Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis
Abstract
:1. Introduction
2. Viral-Infections-Induced Human Cancers
3. HCV Pathogenesis
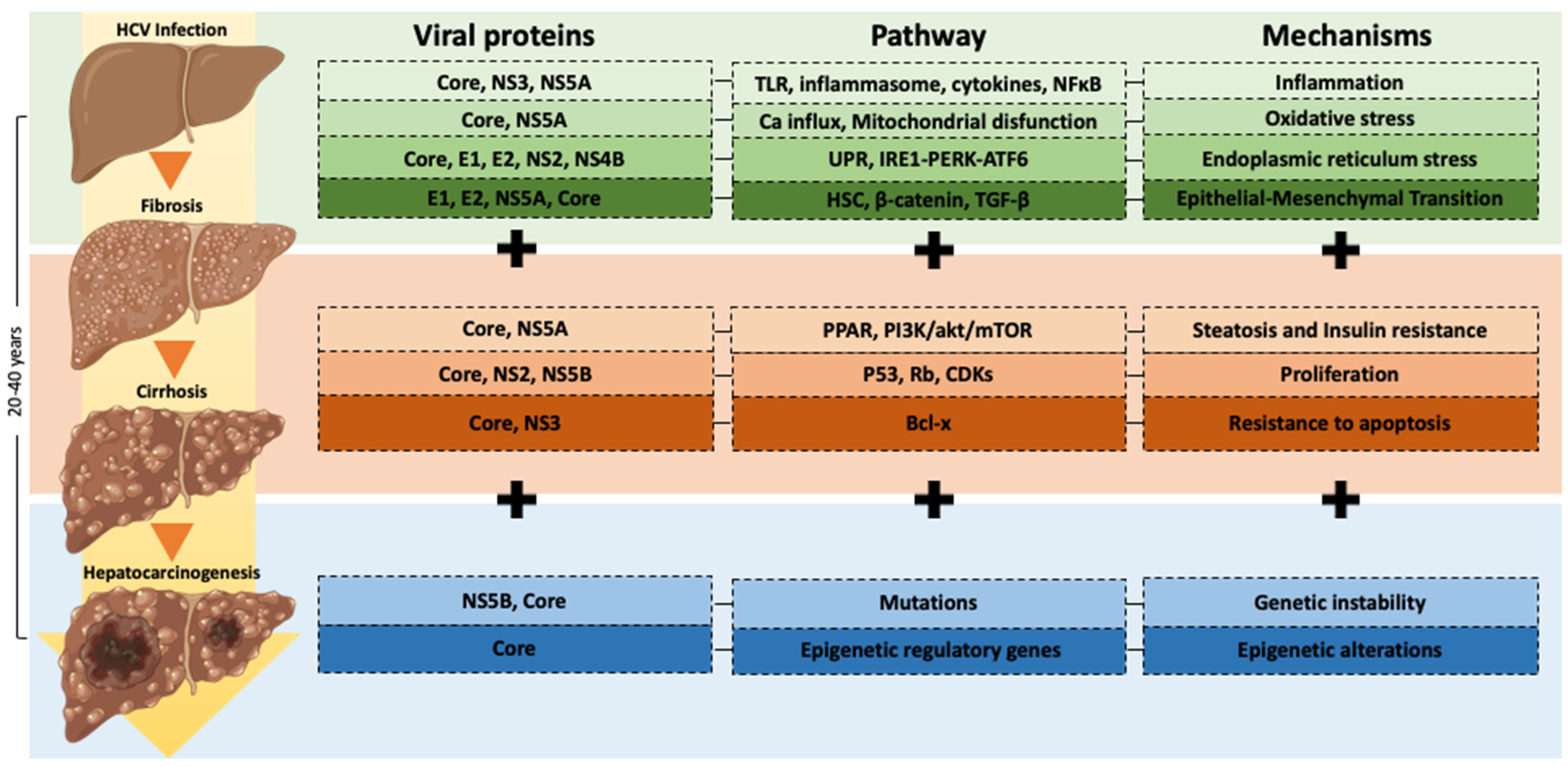
4. Molecular Mechanisms in HCV Infection-Induced Carcinogenesis
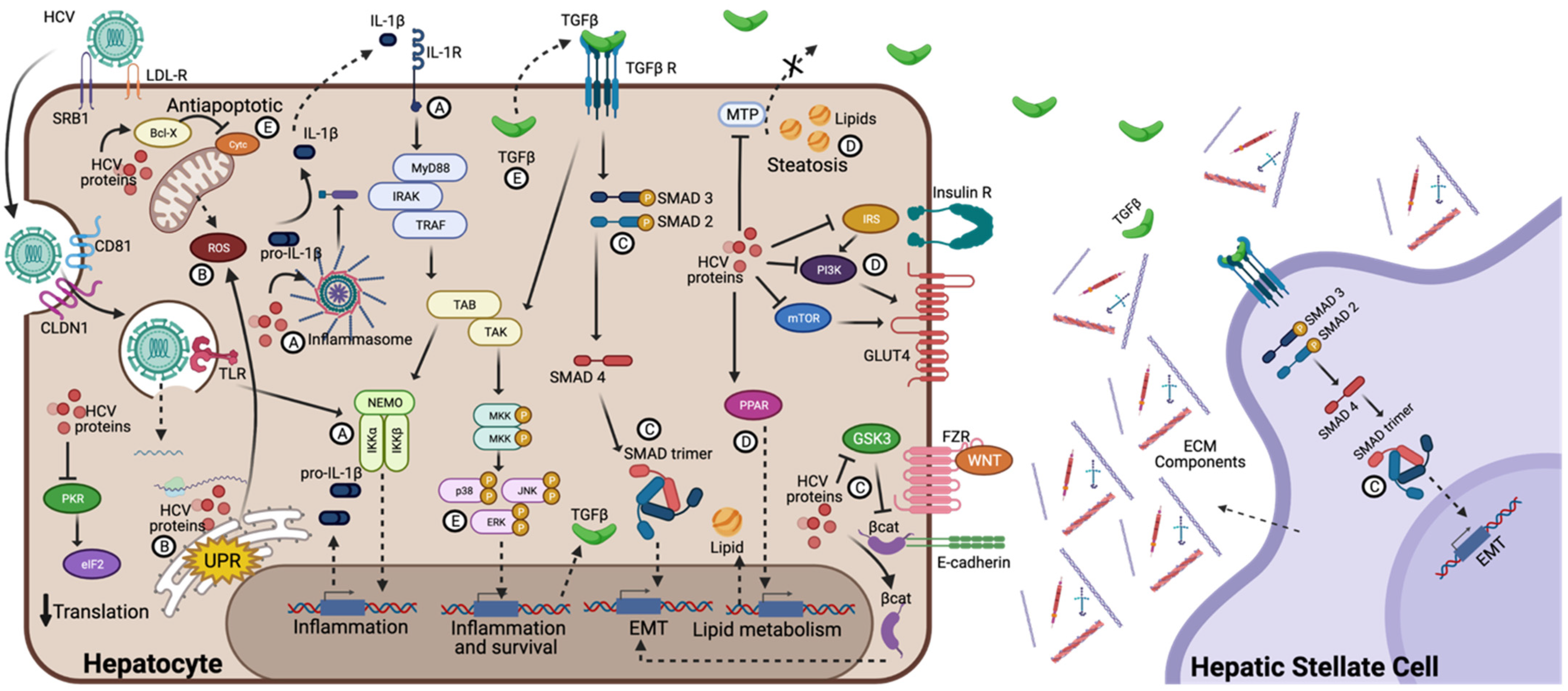
4.1. Chronic Inflammation and Oxidative Stress
4.2. Epithelial-Mesenchymal Transition (EMT)
| Molecular Mechanism | Viral Protein | Pathway | Ref. |
|---|---|---|---|
| Cell proliferation | Core | Enhancement of canonical Wnt/beta-catenin. | [21] |
| Overexpression of TGF-β levels and implication of thrombospondin-1 in core-dependent TGF-β activation | [22] | ||
| NS5A | Activation of PI3K, increased Akt/protein kinase B activity and provided protection against apoptosis | [23] | |
| Activation of the c-Myc promoter and increased c-Myc transcription | [24] | ||
| NS5B | pRb is ubiquitinated and degraded in a proteasome-dependent manner | [25] | |
| Epithelial-Mesenchymal Transition | Core | Increase Snail expression and induce EMT via STAT3 | [26] |
| NS5A | Activate Twist2 and induce upregulation of Vimentin and N-cadherin and downregulates E-cadherin expression | [19] | |
| NS5B | Induce upregulation of N-cadherin via Snail | [27] | |
| Oxidative Stress | NS5A | Alters intracellular calcium levels, induces oxidative stress, and activates STAT3 and NF-κB. | [28] |
| Core | Increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity | [29] | |
| Genetic alterations | NS3/NS4 | Interacts with cellular protein that plays role in double-strand DNA breaks | [30] |
4.3. EMT and Inflammasome Activation
4.4. Steatosis and Insulin Resistance
4.5. Endoplasmic Reticulum Stress
4.6. Proliferation and Apoptosis
4.7. Epigenetic and Genetic Alterations
5. Carcinogenesis after Clearance of HCV Infection
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global Burden of Cancer Attributable to Infections in 2018: A Worldwide Incidence Analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [Green Version]
- Petrick, J.L.; Florio, A.A.; Znaor, A.; Ruggieri, D.; Laversanne, M.; Alvarez, C.S.; Ferlay, J.; Valery, P.C.; Bray, F.; McGlynn, K.A. International Trends in Hepatocellular Carcinoma Incidence, 1978–2012. Int. J. Cancer 2020, 147, 317–330. [Google Scholar] [CrossRef]
- Terrault, N. Global Prevalence and Genotype Distribution of Hepatitis C Virus Infection in 2015: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018, 2, 161–176. [Google Scholar]
- Wu, T.-C.; Chang, M.-H.; Jeang, K.-T. Viruses and Human Cancer, 2nd ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; ISBN 978-3-030-57362-1. [Google Scholar]
- Uchi, H. Merkel Cell Carcinoma: An Update and Immunotherapy. Front. Oncol. 2018, 8, 48. [Google Scholar] [CrossRef]
- Dubuisson, J.; Cosset, F.L. Virology and Cell Biology of the Hepatitis C Virus Life Cycle—An Update. J. Hepatol. 2014, 61, S3–S13. [Google Scholar] [CrossRef] [Green Version]
