The Role of Bacterial and Fungal Superinfection in Critical COVID-19
Abstract
1. Introduction
Aims
2. Methods
- (1)
- Contamination.
- (2)
- Blood stream infection (BSI), including catheter-related blood stream infection (CRBSI) and IC (invasive candidiasis).
- (3)
- Bacterial pneumonia, subdivided into community-acquired pneumonia (CAP), hospital-acquired pneumonia (HAP) and ventilator-associated pneumonia (VAP).
- (4)
- COVID-19-associated pulmonary aspergillosis (CAPA), subdivided into highly likely and likely CAPA.
2.1. Blood Stream Infections
- A colony count of microbes grown from blood obtained through the catheter hub;
- is at least 3-fold greater than the colony count from blood obtained from a peripheral vein
- OR
- Growth in microbes from a blood sample drawn from a catheter hub is detected at least 2 h before microbial growth in a blood sample obtained from a peripheral vein
2.2. Bacterial Pneumonia
- 1.
- Radiological
- New or worsening infiltrates on Chest X-Rax or CT Thorax.
- 2.
- Clinical
- Temperature > 38 °C without other cause.and/or
- Leukopenia (<4000 WBC/mm3) or leucocytosis (>12,000 WBC/mm3)and at least one of the following:
- New onset of purulent sputum or change in characteristics.
- Suggestive auscultation.
- Worsening gas exchange.
- 3.
- Microbiological
- Positive culture of sputum, tracheal aspirates or bronchoalveolar lavage (BAL) with a threshold ≥104 CFU/mL.Or
- Positive qualitative result in RT-PCR of tracheal aspirates or bronchoalveolar lavage (BAL).
2.3. CAPA
2.4. Statistical Analysis
3. Results
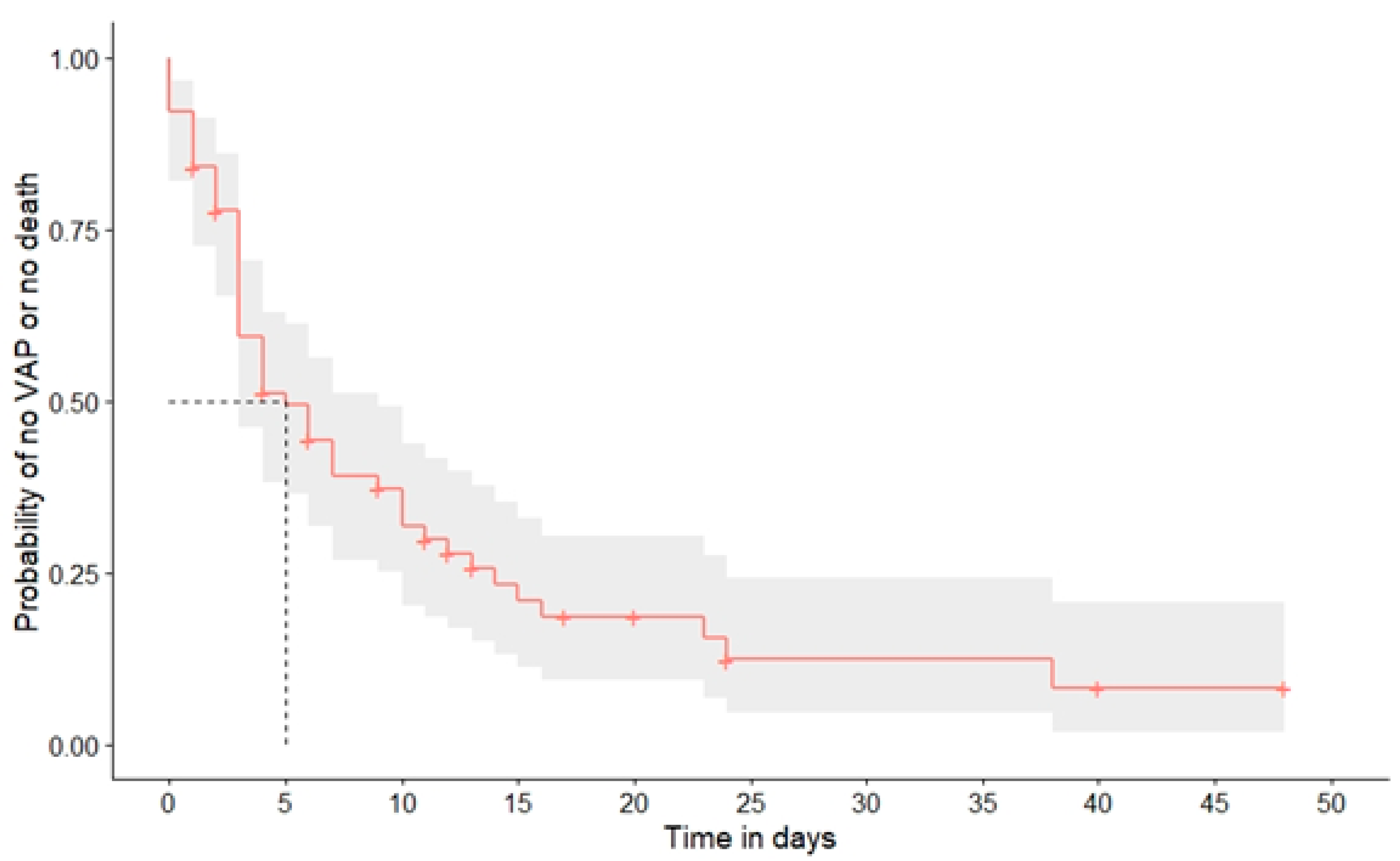
3.1. Rate of Superinfections
3.1.1. Bacterial Pneumonia
3.1.2. CAPA
3.1.3. Blood-Stream Infections
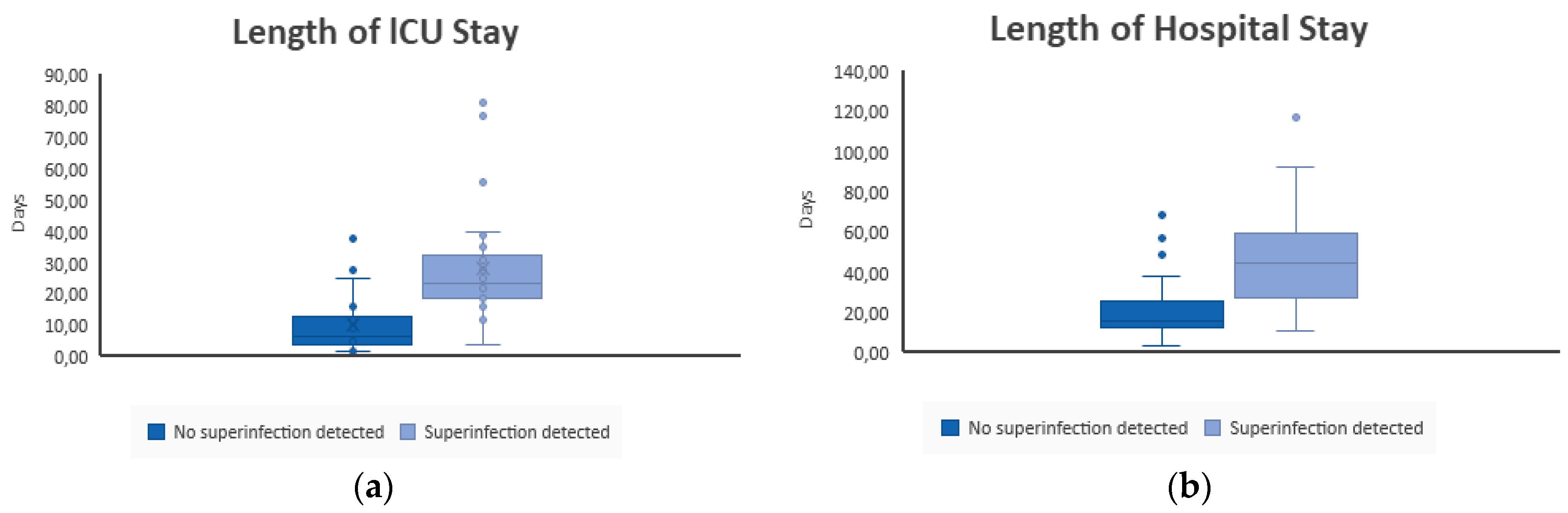
3.1.4. Influence of Superinfections on Clinical Outcome
