Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome
Abstract
:1. Introduction
1.1. Therapeutic Exercise
1.2. Post-COVID-19 Syndrome
1.3. Exercise and Post-COVID-19
2. Method
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
3. Results
3.1. Characteristics of the Participants and Interventions
3.2. Outcome Evaluation
3.2.1. Strength
3.2.2. Respiratory Function
3.2.3. Physical Capacity
3.2.4. Quality of Life
3.2.5. Other Biomarkers
4. Discussion
4.1. Strength
4.2. Respiratory Function
4.3. Fatigue and Physical Capacity
4.4. Quality of Life
4.5. Telemedicine
4.6. Considerations on Therapeutic Exercise
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fernández-Lázaro, D.; Garrosa, M. Identification, mechanism, and treatment of skin lesions in COVID-19: A review. Viruses 2021, 13, 1916. [Google Scholar] [CrossRef]
- COVID-19 Map—Johns Hopkins Coronavirus Resource Center. Available online: https://coronavirus.jhu.edu/map.html (accessed on 3 September 2022).
- Fernández-Lázaro, D.; Sánchez-Serrano, N.; Mielgo-Ayuso, J.; García-Hernández, J.L.; González-Bernal, J.J.; Seco-Calvo, J. Long COVID a new derivative in the chaos of SARS-CoV-2 infection: The emergent pandemic? J. Clin. Med. 2021, 10, 5799. [Google Scholar] [CrossRef] [PubMed]
- Rooney, S.; Webster, A.; Paul, L. Systematic Review of Changes and Recovery in Physical Function and Fitness after Severe Acute Respiratory Syndrome-Related Coronavirus Infection: Implications for COVID-19 Rehabilitation. Phys. Ther. 2020, 100, 1717–1729. [Google Scholar] [CrossRef]
- Patel, K.P.; Patel, P.A.; Vunnam, R.R.; Hewlett, A.T.; Jain, R.; Jing, R.; Vunnam, S.R. Gastrointestinal, hepatobiliary, and pancreatic manifestations of COVID-19. J. Clin. Virol. 2020, 128, 104386. [Google Scholar] [CrossRef]
- Boban, M. Novel coronavirus disease (COVID-19) update on epidemiology, pathogenicity, clinical course and treatments. Int. J. Clin. Pract. 2021, 75, e13868. [Google Scholar] [CrossRef]
- Whittaker, A.; Anson, M.; Harky, A. Neurological Manifestations of COVID-19: A systematic review and current update. Acta Neurol. Scand. 2020, 142, 14–22. [Google Scholar] [CrossRef]
- Agarwal, S.; Agarwal, S.K. Endocrine changes in SARS-CoV-2 patients and lessons from SARS-CoV. Postgrad. Med. J. 2020, 96, 412–416. [Google Scholar] [CrossRef]
- Behzad, S.; Aghaghazvini, L.; Radmard, A.R.; Gholamrezanezhad, A. Extrapulmonary manifestations of COVID-19: Radiologic and clinical overview. Clin. Imaging 2020, 66, 35–41. [Google Scholar] [CrossRef]
- Long, B.; Brady, W.J.; Koyfman, A.; Gottlieb, M. Cardiovascular complications in COVID-19. Am. J. Emerg. Med. 2020, 38, 1504–1507. [Google Scholar] [CrossRef]
- Ahmadian, E.; Hosseiniyan, S.M.; Soofiyani, S.R.; Abediazar, S.; Shoja, M.M.; Ardalan, M.; Vahed, S.Z. COVID-19 and kidney injury: Pathophysiology and molecular mechanisms. Rev. Med. Virol. 2021, 31, e2176. [Google Scholar] [CrossRef]
- Tanriverdi, A.; Savci, S.; Kahraman, B.O.; Ozpelit, E. Extrapulmonary features of post-COVID-19 patients: Muscle function, physical activity, mood, and sleep quality. Ir. J. Med. Sci. 2022, 191, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.S.; Magdum, M.; Novotny, J. COVID-19 impact on reproduction and fertility. JBRA Assist. Reprod. 2021, 25, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Jimeno-Almazán, A.; Pallarés, J.G.; Buendía-Romero, A.; Martínez-Cava, A.; Franco-López, F.; Sánchez-Alcaraz, B.J.; Bernal-Morel, E.; Courel-Ibánez, J. Post-COVID-19 Syndrome and the Potential Benefits of Exercise. Int. J. Environ. Res. Public Health 2021, 18, 5329. [Google Scholar] [CrossRef]
