Using CSF Proteomics to Investigate Herpesvirus Infections of the Central Nervous System
Abstract
:1. Introduction
2. Materials and Methods
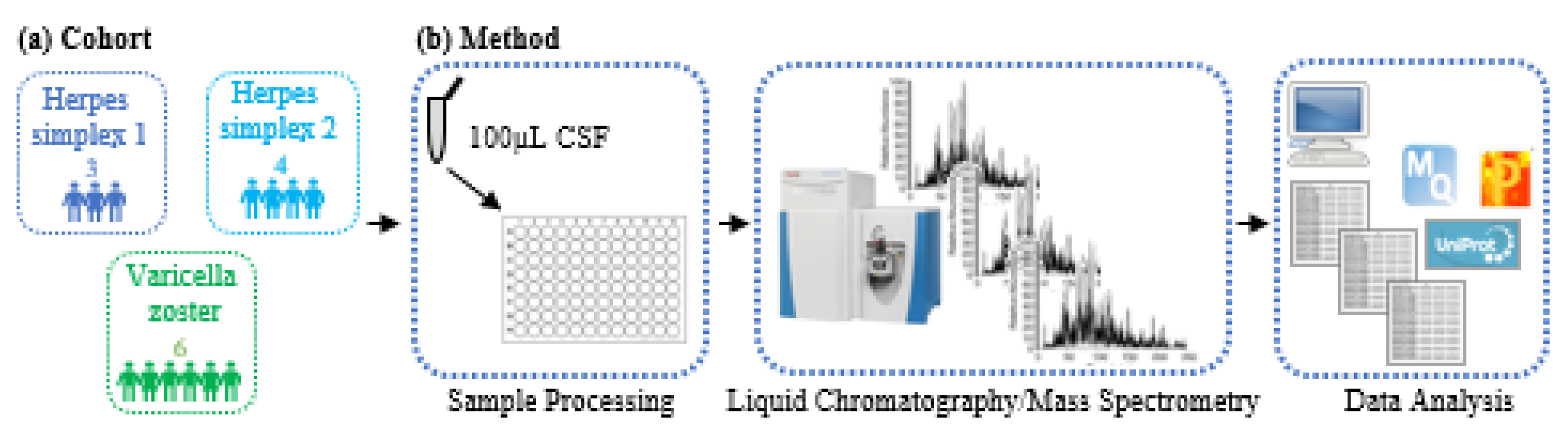
2.1. Patient Samples
2.2. Sample Processing Method
2.3. LC/MS-based CSF Proteome Mapping
2.4. LC/MS Data Analysis
3. Results
3.1. Characteristics of CSF Proteomics Study Population
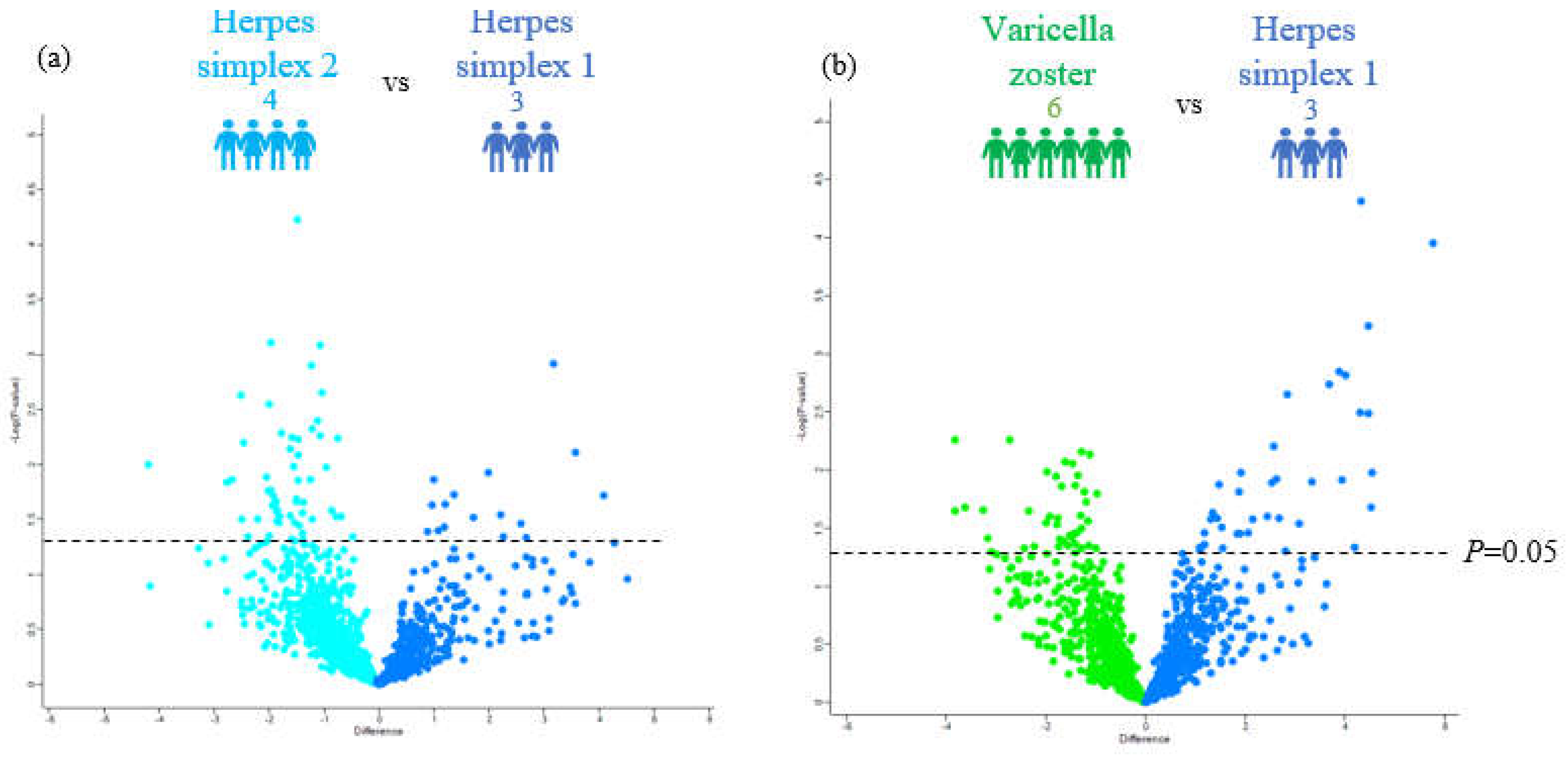
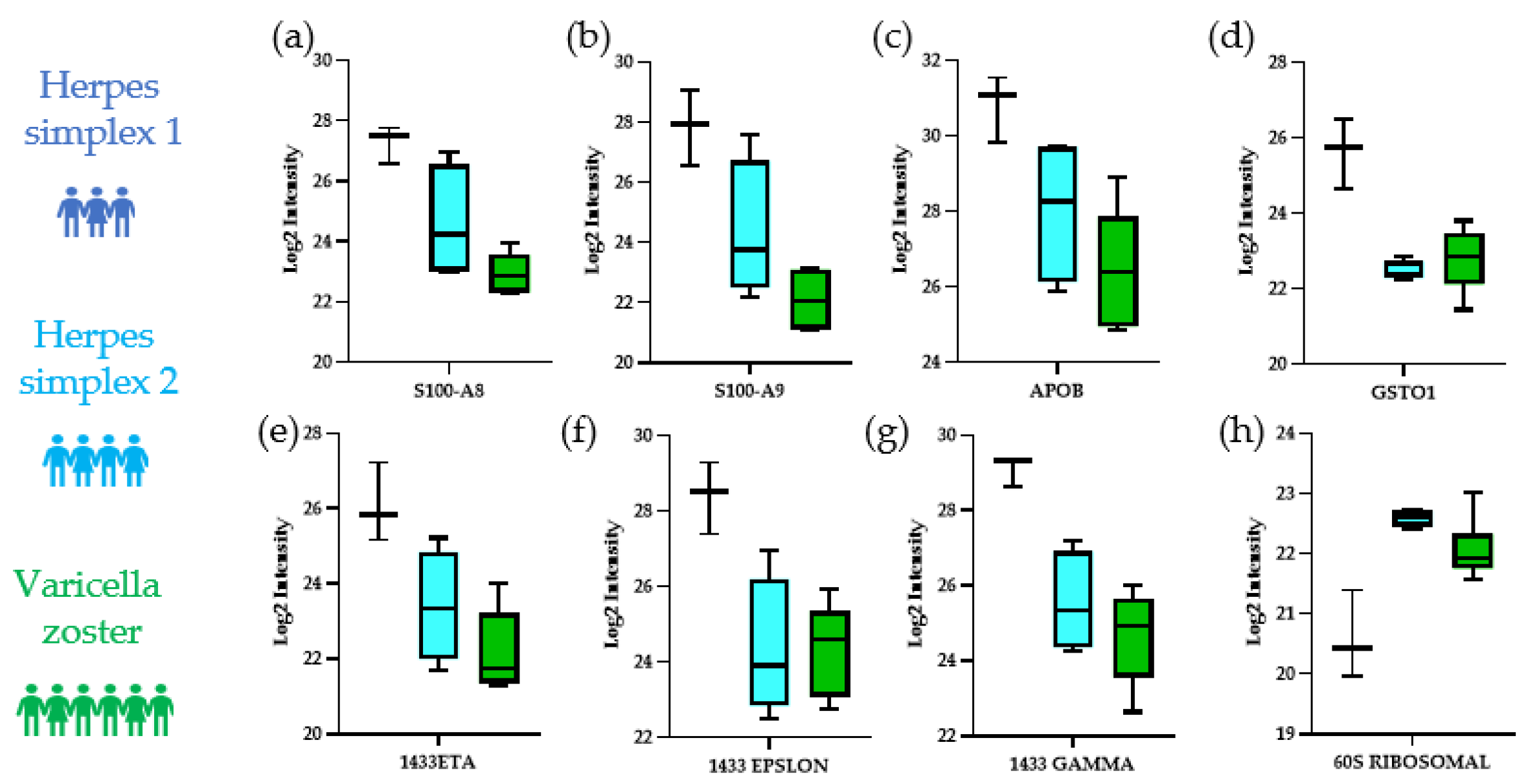
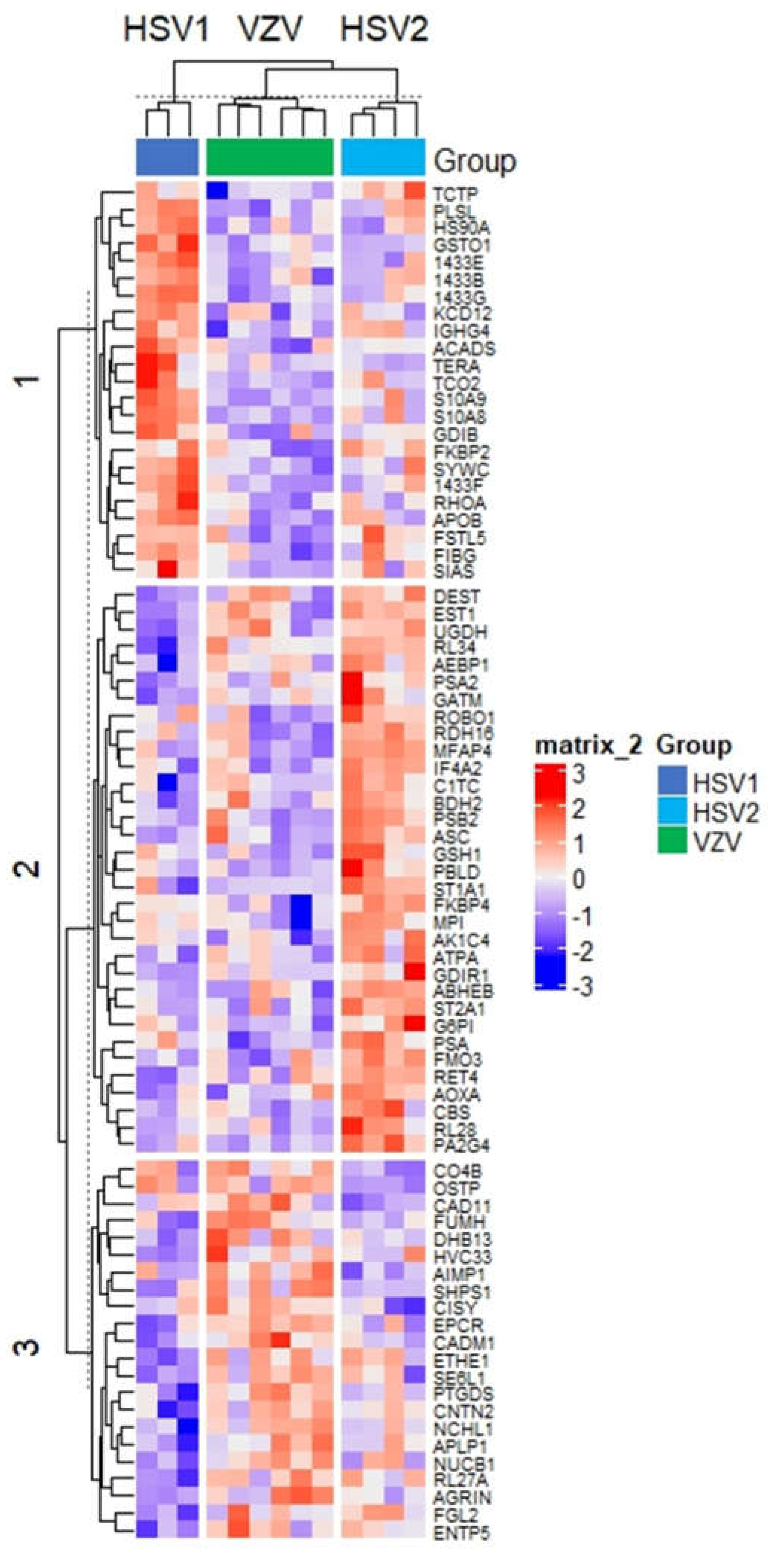
3.2. Distinguishing the CSF Proteomes of Patients with HSV-1, HSV-2, and VZV
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilden, D.H.; Mahalingam, R.; Cohrs, R.J.; Tyler, K.L. Herpesvirus infections of the nervous system. Nat. Clin. Pract. Neurol. 2007, 3, 82–94. [Google Scholar] [CrossRef]
- Steiner, I.; Kennedy, P.G.; Pachner, A.R. The neurotropic herpes viruses: Herpes simplex and varicella-zoster. Lancet Neurol. 2007, 6, 1015–1028. [Google Scholar] [CrossRef] [PubMed]
- Gilden, D.; Nagel, M.A.; Cohrs, R.J. Varicella-zoster. Handb. Clin. Neurol. 2014, 123, 265–283. [Google Scholar] [PubMed] [Green Version]
- Maher, M.D.; Douglas, V.P.; Douglas, K.A.A.; Collens, S.I.; Gilbert, A.L.; Torun, N.; Klein, J.P.; Sobrin, L.; Buchbinder, B.R.; Gupta, R.; et al. Clinical and neuroradiologic characteristics in varicella zoster virus reactivation with central nervous system involvement. J. Neurol. Sci. 2022, 437, 120262. [Google Scholar] [CrossRef]
- Piantadosi, A.; Mukerji, S.S.; Ye, S.; Leone, M.J.; Freimark, L.M.; Park, D.; Adams, G.; Lemieux, J.; Kanjilal, S.; Solomon, I.H.; et al. Enhanced Virus Detection and Metagenomic Sequencing in Patients with Meningitis and Encephalitis. mBio 2021, 12, e0114321. [Google Scholar] [CrossRef]