- Arzumanyan, A.; Reis, H.M.G.P.V.; Feitelson, M.A. Pathogenic Mechanisms in HBV-and HCV-Associated Hepatocellular Carcinoma. Nat. Rev. Cancer 2013, 13, 123–135. [Google Scholar] [CrossRef]
- Mitchell, J.K.; Lemon, S.M.; McGivern, D.R. How Do Persistent Infections with Hepatitis C Virus Cause Liver Cancer? Curr. Opin. Virol. 2015, 14, 101–108. [Google Scholar] [CrossRef] [Green Version]
- Irshad, M.; Gupta, P.; Irshad, K. Molecular Basis of Hepatocellular Carcinoma Induced by Hepatitis C Virus Infection. World J. Hepatol. 2017, 9, 1305–1314. [Google Scholar] [CrossRef] [Green Version]
- Marukian, S.; Andrus, L.; Sheahan, T.P.; Jones, C.T.; Charles, E.D.; Ploss, A.; Rice, C.M.; Dustin, L.B. Hepatitis C Virus Induces Interferon-λ and Interferon-Stimulated Genes in Primary Liver Cultures. Hepatology 2011, 54, 1913–1923. [Google Scholar] [CrossRef] [Green Version]
- Negash, A.A.; Ramos, H.J.; Crochet, N.; Lau, D.T.Y.; Doehle, B.; Papic, N.; Delker, D.A.; Jo, J.; Bertoletti, A.; Hagedorn, C.H.; et al. IL-1β Production through the NLRP3 Inflammasome by Hepatic Macrophages Links Hepatitis C Virus Infection with Liver Inflammation and Disease. PLoS Pathog. 2013, 9, e1003330. [Google Scholar] [CrossRef]
- Nishitsuji, H.; Funami, K.; Shimizu, Y.; Ujino, S.; Sugiyama, K.; Seya, T.; Takaku, H.; Shimotohno, K. Hepatitis C Virus Infection Induces Inflammatory Cytokines and Chemokines Mediated by the Cross Talk between Hepatocytes and Stellate Cells. J. Virol. 2013, 87, 8169–8178. [Google Scholar] [CrossRef] [PubMed]
- Paracha, U.Z.; Fatima, K.; Alqahtani, M.; Chaudhary, A.; Abuzenadah, A.; Damanhouri, G.; Qadri, I. Oxidative Stress and Hepatitis C Virus. Virol. J. 2013, 10, 251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ko, K.S.; Tomasi, M.L.; Iglesias-Ara, A.; French, B.A.; French, S.W.; Ramani, K.; Lozano, J.J.; Oh, P.; He, L.; Stiles, B.L.; et al. Liver-Specific Deletion of Prohibitin 1 Results in Spontaneous Liver Injury, Fibrosis, and Hepatocellular Carcinoma in Mice. Hepatology 2010, 52, 2096–2108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thimme, R. T Cell Immunity to Hepatitis C Virus: Lessons for a Prophylactic Vaccine. J. Hepatol. 2021, 74, 220–229. [Google Scholar] [CrossRef]
- Lin, W.; Tsai, W.L.; Shao, R.X.; Wu, G.; Peng, L.F.; Barlow, L.L.; Chung, W.J.; Zhang, L.; Zhao, H.; Jang, J.Y.; et al. Hepatitis C Virus Regulates Transforming Growth Factor Β1 Production Through the Generation of Reactive Oxygen Species in a Nuclear Factor ΚB-Dependent Manner. Gastroenterology 2010, 138, 2509–2518. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.J.; Kim, K.H.; Park, K.K. Mechanisms of Fibrogenesis in Liver Cirrhosis: The Molecular Aspects of Epithelial-Mesenchymal Transition. World J. Hepatol. 2014, 6, 207–216. [Google Scholar] [CrossRef]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular Mechanisms of Epithelial-Mesenchymal Transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [Green Version]