3.1.5. Risk Factors of Superinfection
3.1.6. Blood-Stream Infections
3.1.7. Bacterial Pneumonia
3.1.8. CAPA
4. Discussion
4.1. Fungal Infections
4.2. Bacterial Pneumonia
4.3. Pathogenesis of Bacterial and Fungal Superinfection
4.4. Risk Factors for the Development of Superinfection
4.5. Limitations and Strength
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ripa, M.; Galli, L.; Poli, A.; Oltolini, C.; Spagnuolo, V.; Mastrangelo, A.; Muccini, C.; Monti, G.; De Luca, G.; Landoni, G.; et al. Secondary infections in patients hospitalized with COVID-19: Incidence and predictive factors. Clin. Microbiol. Infect. 2021, 27, 451–457. [Google Scholar] [CrossRef]
- Sang, L.; Xi, Y.; Lin, Z.; Pan, Y.; Song, B.; Li, C.A.; Zheng, X.; Zhong, M.; Jiang, L.; Pan, C.; et al. Secondary infection in severe and critical COVID-19 patients in China: A multicenter retrospective study. Ann. Palliat Med. 2021, 10, 8557–8570. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Pickens, C.O.; Gao, C.A.; Cuttica, M.J.; Smith, S.B.; Pesce, L.L.; Grant, R.A.; Kang, M.; Morales-Nebreda, L.; Bavishi, A.A.; Arnold, J.M.; et al. Bacterial Superinfection Pneumonia in Patients Mechanically Ventilated for COVID-19 Pneumonia. Am. J. Respir. Crit. Care Med. 2021, 204, 921–932. [Google Scholar] [CrossRef]
- Bendala Estrada, A.D.; Calderon Parra, J.; Fernandez Carracedo, E.; Muino Miguez, A.; Ramos Martinez, A.; Munez Rubio, E.; Rubio-Rivas, M.; Agudo, P.; Arnalich Fernandez, F.; Estrada Perez, V.; et al. Inadequate use of antibiotics in the covid-19 era: Effectiveness of antibiotic therapy. BMC Infect. Dis. 2021, 21, 1–23. [Google Scholar] [CrossRef]