- Peramo-Álvarez, F.P.; López-Zúñiga, M.Á.; López-Ruz, M.Á. Medical sequels of COVID-19. Med. Clin. 2021, 157, 388–394. [Google Scholar] [CrossRef]
- Kamal, M.; Abo-Omirah, M.; Hussein, A.; Saeed, H. Assessment and characterisation of post-COVID-19 manifestations. Int. J. Clin. Pract. 2021, 75, e13746. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of post-acute COVID-19 in primary care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef]
- Belli, S.; Balbi, B.; Prince, I.; Cattaneo, D.; Masocco, F.; Zaccaria, S.; Bertalli, L.; Cattini, F.; Lomazzo, A.; Dal Negro, F.; et al. Low physical functioning and impaired performance of activities of daily life in COVID-19 patients who survived hospitalisation. Eur. Respir. J. 2020, 56, 2002096. [Google Scholar] [CrossRef]
- Kurtais, Y.; Füsun, B.; Özyemisçi, Ö.; Kutay, N.; Ünsal, S.; Sonel, B.; Sarikaya, S.; Sirzai, H.; Tekdemir, T.; Alemdaroglu, E.; et al. Pulmonary rehabilitation principles in SARS-CoV-2 infection (COVID-19): A guideline for the acute and subacute rehabilitation. Turk. J. Phys. Med. Rehab. 2020, 66, 104–120. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Lázaro, M.P.; Córdoba, A.; Caballero-García, A.; Fernández-Lázaro, C.I. Intradialytic physical exercise in chronic kidney disease: A systematic review of health outcomes. Arch. Med. Deporte 2020, 37, 419–429. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Hernández-Burgos, N.; Cobreros, R.; García-Lázaro, S. Evaluation of physical activity as a therapeutic adjuvant for patients with inflammatory bowel disease: A review. Investig. Clin. 2022, 63, 304–322. [Google Scholar] [CrossRef]
- Fernández-Lázaro, D.; Mielgo-Ayuso, J.; Caballero-García, A.; Córdoba, C.; Lázaro, M.P.; Fernández-Lázaro, C.I. Physical activity in oncology patients with breast cancer: Non-pharmacological sports-based medical therapy? Systematic review. Arch. Med. Deporte 2020, 37, 266–274. [Google Scholar]
- Arena, E.B.; Sáez, M.E.; Buenavista, T.C.S. Benefits of exercise in adults. Enfermería Comunitaria 2014, 2, 21–30. [Google Scholar]
- Varo, J.J.; Martínez, J.A.; Martínez-González, M.Á. Benefits of physical activity and risks of a sedentary lifestyle. Med. Clin. 2003, 121, 665–672. [Google Scholar]
- Mikkelsen, K.; Stojanovska, L.; Polenakovic, M.; Bosevski, M.; Apostolopoulos, V. Exercise and mental health. Maturitas 2017, 106, 48–56. [Google Scholar] [CrossRef]
- Bull, F.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. J. Sport Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Cordero, A.; Masiá, M.D.; Galve, E. Physical Exercise and Health. Rev. Española Cardiol. 2014, 67, 748–753. [Google Scholar] [CrossRef]
- Puiggneró, V.; Barbany, J.R. Effects of physical activity and training on the various expressions of immune defense mechanisms. Educ. Física Y Deportes 1995, 39, 111–120. [Google Scholar]
- Fragala, M.S.; Kraemer, W.J.; Denegar, C.R.; Maresh, C.M.; Mastro, A.M.; Volek, J.S. Neuroendocrine-immune interactions and responses to exercise. Sport Med. 2011, 41, 621–639. [Google Scholar] [CrossRef]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Elsevier Inc. 2015, 135, 355–380. [Google Scholar]