- Akkari, L.; Grégoire, D.; Floc’H, N.; Moreau, M.; Hernandez, C.; Simonin, Y.; Rosenberg, A.R.; Lassus, P.; Hibner, U. Hepatitis C Viral Protein NS5A Induces EMT and Participates in Oncogenic Transformation of Primary Hepatocyte Precursors. J. Hepatol. 2012, 57, 1021–1028. [Google Scholar] [CrossRef]
- Nie, D.; Shan, X.; Nie, L.; Duan, Y.; Chen, Z.; Yang, Y.; Li, Z.; Tian, L.; Gao, Q.; Shan, Y.; et al. Hepatitis C Virus Core Protein Interacts with Snail and Histone Deacetylases to Promote the Metastasis of Hepatocellular Carcinoma. Oncogene 2016, 35, 3626–3635. [Google Scholar] [CrossRef]
- Liu, J.; Ding, X.; Tang, J.; Cao, Y.; Hu, P.; Zhou, F.; Shan, X.; Cai, X.; Chen, Q.; Ling, N.; et al. Enhancement of Canonical Wnt/β-Catenin Signaling Activity by Hcv Core Protein Promotes Cell Growth of Hepatocellular Carcinoma Cells. PLoS ONE 2011, 6, e27496. [Google Scholar] [CrossRef]
- Benzoubir, N.; Lejamtel, C.; Battaglia, S.; Testoni, B.; Benassi, B.; Gondeau, C.; Perrin-Cocon, L.; Desterke, C.; Thiers, V.; Samuel, D.; et al. HCV Core-Mediated Activation of Latent TGF-β via Thrombospondin Drives the Crosstalk between Hepatocytes and Stromal Environment. J. Hepatol. 2013, 59, 1160–1168. [Google Scholar] [CrossRef] [PubMed]
- Street, A.; Macdonald, A.; Crowder, K.; Harris, M. The Hepatitis C Virus NS5A Protein Activates a Phosphoinositide 3-Kinase-Dependent Survival Signaling Cascade. J. Biol. Chem. 2004, 279, 12232–12241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgs, M.R.; Lerat, H.; Pawlotsky, J.M. Hepatitis C Virus-Induced Activation of β-Catenin Promotes c-Myc Expression and a Cascade of pro-Carcinogenetic Events. Oncogene 2013, 32, 4683–4693. [Google Scholar] [CrossRef] [Green Version]
- Munakata, T.; Liang, Y.; Kim, S.; McGivern, D.R.; Huibregtse, J.; Nomoto, A.; Lemon, S.M. Hepatitis C Virus Induces E6AP-Dependent Degradation of the Retinoblastoma Protein. PLoS Pathog. 2007, 3, 1335–1347. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.J.; Meng, Z.; He, X.Y.; Cheng, D.; Ye, H.L.; Deng, X.G.; Chen, R.F. Hepatitis C Virus Core Protein Increases Snail Expression and Induces Epithelial–Mesenchymal Transition through the Signal Transducer and Activator of Transcription 3 Pathway in Hepatoma Cells. Hepatol. Res. 2017, 47, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Xie, S.; Hu, Y.; Chen, W.; Chen, X.; Zheng, Y.; Wu, X. Hepatitis C Virus NS4B Protein Induces Epithelial-Mesenchymal Transition by Upregulation of Snail. Virol. J. 2017, 14, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, G.; Waris, G.; Tanveer, R.; Siddiqui, A. Human Hepatitis C Virus NS5A Protein Alters Intracellular Calcium Levels, Induces Oxidative Stress, and Activates STAT-3 and NF-ΚB. Proc. Natl. Acad. Sci. USA 2001, 98, 9599–9604. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Boehning, D.F.; Qian, T.; Popov, V.L.; Weinman, S.A. Hepatitis C Virus Core Protein Increases Mitochondrial ROS Production by Stimulation of Ca2+ Uniporter Activity. FASEB J. 2007, 21, 2474–2485. [Google Scholar] [CrossRef]