- Costa Sa, A.C.; Madsen, H.; Brown, J.R. Shared Molecular Signatures Across Neurodegenerative Diseases and Herpes Virus Infections Highlights Potential Mechanisms for Maladaptive Innate Immune Responses. Sci. Rep. 2019, 9, 8795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Fatou, B.; Mehta, N.M.; Bennike, T.B.; Steen, H. Sample Preparation for High-Throughput Urine Proteomics Using 96-Well Polyvinylidene Fluoride (PVDF) Membranes. Adv. Exp. Med. Biol. 2021, 1306, 1–12. [Google Scholar] [PubMed]
- Bennike, T.B.; Fatou, B.; Angelidou, A.; Diray-Arce, J.; Falsafi, R.; Ford, R.; Gill, E.E.; van Haren, S.D.; Idoko, O.T.; Lee, A.H.; et al. Preparing for Life: Plasma Proteome Changes and Immune System Development During the First Week of Human Life. Front. Immunol. 2020, 11, 578505. [Google Scholar] [CrossRef]
- IMPACC Manuscript Writing Team; IMPACC Network Steering Committee. Immunophenotyping assessment in a COVID-19 cohort (IMPACC): A prospective longitudinal study. Sci. Immunol. 2021, 6, eabf3733. [Google Scholar] [CrossRef]
- Kentsis, A.; Shulman, A.; Ahmed, S.; Brennan, E.; Monuteaux, M.C.; Lee, Y.H.; Lipsett, S.; Paulo, J.A.; Dedeoglu, F.; Fuhlbrigge, R.; et al. Urine proteomics for discovery of improved diagnostic markers of Kawasaki disease. EMBO Mol. Med. 2013, 5, 210–220. [Google Scholar] [CrossRef]
- Chau, K.F.; Springel, M.W.; Broadbelt, K.G.; Park, H.Y.; Topal, S.; Lun, M.P.; Mullan, H.; Maynard, T.; Steen, H.; LaMantia, A.S.; et al. Progressive Differentiation and Instructive Capacities of Amniotic Fluid and Cerebrospinal Fluid Proteomes following Neural Tube Closure. Dev. Cell. 2015, 35, 789–802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dayon, L.; Cominetti, O.; Affolter, M. Proteomics of human biological fluids for biomarker discoveries: Technical advances and recent applications. Expert Rev. Proteom. 2022, 19, 131–151. [Google Scholar] [CrossRef] [PubMed]