- Fernández-Lázaro, D.; González-Bernal, J.J.; Sánchez-Serrano, N.; Navascués, L.J.; Del Río, A.A.; Mielgo-Ayuso, J. Physical exercise as a multimodal tool for COVID-19: Could it be used as a preventive strategy? Int. J. Environ. Res. Public Health 2020, 17, 8496. [Google Scholar] [CrossRef]
- Vijayaraghava, A.; Radhika, K. Alteration of interferon Gamma (IFN-γ) in human plasma with graded physical activity. J. Clin. Diagnostic Res. 2014, 8, 13–15. [Google Scholar]
- Moldoveanu, A.I.; Shephard, R.J.; Shek, P.N. The cytokine response to physical activity and training. Sport Med. 2001, 31, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Fossati, C.; Torre, G.; Vasta, S.; Giombini, A.; Quaranta, F.; Papalia, R.; Pigozzi, F. Physical Exercise and Mental Health: The Routes of a Reciprocal Relation. Int. J. Environ. Res. Public Health 2021, 18, 12364. [Google Scholar] [CrossRef] [PubMed]
- Amorosi, M. Correlation between sport and depression. Psychiatr. Danub. 2014, 26, 208–210. [Google Scholar]
- World Health Organization. Coronavirus Disease (COVID-19): Post-COVID-19 Condition. Available online: https://www.who.int/es/news-room/questions-and-answers/item/coronavirus-disease-(covid-19)-post-covid-19-condition (accessed on 23 September 2022).
- Veronese, N.; Bonica, R.; Cotugno, S.; Tulone, O.; Camporeale, M.; Smith, L.; Trott, M.; Bruyere, O.; Mirarchi, L.; Rizzo, G.; et al. Interventions for Improving Long COVID-19 Symptomatology: A Systematic Review. Viruses 2022, 14, 1863. [Google Scholar] [CrossRef]
- Chen, P.; Mao, L.; Nassis, G.P.; Harmer, P.; Ainsworth, B.E.; Li, F. Coronavirus disease (COVID-19): The need to maintain regular physical activity while taking precautions. J. Sport Health Sci. 2020, 9, 103–104. [Google Scholar] [CrossRef]
- Postigo-Martin, P.; Cantarero-Villanueva, I.; Lista-Paz, A.; Castro-Martín, E.; Arroyo-Morales, M.; Seco-Calvo, J. A COVID-19 Rehabilitation Prospective Surveillance Model for Use by Physiotherapists. J. Clin. Med. 2021, 10, 1691. [Google Scholar] [CrossRef]
- Liu, K.; Zhang, W.; Yang, Y.; Zhang, J.; Li, Y.; Chen, Y. Respiratory rehabilitation in elderly patients with COVID-19: A randomized controlled study. Complement. Ther. Clin. Pract. 2020, 39, 101166. [Google Scholar] [CrossRef]
- Longobardi, I.; do Prado, D.M.L.; Goessler, K.F.; de Oliveira, G.N.; de Andrade, D.C.O.; Gualano, B.; Roschel, H. Benefits of Home-Based Exercise Training Following Critical SARS-CoV-2 Infection: A Case Report. Front. Sport Act. Living 2022, 3, 791703. [Google Scholar] [CrossRef]
- Mayer, K.P.; Steele, A.K.; Soper, M.K.; Branton, J.D.; Lusby, M.L.; Kalema, A.G.; Dupont-Versteegden, E.E.; Montgomery-Yates, A.A. Physical Therapy Management of an Individual With Post-COVID Symdrome: A Case Report. Phys. Ther. 2021, 101, pzab098. [Google Scholar] [CrossRef]
- McNarry, M.A.; Berg, R.M.G.; Shelley, J.; Hudson, J.; Saynor, Z.L.; Duckers, J.; Lewis, K.; Davies, G.A.; Mackintosh, K.A. Inspiratory Muscle Training Enhances Recovery Post COVID-19: A Randomised Controlled Trial. Eur. Respir. J. 2022, 60, 2103101. [Google Scholar] [CrossRef] [PubMed]
- Nambi, G.; Abdelbasset, W.K.; Alrawaili, S.M.; Elsayed, S.H.; Verma, A.; Vellaiyan, A.; Eid, M.M.; Aldhafian, O.R.; Nwihadh, N.B.; Saleh, A.K. Comparative effectiveness study of low versus high-intensity aerobic training with resistance training in community-dwelling older men with post-COVID 19 sarcopenia: A randomized controlled trial. Clin. Rehabil. 2022, 36, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Pancera, S.; Galeri, S.; Porta, R.; Pietta, I.; Bianchi, L.N.C.; Carrozza, M.C.; Villafañe, J.H. Feasibility and Efficacy of the Pulmonary Rehabilitation Program in a Rehabilitation Center: Case report of a young patients developing severe COVID-19 acute respiratory distress syndrome. J. Cardiopulm. Rehabil. Prev. 2020, 40, 205–208. [Google Scholar] [CrossRef]