- Lai, C.K.; Jeng, K.S.; Machida, K.; Cheng, Y.S.; Lai, M.M.C. Hepatitis C Virus NS3/4A Protein Interacts with ATM, Impairs DNA Repair and Enhances Sensitivity to Ionizing Radiation. Virology 2008, 370, 295–309. [Google Scholar] [CrossRef] [Green Version]
- Negash, A.A.; Olson, R.M.; Griffin, S.; Gale, M. Modulation of Calcium Signaling Pathway by Hepatitis C Virus Core Protein Stimulates NLRP3 Inflammasome Activation. PLoS Pathog. 2019, 15, e1007593. [Google Scholar] [CrossRef]
- Alyaseer, A.A.A.; de Lima, M.H.S.; Braga, T.T. The Role of NLRP3 Inflammasome Activation in the Epithelial to Mesenchymal Transition Process During the Fibrosis. Front. Immunol. 2020, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Dewidar, B.; Meyer, C.; Dooley, S.; Meindl-Beinker, A.N. TGF-β in Hepatic Stellate Cell Activation and Liver Fibrogenesis—Updated 2019. Cells 2019, 8, 1419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perlemuter, G.; Sabile, A.; Letteron, P.; Vona, G.; Topilco, A.; Chrétien, Y.; Koike, K.; Pessayre, D.; Chapman, J.; Barba, G.; et al. Hepatitis C Virus Core Protein Inhibits Microsomal Triglyceride Transfer Protein Activity and Very Low Density Lipoprotein Secretion: A Model of Viral-related Steatosis. FASEB J. 2002, 16, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Aytug, S.; Reich, D.; Sapiro, L.E.; Bernstein, D.; Begum, N. Impaired IRS-1/PI3-Kinase Signaling in Patients with HCV: A Mechanism for Increased Prevalence of Type 2 Diabetes. Hepatology 2003, 38, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.K.; Shrivastava, S.; Meyer, K.; Ray, R.B.; Ray, R. Hepatitis C Virus Activates the MTOR/S6K1 Signaling Pathway in Inhibiting IRS-1 Function for Insulin Resistance. J. Virol. 2012, 86, 6315–6322. [Google Scholar] [CrossRef] [Green Version]
- Deng, L.; Shoji, I.; Ogawa, W.; Kaneda, S.; Soga, T.; Jiang, D.-P.; Ide, Y.-H.; Hotta, H. Hepatitis C Virus Infection Promotes Hepatic Gluconeogenesis through an NS5A-Mediated, FoxO1-Dependent Pathway. J. Virol. 2011, 85, 8556–8568. [Google Scholar] [CrossRef] [Green Version]
- Okamoto, K.; Moriishi, K.; Miyamura, T.; Matsuura, Y. Intramembrane Proteolysis and Endoplasmic Reticulum Retention of Hepatitis C Virus Core Protein. J. Virol. 2004, 78, 6370–6380. [Google Scholar] [CrossRef] [Green Version]
- Austin, R.C. The Unfolded Protein Response in Health and Disease. Antioxid. Redox Signal. 2009, 11, 2279–2287. [Google Scholar] [CrossRef]
- Benali-Furet, N.L.; Chami, M.; Houel, L.; De Giorgi, F.; Vernejoul, F.; Lagorce, D.; Buscail, L.; Bartenschlager, R.; Ichas, F.; Rizzuto, R.; et al. Hepatitis C Virus Core Triggers Apoptosis in Liver Cells by Inducing ER Stress and ER Calcium Depletion. Oncogene 2005, 24, 4921–4933. [Google Scholar] [CrossRef] [Green Version]
- Tardif, K.D.; Mori, K.; Kaufman, R.J.; Siddiqui, A. Hepatitis C Virus Suppresses the IRE1-XBP1 Pathway of the Unfolded Protein Response. J. Biol. Chem. 2004, 279, 17158–17164. [Google Scholar] [CrossRef] [Green Version]