- Lee, G.H.; Kim, J.; Kim, H.W.; Cho, J.W. Herpes simplex viruses (1 and 2) and varicella-zoster virus infections in an adult population with aseptic meningitis or encephalitis: A nine-year retrospective clinical study. Medicine 2021, 100, e27856. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Gable, A.L.; Lyon, D.; Junge, A.; Wyder, S.; Huerta-Cepas, J.; Simonovic, M.; Doncheva, N.T.; Morris, J.H.; Bork, P.; et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019, 47, D607–D613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thaker, S.K.; Ch’ng, J.; Christofk, H.R. Viral hijacking of cellular metabolism. BMC Biol. 2019, 17, 59. [Google Scholar] [CrossRef]
- Vastag, L.; Koyuncu, E.; Grady, S.L.; Shenk, T.E.; Rabinowitz, J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011, 7, e1002124. [Google Scholar] [CrossRef] [Green Version]
- Abrantes, J.L.; Alves, C.M.; Costa, J.; Almeida, F.C.; Sola-Penna, M.; Fontes, C.F.; Souza, T.M. Herpes simplex type 1 activates glycolysis through engagement of the enzyme 6-phosphofructo-1-kinase (PFK-1). Biochim. Biophys. Acta 2012, 1822, 1198–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chattopadhyay, D.; Mukhopadhyay, A.; Ojha, D.; Sadhukhan, P.; Dutta, S. Immuno-metabolic changes in herpes virus infection. Cytokine 2018, 112, 52–62. [Google Scholar] [CrossRef]