- Piquet, V.; Luczak, C.; Seiler, F.; Monaury, J.; Martini, A.; Ward, A.B.; Gracies, J.M.; Motavasseli, D. Do Patients With COVID-19 Benefit from Rehabilitation? Functional Outcomes of the First 100 Patients in a COVID-19 Rehabilitation Unit. Arch. Phys. Med. Rehabil. 2021, 102, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Santos, S.; Flores, J.A. Musculoskeletal physiotherapy in physical sequelae of SARS-CoV-2 infection: A case report. Physiother. Res. Int. 2022, 27, e1938. [Google Scholar] [CrossRef] [PubMed]
- Udina, C.; Ars, J.; Morandi, A.; Vilaró, J.; Cáceres, C.; Inzitari, M. Rehabilitation in adult post-COVID-19 patients in post-acute care with Therapeutic Exercise. J. Frailty Aging 2021, 10, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Coats, A.J.S.; Morley, J.E.; Rosano, G.; Bernabei, R.; von Haehling, S.; Kalantar-Zadeh, K. Muscle wasting disease: A proposal for a new disease classification. J. Cachexia Sarcopenia Muscle 2014, 5, 1–3. [Google Scholar] [CrossRef]
- Levy, D.; Giannini, M.; Oulehri, W.; Riou, M.; Marcot, C.; Pizzimenti, M.; Debrut, L.; Charloux, A.; Geny, B.; Meyer, A. Long Term Follow-Up of Sarcopenia and Malnutrition after Hospitalization for COVID-19 in Conventional or Intensive Care Units. Nutrients 2022, 14, 912. [Google Scholar] [CrossRef]
- Chaabene, H.; Prieske, O.; Herz, M.; Moran, J.; Höhne, J.; Kliegl, R.; Ramirez-Campillo, R.; Behm, D.G.; Hortobágyi, T.; Granacher, U. Home-based exercise programmes improve physical fitness of healthy older adults: A PRISMA-compliant systematic review and meta-analysis with relevance for COVID-19. Ageing Res. Rev. 2021, 67, 101265. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Skeletal Muscle Damage in COVID-19: A Call for Action. Medicina 2021, 57, 372. [Google Scholar] [CrossRef]
- Tuzun, S.; Keles, A.; Okutan, D.; Yildiran, T.; Palamar, D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Jt. Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Malhotra, S.; Kumar, A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 2008, 86, 1113–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, O.; María, S.; Velázquez-Alva, M.; Cabrera-Rosales, M.F. Malnutrition in COVID-19 patients and loss of muscle mass. Med. Int. Mex. 2020, 36 (Suppl. 4), 14–17. [Google Scholar]
- Johnsen, S.; Sattler, S.M.; Miskowiak, K.W.; Kunalan, K.; Victor, A.; Pedersen, L.; Andreassen, H.F.; Jorgensen, B.J.; Heeboll, H.; Andersen, M.B.; et al. Descriptive analysis of long COVID sequelae identified in a multidisciplinary clinic serving hospitalised and non-hospitalised patients. ERJ Open Res. 2021, 7, 00205. [Google Scholar] [CrossRef]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients without Previous Disabilities Recovering from COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef]
- Rodriguez, P. Clinical Guide for Long COVID/Persistent COVID Patient Care. Spanish Society of Rheumatology. 2021. Available online: https://policycommons.net/artifacts/1692997/guia-clinica-para-la-atencion-al-paciente-long-covidcovid-persistente/2424645/ (accessed on 26 September 2022).