- Jordana-Lluch, E.; Rivaya, B.; Marcó, C.; Giménez, M.; Quesada, M.D.; Escobedo, A.; Batlle, M.; Martró, E.; Ausina, V. Molecular diagnosis of bloodstream infections in onco-haematology patients with PCR/ESI-MS technology. J. Infect. 2017, 74, 187–194. [Google Scholar] [CrossRef]
- Roudbary, M.; Kumar, S.; Kumar, A.; Černáková, L.; Nikoomanesh, F.; Rodrigues, C.F. Overview on the Prevalence of Fungal Infections, Immune Response, and Microbiome Role in COVID-19 Patients. J. Fungi 2021, 7, 720. [Google Scholar] [CrossRef]
- Nucci, M.; Barreiros, G.; Guimarães, L.F.; Deriquehem, V.A.S.; Castiñeiras, A.C.; Nouér, S.A. Increased incidence of candidemia in a tertiary care hospital with the COVID-19 pandemic. Mycoses 2021, 64, 152–156. [Google Scholar] [CrossRef]
- Lai, C.C.; Yu, W.L. COVID-19 associated with pulmonary aspergillosis: A literature review. J. Microbiol. Immunol. Infect. 2021, 54, 46–53. [Google Scholar] [CrossRef]
- Pierce, J.; Stevens, M.P. COVID-19 and antimicrobial stewardship: Lessons learned, best practices, and future implications. Int. J. Infect. Dis. 2021, 113, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Tortorano, A.M.; Peman, J.; Bernhardt, H.; Klingspor, L.; Kibbler, C.C.; Faure, O.; Biraghi, E.; Canton, E.; Zimmermann, K.; Seaton, S.; et al. Epidemiology of candidaemia in Europe: Results of 28-month European Confederation of Medical Mycology (ECMM) hospital-based surveillance study. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Musuuza, J.S.; Watson, L.; Parmasad, V.; Putman-Buehler, N.; Christensen, L.; Safdar, N. Prevalence and outcomes of co-infection and superinfection with SARS-CoV-2 and other pathogens: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0251170. [Google Scholar] [CrossRef]
- T2MR Technology|T2 Biosystems, n.d. Available online: https://www.t2biosystems.com/products-technology/t2mr-technology/ (accessed on 11 December 2022).