- Reed, L.J.; Lasserson, D.; Marsden, P.; Bright, P.; Stanhope, N.; Kopelman, M.D. Correlations of regional cerebral metabolism with memory performance and executive function in patients with herpes encephalitis or frontal lobe lesions. Neuropsychology 2005, 19, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.; Mohr, I. Viral subversion of the host protein synthesis machinery. Nat. Rev. Microbiol. 2011, 9, 860–875. [Google Scholar] [CrossRef]
- Banerjee, A.; Kulkarni, S.; Mukherjee, A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020, 11, 733. [Google Scholar] [CrossRef] [PubMed]
- Hennig, T.; Djakovic, L.; Dolken, L.; Whisnant, A.W. A Review of the Multipronged Attack of Herpes Simplex Virus 1 on the Host Transcriptional Machinery. Viruses 2021, 13, 1836. [Google Scholar] [CrossRef] [PubMed]
- Dauber, B.; Saffran, H.A.; Smiley, J.R. The herpes simplex virus 1 virion host shutoff protein enhances translation of viral late mRNAs by preventing mRNA overload. J. Virol. 2014, 88, 9624–9632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mertens, M.E.; Knipe, D.M. Herpes Simplex Virus 1 Manipulates Host Cell Antiviral and Proviral DNA Damage Responses. mBio 2021, 12, e03552-20. [Google Scholar] [CrossRef]
- Vink, E.I.; Andrews, J.; Duffy, C.; Mohr, I. Preventing translational inhibition from ribosomal protein insufficiency by a herpes simplex virus-encoded ribosome-associated protein. Proc. Natl. Acad. Sci. USA 2021, 118, e2025546118. [Google Scholar] [CrossRef]
- Nathan, K.G.; Lal, S.K. The Multifarious Role of 14-3-3 Family of Proteins in Viral Replication. Viruses 2020, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, J.C.; Min, J.S.; Kang, I.; Oh, J.; Ahn, J.K. HSV-1 ICP27 induces apoptosis by promoting Bax translocation to mitochondria through interacting with 14-3-3theta. BMB Rep. 2017, 50, 257–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, C.R.; Melo, B.M.S.; Silva, J.R.; Lopes, A.H.; Pereira, J.A.; Cecilio, N.T.; Berlink, J.; Souza, G.G.; Lucas, G.; Vogl, T.; et al. S100A9 plays a pivotal role in a mouse model of herpetic neuralgia via TLR4/TNF pathway. Brain Behav. Immun. 