- Chan, S.W. Unfolded Protein Response in Hepatitis C Virus Infection. Front. Microbiol. 2014, 5, 233. [Google Scholar] [CrossRef] [PubMed]
- Tavakolian, S.; Goudarzi, H.; Faghihloo, E. Cyclin-Dependent Kinases and CDK Inhibitors in Virus-Associated Cancers. Infect. Agent. Cancer 2020, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Tsutsumi, T.; Suzuki, T.; Moriya, K.; Shintani, Y.; Fujie, H.; Miyoshi, H.; Matsuura, Y.; Koike, K.; Miyamura, T. Hepatitis C Virus Core Protein Activates ERK and P38 MAPK in Cooperation with Ethanol in Transgenic Mice. Hepatology 2003, 38, 820–828. [Google Scholar] [CrossRef] [PubMed]
- Kwun, H.J.; Jang, K.L. Dual Effects of Hepatitis C Virus Core Protein on the Transcription of Cyclin-Dependent Kinase Inhibitor P21 Gene. J. Viral Hepat. 2003, 10, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Munakata, T.; Nakamura, M.; Liang, Y.; Li, K.; Lemon, S.M. Down-Regulation of the Retinoblastoma Tumor Suppressor by the Hepatitis C Virus NS5B RNA-Dependent RNA Polymerase. Proc. Natl. Acad. Sci. USA 2005, 102, 18159–18164. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Wang, Y.; Xu, Y.; Tong, W.; Pan, T.; Li, J.; Sun, S.; Shao, J.; Ding, H.; Toyoda, T.; et al. Hepatitis C Virus NS5B Protein Delays s Phase Progression in Human Hepatocyte-Derived Cells by Relocalizing Cyclin-Dependent Kinase 2-Interacting Protein (CINP). J. Biol. Chem. 2011, 286, 26603–26615. [Google Scholar] [CrossRef] [Green Version]
- Machida, K.; Tsukiyama-Kohara, K.; Seike, E.; Toné, S.; Shibasaki, F.; Shimizu, M.; Takahashi, H.; Hayashi, Y.; Funata, N.; Taya, C.; et al. Inhibition of Cytochrome c Release in Fas-Mediated Signaling Pathway in Transgenic Mice Induced to Express Hepatitis C Viral Proteins. J. Biol. Chem. 2001, 276, 12140–12146. [Google Scholar] [CrossRef] [Green Version]
- Otsuka, M.; Kato, N.; Taniguchi, H.; Yoshida, H.; Goto, T.; Shiratori, Y.; Omata, M. Hepatitis C Virus Core Protein Inhibits Apoptosis via Enhanced Bcl-XL Expression. Virology 2002, 296, 84–93. [Google Scholar] [CrossRef] [Green Version]
- Meylan, E.; Curran, J.; Hofmann, K.; Moradpour, D.; Binder, M.; Bartenschlager, R.; Tschopp, J. Cardif Is an Adaptor Protein in the RIG-I Antiviral Pathway and Is Targeted by Hepatitis C Virus. Nature 2005, 437, 1167–1172. [Google Scholar] [CrossRef]
- Zhao, P.; Malik, S.; Xing, S. Epigenetic Mechanisms Involved in HCV-Induced Hepatocellular Carcinoma (HCC). Front. Oncol. 2021, 11, 677926. [Google Scholar] [CrossRef]
- Feng, Q.; Stern, J.E.; Hawes, S.E.; Lu, H.; Jiang, M.; Kiviat, N.B. DNA Methylation Changes in Normal Liver Tissues and Hepatocellular Carcinoma with Different Viral Infection. Exp. Mol. Pathol. 2010, 88, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Calvisi, D.F.; Ladu, S.; Conner, E.A.; Seo, D.; Hsieh, J.T.; Factor, V.M.; Thorgeirsson, S.S. Inactivation of Ras GTPase-Activating Proteins Promotes Unrestrained Activity of Wild-Type Ras in Human Liver Cancer. J. Hepatol. 2011, 54, 311–319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erez, N.; Milyavsky, M.; Eilam, R.; Shats, I.; Goldfinger, N.; Rotter, V. Expression of Prolyl-Hydroxylase-1 (PHD1/EGLN2) Suppresses Hypoxia Inducible Factor-1α Activation and Inhibits Tumor Growth. Cancer Res. 2003, 63, 8777–8783. [Google Scholar] [PubMed]