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. IDSA Guidelines for Intravascular Catheter-Related Infection Clinical Practice Guidelines for the Diagnosis and Management of Intravascular Catheter-Related Infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef]
- Guidelines for the management of adults with hospital-acquired, ventilator-associated, and healthcare-associated pneumonia. Am. J. Respir. Crit. Care Med. 2005, 171, 388–416. [CrossRef]
- Armstrong-James, D.; Youngs, J.; Bicanic, T.; Abdolrasouli, A.; Denning, D.W.; Johnson, E.; Mehra, V.; Pagliuca, T.; Patel, B.; Rhodes, J.; et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis. Eur. Respir. J. 2020, 56, 2002554. [Google Scholar] [CrossRef]
- Grasselli, G.; Scaravilli, V.; Mangioni, D.; Scudeller, L.; Alagna, L.; Bartoletti, M.; Bellani, G.; Biagioni, E.; Bonfanti, P.; Bottino, N.; et al. Hospital-Acquired Infections in Critically Ill Patients With COVID-19. Chest 2021, 160, 454–465. [Google Scholar] [CrossRef]
- Falcone, M.; Tiseo, G.; Giordano, C.; Leonildi, A.; Menichini, M.; Vecchione, A.; Pistello, M.; Guarracino, F.; Ghiadoni, L.; Forfori, F.; et al. Predictors of hospital-acquired bacterial and fungal superinfections in COVID-19: A prospective observational study. J. Antimicrob. Chemother. 2021, 76, 1078–1084. [Google Scholar] [CrossRef]
- Boulos, M.; Bassal, T.; Layyous, A.; Basheer, M.; Assy, N. Inflammation in COVID-19: A Risk for Superinfections. COVID 2022, 2, 1609–1624. [Google Scholar] [CrossRef]
- Kayaaslan, B.; Eser, F.; Kaya Kalem, A.; Bilgic, Z.; Asilturk, D.; Hasanoglu, I.; Ayhan, M.; Tezer Tekce, Y.; Erdem, D.; Turan, S.; et al. Characteristics of candidemia in COVID-19 patients; increased incidence, earlier occurrence and higher mortality rates compared to non-COVID-19 patients. Mycoses 2021, 64, 1083–1091. [Google Scholar] [CrossRef] [PubMed]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The Landscape of Candidemia During the Coronavirus Disease 2019 (COVID-19) Pandemic. Clin. Infect. Dis. 2022, 74, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Omrani, A.S.; Koleri, J.; Ben Abid, F.; Daghfel, J.; Odaippurath, T.; Peediyakkal, M.Z.; Baiou, A.; Sarsak, E.; Elayana, M.; Kaleeckal, A.; et al. Clinical characteristics and risk factors for COVID-19-associated Candidemia. Med. Mycol. 2021, 59, 1262–1266. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef]
- Hartl, B.; Zeller, I.; Manhart, A.; Selitsch, B.; Lass-Flörl, C.; Willinger, B. A Retrospective Assessment of Four Antigen Assays for the Detection of Invasive Candidiasis Among High-Risk Hospitalized Patients. Mycopathologia 2018, 183, 513. [Google Scholar] [CrossRef]
- Mylonakis, E.; Zacharioudakis, I.M.; Clancy, C.J.; Hong Nguyen, M.; Pappas, P.G. Efficacy of T2 Magnetic Resonance Assay in Monitoring Candidemia after Initiation of Antifungal Therapy: The Serial Therapeutic and Antifungal Monitoring Protocol (STAMP) Trial. J. Clin. Microbiol. 2018, 56, e01756-17. [Google Scholar] [CrossRef]