- Fernández-Lázaro, D.; Garrosa, E.; Seco-Calvo, J.; Garrosa, M. Potential Satellite Cell-Linked Biomarkers in Aging Skeletal Muscle Tissue: Proteomics and Proteogenomics to Monitor Sarcopenia. Proteomes 2022, 10, 29. [Google Scholar] [CrossRef]
- Song, Y.H.; Song, J.L.; Delafontaine, P.; Godard, M.P. The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol. Metab. 2013, 24, 310–319. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Chen, C.M.; Huang, W.C.; Chiang, S.L.; Hsieh, P.C.; Lin, K.L.; Chen, Y.J.; Fu, T.C.; Huang, S.C.; Chen, S.Y.; et al. Rehabilitation programs for patients with COronaVIrus Disease 2019: Consensus statements of Taiwan Academy of Cardiovascular and Pulmonary Rehabilitation. J. Formos. Med. Assoc. 2021, 120, 83–92. [Google Scholar] [CrossRef]
- Swaminathan, N.; Jiandani, M.; Surendran, P.J.; Jacob, P.; Bhise, A.; Baxi, G.; Devani, P.; Agarwal, B.; Kumar, V.S.; Pinto, N.M.; et al. Beyond COVID-19: Evidence-Based Consensus Statement on the Role of Physiotherapy in Pulmonary Rehabilitation in the Indian Context. J. Assoc. Physicians India 2020, 68, 82–89. [Google Scholar]
- Nunes, R.; Da Luz, C.; Rezende, M.; Yassuyuki, G.; Dionir, G.; Severin, R.; Faghy, M.A.; Arena, R.; Borghi-Silva, A. Cardiorespiratory and skeletal muscle damage due to COVID-19: Making the urgent case for rehabilitation. Expert Rev. Respir. Med. 2021, 15, 1107–1120. [Google Scholar]
- Shi, Z.; De Vries, H.J.; Vlaar, A.P.J.; Van Der Hoeven, J.; Boon, R.A.; Heunks, L.M.A.; Ottenheijm, C.A.C. Diaphragm Pathology in Critically Ill Patients with COVID-19 and Postmortem Findings from 3 Medical Centers. JAMA Intern. Med. 2021, 181, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Jian, W.; Su, Z.; Chen, M.; Peng, H.; Peng, P.; Lei, C.; Chen, R.; Zhong, N.; Li, S. Abnormal pulmonary function in COVID-19 patients at time of hospital discharge. Eur. Respir. J. 2020, 55, 2001217. [Google Scholar] [CrossRef] [PubMed]
- Barbagelata, L.; Masson, W.; Iglesias, D.; Lillo, E.; Migone, J.F.; Orazi, M.L.; Furcada, J.M. Cardiopulmonary Exercise Testing in Patients with Post-COVID-19 Syndrome. Med. Clin. 2022, 159, 6–11. [Google Scholar] [CrossRef]
- Imamura, M.; Mirisola, A.R.; Ribeiro, F.Q.; de Pretto, L.R.; Alfieri, F.M.; Delgado, V.R.; Battistella, L.R. Rehabilitation of patients after COVID-19 recovery: An experience at the Physical and Rehabilitation Medicine Institute and Lucy Montoro Rehabilitation Institute. Clinics 2021, 76, e2804. [Google Scholar] [CrossRef]
- Moreno, J.E.; Pinzón-Ríos, I.D.; Rodríguez, L.C.; Reyes, M.M.; Torres, J.I. Respiratory physiotherapy in the functionality of the COVID-19 patient. Arch. Med. 2021, 21, 266–278. [Google Scholar]
- Fernández-Lázaro, D.; Gallego-Gallego, D.; Corchete, L.A.; Fernández, D.; González-Bernal, J.J.; García, B.; Mielgo-Ayuso, J. Inspiratory Muscle Training Program Using the PowerBreath®: Does It Have Ergogenic Potential for Respiratory and/or Athletic Performance? A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 6703. [Google Scholar] [CrossRef]