- Rayess, H.; Wang, M.B.; Srivatsan, E.S. Cellular Senescence and Tumor Suppressor Gene P16. Int. J. Cancer 2012, 130, 1715–1725. [Google Scholar] [CrossRef] [Green Version]
- Dang, S.; Zhou, J.; Chen, Y.; Chen, P.; Ji, M.; Shi, B.; Yang, Q.; Hou, P. Dynamic Expression of ZNF382 and Its Tumor-Suppressor Role in Hepatitis B Virus-Related Hepatocellular Carcinogenesis. Oncogene 2019, 38, 4804–4819. [Google Scholar] [CrossRef]
- Umezaki, N.; Nakagawa, S.; Yamashita, Y.I.; Kitano, Y.; Arima, K.; Miyata, T.; Hiyoshi, Y.; Okabe, H.; Nitta, H.; Hayashi, H.; et al. Lysyl Oxidase Induces Epithelial-Mesenchymal Transition and Predicts Intrahepatic Metastasis of Hepatocellular Carcinoma. Cancer Sci. 2019, 110, 2033–2043. [Google Scholar] [CrossRef]
- Anwar, S.L.; Krech, T.; Hasemeier, B.; Schipper, E.; Schweitzer, N.; Vogel, A.; Kreipe, H.; Lehmann, U. Deregulation of RB1 Expression by Loss of Imprinting in Human Hepatocellular Carcinoma. J. Pathol. 2014, 233, 392–401. [Google Scholar] [CrossRef]
- Adamek, A.; Kasprzak, A. Insulin-like Growth Factor (IGF) System in Liver Diseases. Int. J. Mol. Sci. 2018, 19, 1308. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Z.; Ye, M.; Qie, J.; Ye, T. Foxa1 Promotes Cell Proliferation and Suppresses Apoptosis in HCC by Directly Regulating MiR-212-3p/FOXA1/AGR2 Signaling Pathway. OncoTargets Ther. 2020, 13, 5231–5240. [Google Scholar] [CrossRef]
- Ning, B.F.; Ding, J.; Liu, J.; Yin, C.; Xu, W.P.; Cong, W.M.; Zhang, Q.; Chen, F.; Han, T.; Deng, X.; et al. Hepatocyte Nuclear Factor 4α-Nuclear Factor-ΚB Feedback Circuit Modulates Liver Cancer Progression. Hepatology 2014, 60, 1607–1619. [Google Scholar] [CrossRef]
- Lourenço, A.R.; Coffer, P.J. A Tumor Suppressor Role for C/EBPα in Solid Tumors: More than Fat and Blood. Oncogene 2017, 36, 5221–5230. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hlady, R.A.; Joyce, B.T.; Robertson, K.D.; He, C.; Nannini, D.R.; Kibbe, W.A.; Achenbach, C.J.; Murphy, R.L.; Roberts, L.R.; et al. DNA Methylation of Individual Repetitive Elements in Hepatitis C Virus Infection-Induced Hepatocellular Carcinoma. Clin. Epigenetics 2019, 11, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattori, N.; Ushijima, T. Epigenetic Impact of Infection on Carcinogenesis: Mechanisms and Applications. Genome Med. 2016, 8, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dash, S.; Aydin, Y.; Widmer, K.E.; Nayak, L. Hepatocellular Carcinoma Mechanisms Associated with Chronic HCV Infection and the Impact of Direct-Acting Antiviral Treatment. J. Hepatocell. Carcinoma 2020, 7, 45–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conti, F.; Buonfiglioli, F.; Scuteri, A.; Crespi, C.; Bolondi, L.; Caraceni, P.; Foschi, F.G.; Lenzi, M.; Mazzella, G.; Verucchi, G.; et al. Early Occurrence and Recurrence of Hepatocellular Carcinoma in HCV-Related Cirrhosis Treated with Direct-Acting Antivirals. J. Hepatol. 