- Seitz, T.; Holbik, J.; Hind, J.; Gibas, G.; Karolyi, M.; Pawelka, E.; Traugott, M.; Wenisch, C.; Zoufaly, A. Rapid Detection of Bacterial and Fungal Pathogens Using the T2MR versus Blood Culture in Patients with Severe COVID-19. Microbiol. Spectr. 2022, 10, e00140-22. [Google Scholar] [CrossRef]
- Ceccarelli, M.; Marino, A.; Pulvirenti, S.; Coco, V.; Busà, B.; Nunnari, G.; Cacopardo, B.S. Bacterial and Fungal Co-Infections and Superinfections in a Cohort of COVID-19 Patients: Real-Life Data from an Italian Third Level Hospital. Infect. Dis. Rep. 2022, 14, 372–382. [Google Scholar] [CrossRef]
- Russo, A.; Olivadese, V.; Trecarichi, E.M.; Torti, C. Bacterial Ventilator-Associated Pneumonia in COVID-19 Patients: Data from the Second and Third Waves of the Pandemic. J. Clin. Med. 2022, 11, 2279. [Google Scholar] [CrossRef]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Patil, H.V.; Patil, V.C. Incidence, bacteriology, and clinical outcome of ventilator-associated pneumonia at tertiary care hospital. J. Nat. Sci. Biol. Med. 2017, 8, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Liang, G.; Liu, W. Fungal Co-infections Associated with Global COVID-19 Pandemic: A Clinical and Diagnostic Perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., III; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Cao, Q.; Qin, L.E.; Wang, X.; Cheng, Z.; Pan, A.; Dai, J.; Sun, Q.; Zhao, F.; Qu, J.; et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19):A multi-center study in Wenzhou city, Zhejiang, China. J. Infect. 2020, 80, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Netea, M.G.; Giamarellos-Bourboulis, E.J.; Domínguez-Andrés, J.; Curtis, N.; van Crevel, R.; van de Veerdonk, F.L.; Bonten, M. Trained Immunity: A Tool for Reducing Susceptibility to and the Severity of SARS-CoV-2 Infection. Cell 2020, 181, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Stanzani, M.; Vianelli, N.; Cavo, M.; Kontoyiannis, D.P.; Lewis, R.E. Development and internal validation of a model for predicting 60-day risk of invasive mould disease in patients with haematological malignancies. J. Infect. 2019, 78, 484–490. [Google Scholar] [CrossRef]
- Lamoth, F.; Lewis, R.E.; Walsh, T.J.; Kontoyiannis, D.P. Navigating the Uncertainties of COVID-19–Associated Aspergillosis: A Comparison With Influenza-Associated Aspergillosis. J. Infect. Dis. 2021, 224, 1631–1640. [Google Scholar] [CrossRef]
- Soltani, S.; Zakeri, A.; Zandi, M.; Kesheh, M.M.; Tabibzadeh, A.; Dastranj, M.; Faramarzi, S.; Didehdar, M.; Hafezi, H.; Hosseini, P.; et al. The Role of Bacterial and Fungal Human Respiratory Microbiota in COVID-19 Patients. Biomed Res. Int. 2021, 2021, 6670798. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Mantovani, A.; Byrne, C.D.; Zheng, M.H.; Targher, G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: A meta-analysis of observational studies. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 1236–1248. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.A.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Gold, M.S.; Sehayek, D.; Gabrielli, S.; Zhang, X.; McCusker, C.; Ben-Shoshan, M. COVID-19 and Comorbidities: A Systematic Review and Meta-Analysis. Postgrad. Med. 2020, 132, 749–755. [Google Scholar] [CrossRef] [PubMed]
