, 253, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Yang, R.; Guo, L.; Qu, J.; Wang, J.; Hung, T. Apoptosis induced by the SARS-associated coronavirus in Vero cells is replication-dependent and involves caspase. DNA Cell Biol. 2005, 24, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Fehr, A.R.; Perlman, S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015, 1282, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Oh, J.S.; Song, D.S.; Park, B.K. Identification of a putative cellular receptor 150 kDa polypeptide for porcine epidemic diarrhea virus in porcine enterocytes. J. Vet. Sci. 2003, 4, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Li, B.X.; Ge, J.W.; Li, Y.J. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology 2007, 365, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Luo, R.; He, Q.; van Kuppeveld, F.J.M.; Rottier, P.J.M.; Bosch, B.J. Aminopeptidase N is not required for porcine epidemic diarrhea virus cell entry. Virus Res. 2017, 235, 6–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, C.M.; Wang, B.; Zhou, J.; Huang, Y.W. Aminopeptidase-N-independent entry of porcine epidemic diarrhea virus into Vero or porcine small intestine epithelial cells. Virology 2018, 517, 16–23. [Google Scholar] [CrossRef]
- Hu, Y.; Xie, X.; Yang, L.; Wang, A. A comprehensive view on the host factors and viral proteins associated with porcine epidemic diarrhea virus infection. Front. Microbiol. 2021, 12, 762358. [Google Scholar] [CrossRef]
- Herbein, G.; Montaner, L.J.; Gordon, S. Tumor necrosis factor alpha inhibits entry of human immunodeficiency virus type 1 into primary human macrophages: A selective role for the 75-kilodalton receptor. J. Virol. 1996, 70, 7388–7397. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-Z.; Tian, W.-J.; Wang, J.; You, J.-L.; Wang, X.-J. Death Receptor DR5 as a Proviral Factor for Viral Entry and Replication of Coronavirus PEDV. Viruses 2022, 14, 2724. https://doi.org/10.3390/v14122724
Zhang X-Z, Tian W-J, Wang J, You J-L, Wang X-J. Death Receptor DR5 as a Proviral Factor for Viral Entry and Replication of Coronavirus PEDV. Viruses. 2022; 14(12):2724. https://doi.org/10.3390/v14122724
Chicago/Turabian StyleZhang, Xiu-Zhong, Wen-Jun Tian, Jing Wang, Jing-Ling You, and Xiao-Jia Wang. 2022. "Death Receptor DR5 as a Proviral Factor for Viral Entry and Replication of Coronavirus PEDV" Viruses 14, no. 12: 2724. https://doi.org/10.3390/v14122724