2016, 65, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Calvaruso, V.; Cabibbo, G.; Cacciola, I.; Petta, S.; Madonia, S.; Bellia, A.; Tinè, F.; Distefano, M.; Licata, A.; Giannitrapani, L.; et al. Incidence of Hepatocellular Carcinoma in Patients With HCV-Associated Cirrhosis Treated With Direct-Acting Antiviral Agents. Gastroenterology 2018, 155, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Cammà, C.; Cabibbo, G.; Craxì, A. Direct Antiviral Agents and Risk for HCC Early Recurrence: Much Ado about Nothing. J. Hepatol. 2016, 65, 861–862. [Google Scholar] [CrossRef]
- Meissner, E.G.; Wu, D.; Osinusi, A.; Bon, D.; Virtaneva, K.; Sturdevant, D.; Porcella, S.; Wang, H.; Herrmann, E.; McHutchison, J.; et al. Endogenous Intrahepatic IFNs and Association with IFN-Free HCV Treatment Outcome. J. Clin. Investig. 2014, 124, 3352–3363. [Google Scholar] [CrossRef] [Green Version]
- Villani, R.; Facciorusso, A.; Bellanti, F.; Tamborra, R.; Piscazzi, A.; Landriscina, M.; Vendemiale, G.; Serviddio, G. DAAs Rapidly Reduce Inflammation but Increase Serum VEGF Level: A Rationale for Tumor Risk during Anti-HCV Treatment. PLoS ONE 2016, 11, e0167934. [Google Scholar] [CrossRef] [Green Version]
- Perez, S.; Kaspi, A.; Domovitz, T.; Davidovich, A.; Lavi-Itzkovitz, A.; Meirson, T.; Alison Holmes, J.; Dai, C.-Y.; Huang, C.-F.; Chung, R.T.; et al. Hepatitis C Virus Leaves an Epigenetic Signature Post Cure of Infection by Direct-Acting Antivirals. PLoS Genet. 2019, 15, e1008181. [Google Scholar] [CrossRef]
- Hamdane, N.; Jühling, F.; Crouchet, E.; El Saghire, H.; Thumann, C.; Oudot, M.A.; Bandiera, S.; Saviano, A.; Ponsolles, C.; Roca Suarez, A.A.; et al. HCV-Induced Epigenetic Changes Associated with Liver Cancer Risk Persist After Sustained Virologic Response. Gastroenterology 2019, 156, 2313–2329. [Google Scholar] [CrossRef] [PubMed]

| Virus | Characteristics | Oncogenic Findings | Attributable Cancer Cases |
|---|---|---|---|
| EBV | Enveloped and linear DNA. Tropism: Epithelium and B cell. | Oncoprotein LMP1 induces proliferation and resistance to apoptosis. Encodes virally microRNAs. | 55% of Burkitt’s lymphoma, 50% of Hodgkin’s lymphoma and 84% of Nasopharyngeal carcinoma [1]. |
| HBV | Enveloped and circular partial DNA. Tropism: Hepatocytes. | Chronic inflammation, Tissue injury, oncoprotein HxB induces oxidative DNA damage, metastasis, and proliferation pathways. Insertion of viral genome into host DNA. | 55% Hepatocellular carcinoma [1]. |
| HCV | Enveloped and positive-sense RNA. Tropism: Hepatocytes. | Chronic inflammation, tissue injury, NS5A induce ER stress, Core protein induce steatosis and insulin resistance. | 21% of Hepatocellular carcinoma and 3% Non-Hodgkin lymphomas [1]. |