2020, 88, 353–362. [Google Scholar] [CrossRef]

| Pathology | Age | Sex | Race | PMH | Reason for Lumbar Puncture | Relevant Diagnostic Testing |
|---|---|---|---|---|---|---|
| HSV-1 | 58 | M | Black | Prostate adenocarcinoma, history of pulmonary tuberculosis and syphilis | Altered mental status | CSF PCR: HSV-1: positive, HSV-2: negative, VZV: negative; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| HSV-1 | 25 | M | White | Dental abscesses | Altered mental status, fever, seizure | CSF PCR: HSV-1: positive, HSV-2: negative, VZV: not tested; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| HSV-1 | 57 | M | White | Chronic back pain | Altered mental status | CSF PCR: HSV-1: positive, HSV-2: negative, VZV: not tested; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| HSV-2 | 87 | F | White | Hypertension, hyperlipidemia, peripheral arterial disease, peripheral neuropathy | Altered mental status | CSF PCR: HSV-1: negative, HSV-2: positive, VZV: not tested; CSF Gram stain: no organisms; Bacterial culture: no growth; Skin vesicle VZV DFA: negative; CSF RT-QuIC or 14-3-3: not tested |
| HSV-2 | 85 | M | White | Coronary artery disease, chronic obstructive pulmonary disease, chronic kidney disease, suspected myasthenia gravis s/p thymectomy | Seizure, somnolence, bilateral arm twitching | CSF PCR: HSV-1: negative, HSV-2: positive, VZV: not tested; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| HSV-2 | 39 | F | White | Hypothyroidism, recurrent