| Basis Parameters | |
|---|---|
| Mean age (years ± SD) | 57.2 (±11.9) |
| Female sex (%) | 45 (38.5%) |
| Co-morbidities | |
| Arterial hypertension | 81 (58.3%) |
| Obesity (BMI 30–40) | 57 (41%) |
| Severe obesity (BMI > 40) | 18 (12.9%) |
| Diabetes mellitus II | 48 (35.3%) |
| Chronic lung disease | 27 (19.4%) |
| Hypo/hyperthyroidism | 24 (17.3%) |
| Chronic arterial disease | 19 (13.7%) |
| Chronic renal failure | 9 (6.5%) |
| Chronic heart failure | 7 (5%) |
| Active cancer | 5 (3.6%) |
| Immunosuppression | 4 (2.9%) |
| Days between symptom onset and ICU admission (± SD) | 9.88 (± 6.9) |
| Therapy | |
| Immunomodulating | |
| Dexamethason | 117 (100%) |
| Tocilizumab | 12 (8.6%) |
| Asunercept * | 1 (0.7%) |
| Antiviral therapy | |
| Remdesivir | 51 (43.6%) |
| Camostat | 3 (2.6%) |
| Lopinavir/Ritonavir | 3 (2.6%) |
| Chloroquin/hydroxychloroquin | 1 (0.9%) |
| Parenteral nutrition | 100 (73.5%) |
| Outcome Parameters | |
|---|---|
| Invasive ventilation (%) | 69 (59%) |
| Length of invasive ventilation (days ± SD) | 15 (±10.4) |
| Tracheotomy (%) | 34 (24.5%) |
| ECMO support (%) | 11 (7.9%) |
| Central venous catheter (%) | 109 (80.7%) |
| Catecholamine support (%) | 86 (63.2%) |
| Continuous renal replacement therapy (%) | 10 (7.2%) |
| Length of ICU stay (days ± SD) | 27.3 (±16.14) |
| Length of hospital stay (days ± SD) | 45.6 (±23.23) |
| 28-day mortality (%) | 25 (21.4%) |
| Clinical status at day 28 after ICU admission | |
| Discharged | 48 (41.03%) |
| Normal ward | 21 (17.95%) |
| Still at ICU | 23 (19.66%) |
| Dead | 25 (21.4%) |
| HAP (n = 5) | VAP (n = 45) | CAPA (n = 9) | |
|---|---|---|---|
| Median time since detection (+/−SD) in days | |||
| Since symptom onset of COVID-19 infection | 15 (10.14) | 18 (10.69) | 21 (10.64) |
| Since hospital admission | 7 (7.4) | 8.5 (8.05) | 13.69 (8.67) |
| Since ICU admission | 5 (4.5) | 5 (4.75) | 10.6 (5.6) |
| Since intubation | N/A | 5.5 (4.24) | 8.15 (6.57) |
| Detected pathogens | 17.7% S. aureus 15.6% H. influenzae 11.1% K. pneumoniae 8.8% P. aeruginosa 6.7% S. maltophilia 4.4% E. coli 2.2% M. catarrhalis 33.5% polymicrobial | ||
| 25% MRSA | 44.4% A. fumigatus | ||
| 25% P. aeruginosa | 11.1% A. niger | ||
| 50% polymicrobial | 44.4% unknown |
| All BSI (n = 19) | CRBSI (n = 9) | IC (n = 7) | |
|---|---|---|---|
| Median time since detection (+/−SD) in days | |||
| Since symptom onset | 19 (6.33) | 21 (5.62) | 27 (4.61) |
| Since hospital admission | 12 (7.16) | 13 (4.8) | 14 (4.95) |
| Since ICU admission | 9 (5.41) | 10 (4.57) | 10 (6.87) |