| HPV | Naked and circular DNA. Tropism: Stratified squamous epithelium | Viral genome insertion into host DNA, Oncoproteins E6 and E7 manipulate cell cycle and inhibit apoptosis, E5 induce proliferation pathways. | 100% of Cervix, 30% of Oropharynx, 53% of Penile, 77% of Vaginal and 25%of Vulvar [1]. |
| HTLV-1 | Enveloped and positive-sense RNA. Tropism: T and B cells | Oncoprotein Tax promotes viral replication and activate proliferation, senescence, and genomic instability pathways. | 100% of T-cell leukemia and Lymphoma [1]. |
| KSHV | Enveloped and linear DNA. Tropism: Oropharyngeal epithelium | Encodes viral interleukins and chemokines which promotes proliferation and angiogenesis. Oncoprotein K1 induce cell transformation. LANA protein inhibits cell cycle checkpoints. | 100% of Kaposi’s sarcoma [1] |
| MCPyV | Naked and circular DNA. Tropism: Skin | T-antigen induce cell proliferation and cell transformation. Insertion of viral genome into host DNA | 80% of Merkel cell carcinoma [5]. |
| Methylation Status | Type Genes | Genes | Molecular Mechanisms involved | Ref. |
|---|---|---|---|---|
| Hypermethylated | Tumor suppressor | RASL1, EGLN3, CSMD1, CDKN2A, BCORL1, SFRP1, P73, ZNF382, RUNX3, LOX, RB1 | Proliferation, tumor growth inhibition, cell cycle regulation, apoptosis, EMT. | [51,53,54,55,56,57,58] |
| Hypomethylated | Oncogenic | OTX2, IGF1R, SNCG, ZBTB16, FOXA1, HNF4A, CEBPA | Increasing fibrosis, preneoplastic alterations, EMT, apoptosis inhibition, lipid metabolism, proliferation, inflammation, cell motility. | [51,59,60,61,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heredia-Torres, T.G.; Rincón-Sánchez, A.R.; Lozano-Sepúlveda, S.A.; Galan-Huerta, K.; Arellanos-Soto, D.; García-Hernández, M.; Garza-Juarez, A.d.J.; Rivas-Estilla, A.M. Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis. Viruses 2022, 14, 2762. https://doi.org/10.3390/v14122762
Heredia-Torres TG, Rincón-Sánchez AR, Lozano-Sepúlveda SA, Galan-Huerta K, Arellanos-Soto D, García-Hernández M, Garza-Juarez AdJ, Rivas-Estilla AM. Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis. Viruses. 2022; 14(12):2762. https://doi.org/10.3390/v14122762
Chicago/Turabian StyleHeredia-Torres, Tania Guadalupe, Ana Rosa Rincón-Sánchez, Sonia Amelia Lozano-Sepúlveda, Kame Galan-Huerta, Daniel Arellanos-Soto, Marisela García-Hernández, Aurora de Jesús Garza-Juarez, and Ana María Rivas-Estilla. 2022. "Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis" Viruses 14, no. 12: 2762. https://doi.org/10.3390/v14122762
APA StyleHeredia-Torres, T. G., Rincón-Sánchez, A. R., Lozano-Sepúlveda, S. A., Galan-Huerta, K., Arellanos-Soto, D., García-Hernández, M., Garza-Juarez, A. d. J., & Rivas-Estilla, A. M. (2022). Unraveling the Molecular Mechanisms Involved in HCV-Induced Carcinogenesis. Viruses, 14(12), 2762. https://doi.org/10.3390/v14122762