aseptic meningitis, migraine without aura | Headache, photophobia/phonophobia, nausea, neck stiffness | CSF PCR: HSV-1: negative, HSV-2: positive, VZV: negative; CSF total VZV antibody (ACIF): <1:2; CSF Gram stain: no organisms; Bacterial culture: no growth; Blood HSV-1 IgG negative, HSV-2 IgG positive; CSF RT-QuIC or 14-3-3: not tested |
| HSV-2 | 54 | F | White | Reactive airway disease, obstructive sleep apnea, depression and anxiety, remote migraines, history of HSV-2 meningitis | Worsening headache, neck pain, photophobia, diffuse weakness | CSF PCR: HSV-1: negative, HSV-2: positive, VZV: negative; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| VZV | 39 | F | White | Depression | Fever, headache, neck stiffness and right flank rash/abdominal | CSF PCR: HSV-1: negative, HSV-2: negative, VZV: positive; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| VZV | 74 | F | White | Chronic obstructive pulmonary disease | Right arm/shoulder/back rash, progressive somnolence, event concerning for GTC | CSF PCR: HSV-1: negative, HSV-2: negative, VZV: positive; CSF total VZV antibody (ACIF): 1:32 CSF; Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| VZV | 82 | F | White | Lung and bladder cancer, atrial fibrillation, schizoaffective disorder | Altered mental status, generalized weakness | CSF PCR: HSV-1: negative, HSV-2: positive, VZV: negative; CSF total VZV antibody(ACIF): 1:128; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| VZV | 78 | M | White | Diabetes mellitus, hypertension, hyperlipidemia, coronary artery disease, chronic obstructive pulmonary disease, trigeminal neuralgia | Scalp pain, dysphagia, ataxia, and dizziness | CSF PCR: HSV-1: negative, HSV-2: negative, VZV: negative; CSF VZV total antibody (ACIF): 1:8; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested; Skin vesicle VZV DFA: positive |