| Detected pathogens | 21% C. albicans | 33.3% S. aureus 22.2% C. albicans 22.2% S. epidermidis 11.1% E. faecium 11.1% polymicrobial | 85.7% C. albicans |
| 21% S. aureus | |||
| 16% E. faecium | |||
| 10.5% E. faecalis | 14.3% C. parapsilosis | ||
| 10.5% S. epidermidis | |||
| 12% polymicrobial |
| BSI Diagnosed (n = 19) | BSI Not Diagnosed (n = 98) | p-Value | IC Diagnosed (n = 7) | IC Not Diagnosed (n = 110) | p-Value | |
|---|---|---|---|---|---|---|
| 28-day mortality | ||||||
| 36.8% | 18.4% | p = 0.121 | 57.1% | 19.1% | p = 0.037 | |
| Total length of stay at ICU (days) | ||||||
| Mean | 28.42 | 16.03 | p = 0.046 | 28.67 | 17.40 | ** |
| Median | 27.50 | 10.50 | 32.00 | 12.00 | ||
| SD | 18.49 | 15.27 | 9.45 | 16.33 | ||
| Min-Max | 4–77 | 2–81 | 18–36 | 2–81 | ||
| Total length of stay at hospital (days) | ||||||
| Mean | 46.00 | 29.52 | p = 0.019 | 46.00 | 31.29 | ** |
| Median | 45.00 | 22.00 | 46.00 | 24.00 | ||
| SD | 16.26 | 22.81 | 8.49 | 22.84 | ||
| Min-Max | 11–68 | 4–117 | 40–52 | 4–117 | ||
| VAP Diagnosed (n = 25) | VAP Not Diagnosed (n = 44) | p-Value | CAPA Highly Likely (n = 5) | CAPA Likely (n = 4) | CAPA Not Diagnosed (n = 60) | p-Value | |
|---|---|---|---|---|---|---|---|
| 28-day mortality | |||||||
| 27.3% | 32% | p = 0.784 | 20% | 25% | 31.7% | ** | |
| Total length of stay at ICU (days) | |||||||
| Mean | 27.86 | 26.25 | p = 0.765 | 43.50 | 30.50 | 26.08 | ** |
| Median | 23.00 | 22.50 | 43.50 | 29.50 | 22.50 | ||
| SD | 15.41 | 17.82 | 17.68 | 13.38 | 16.22 | ||
| Min-Max | 12–81 | 7–77 | 31–56 | 19–44 | 7–81 | ||
| Total length of stay at hospital (days) | |||||||
| Mean | 48.73 | 38.70 | 90.00 | 42.25 | 42.69 | ||
| Median | 47.50 | 39.50 | 90.00 | 36.50 | 42.50 | ||
| SD | 24.74 | 18.87 | 38.18 | 19.65 | 19.92 | ||
| Min-Max | 18–117 | 13–69 | 63–117 | 27–69 | 13–93 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seitz, T.; Holbik, J.; Grieb, A.; Karolyi, M.; Hind, J.; Gibas, G.; Neuhold, S.; Zoufaly, A.; Wenisch, C. The Role of Bacterial and Fungal Superinfection in Critical COVID-19. Viruses 2022, 14, 2785. https://doi.org/10.3390/v14122785
Seitz T, Holbik J, Grieb A, Karolyi M, Hind J, Gibas G, Neuhold S, Zoufaly A, Wenisch C. The Role of Bacterial and Fungal Superinfection in Critical COVID-19. Viruses. 2022; 14(12):2785. https://doi.org/10.3390/v14122785
Chicago/Turabian StyleSeitz, Tamara, Johannes Holbik, Alexander Grieb, Mario Karolyi, Julian Hind, Georg Gibas, Stephanie Neuhold, Alexander Zoufaly, and Christoph Wenisch. 2022. "The Role of Bacterial and Fungal Superinfection in Critical COVID-19" Viruses 14, no. 12: 2785. https://doi.org/10.3390/v14122785
APA StyleSeitz, T., Holbik, J., Grieb, A., Karolyi, M., Hind, J., Gibas, G., Neuhold, S., Zoufaly, A., & Wenisch, C. (2022). The Role of Bacterial and Fungal Superinfection in Critical COVID-19. Viruses, 14(12), 2785. https://doi.org/10.3390/v14122785