| VZV | 79 | M | White | Chronic lymphocytic leukemia, chronic obstructive pulmonary disease, interstitial lung disease, chronic kidney disease | Left ptosis, ophthalmoparesis | CSF PCR: HSV-1: negative, HSV-2: negative, VZV: negative; CSF VZV total antibody (ACIF): 1:8; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
| VZV | 85 | M | White | Atrial fibrillation, hypertension, history of resected benign intracranial neoplasm (per report) | Altered mental status and recent history of cutaneous zoster | CSF PCR: HSV-1: negative, HSV-2: negative, VZV: negative; CSF VZV total antibody (ACIF): 1:16; CSF Gram stain: no organisms; Bacterial culture: no growth; CSF RT-QuIC or 14-3-3: not tested |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmed, S.; van Zalm, P.; Rudmann, E.A.; Leone, M.; Keller, K.; Branda, J.A.; Steen, J.; Mukerji, S.S.; Steen, H. Using CSF Proteomics to Investigate Herpesvirus Infections of the Central Nervous System. Viruses 2022, 14, 2757. https://doi.org/10.3390/v14122757
Ahmed S, van Zalm P, Rudmann EA, Leone M, Keller K, Branda JA, Steen J, Mukerji SS, Steen H. Using CSF Proteomics to Investigate Herpesvirus Infections of the Central Nervous System. Viruses. 2022; 14(12):2757. https://doi.org/10.3390/v14122757
Chicago/Turabian StyleAhmed, Saima, Patrick van Zalm, Emily A. Rudmann, Michael Leone, Kiana Keller, John A. Branda, Judith Steen, Shibani S. Mukerji, and Hanno Steen. 2022. "Using CSF Proteomics to Investigate Herpesvirus Infections of the Central Nervous System" Viruses 14, no. 12: 2757. https://doi.org/10.3390/v14122757