- Curci, C.; Pisano, F.; Bonacci, E.; Camozzi, D.M.; Ceravolo, C.; Bergonzi, R.; De Franceschi, S.; Moro, P.; Guarnieri, R.; Ferrillo, M.; et al. Early rehabilitation in post-acute COVID-19 patients: Data from an Italian COVID-19 Rehabilitation Unit and proposal of a treatment protocol. Eur. J. Phys. Rehabil. Med. 2020, 56, 633–641. [Google Scholar] [CrossRef]
- Ortelli, P.; Ferrazzoli, D.; Sebastianelli, L.; Engl, M.; Romanello, R.; Nardone, R.; Bonini, I.; Koch, G.; Saltuari, L.; Quaetarone, A.; et al. Neuropsychological and neurophysiological correlates of fatigue in post-acute patients with neurological manifestations of COVID-19: Insights into a challenging symptom. J. Neurol. Sci. 2021, 420, 117271. [Google Scholar] [CrossRef]
- Van Herck, M.; Goërtz, Y.M.J.; Houben-Wilke, S.; Machado, F.V.C.; Meys, R.; Delbressine, J.M.; Vaes, A.W.; Burtin, C.; Posthuma, R.; Franssen, F.M.E.; et al. Severe Fatigue in Long COVID: Web-Based Quantitative Follow-up Study in Members of Online Long COVID Support Groups. J. Med. Internet Res. 2021, 23, e30274. [Google Scholar] [CrossRef]
- Daher, A.; Balfanz, P.; Cornelissen, C.; Müller, A.; Bergs, I.; Marx, N.; Müller-Wieland, D.; Hartmann, B.; Dreher, M.; Müller, T. Follow up of patients with severe coronavirus disease 2019 (COVID-19): Pulmonary and extrapulmonary disease sequelae. Respir. Med. 2020, 174, 106197. [Google Scholar] [CrossRef] [PubMed]
- Boutou, A.K.; Asimakos, A.; Kortianou, E.; Vogiatzis, I.; Tzouvelekis, A. Long COVID-19 Pulmonary Sequelae and Management Considerations. J. Pers. Med. 2021, 11, 838. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.J.; Barradell, A.C.; Greening, N.J.; Bolton, C.; Jenkins, G.; Preston, L.; Hurst, J.R. British Thoracic Society survey of rehabilitation to support recovery of the post-COVID-19 population. BJM Open 2020, 10, e040213. [Google Scholar] [CrossRef] [PubMed]
- Regional Sports Medicine Center of Castilla y León. Against COVID Prescribes Physical Exercise. 2022. Available online: http://www.saludcastillayleon.es/AulaPacientes/es/videos-aula-pacientes/programa-ejercicio-fisicopersonas-enfermedad-cronica (accessed on 26 September 2022).
- Dixit, S.; Borghi-Silva, A.; Bairapareddy, K.C. Revisiting pulmonary rehabilitation during COVID-19 pandemic: A narrative review. Rev. Cardiovasc. Med. 2021, 22, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Iannaccone, S.; Castellazzi, P.; Tettamanti, A.; Houdayer, E.; Brugliera, L.; de Blasio, F.; Cimino, P.; Ripa, M.; Meloni, C.; Alemanno, F.; et al. Role of Rehabilitation Department for Adult Individuals with COVID-19: The Experience of the San Raffaele Hospital of Milan. Arch. Phys. Med. Rehabil. 2020, 101, 1656–1661. [Google Scholar] [CrossRef]
- Aparisi, Á.; Ybarra-falcón, C.; García-Gómez, M.; Tobar, J.; Iglesias-Echeverría, C.; Jaurrieta-Largo, S.; Ladrón, R.; Uribarri, A.; Catalá, P.; Hinojosa, W.; et al. Exercise Ventilatory Inefficiency in Post-COVID-19 Syndrome: Insights from a Prospective Evaluation. J. Clin. Med. 2021, 10, 2591. [Google Scholar] [CrossRef]
- da Silva, A.G.; Pereira, A.C.; Schneider, B.M.; Caserta, R.A.; Gomes, C.; Kenji, R. Telerehabilitation improves physical function and reduces dyspnoea in people with COVID-19 and post-COVID-19 conditions: A systematic review. J. Physiothrerapy 2022, 68, 90–98. [Google Scholar]
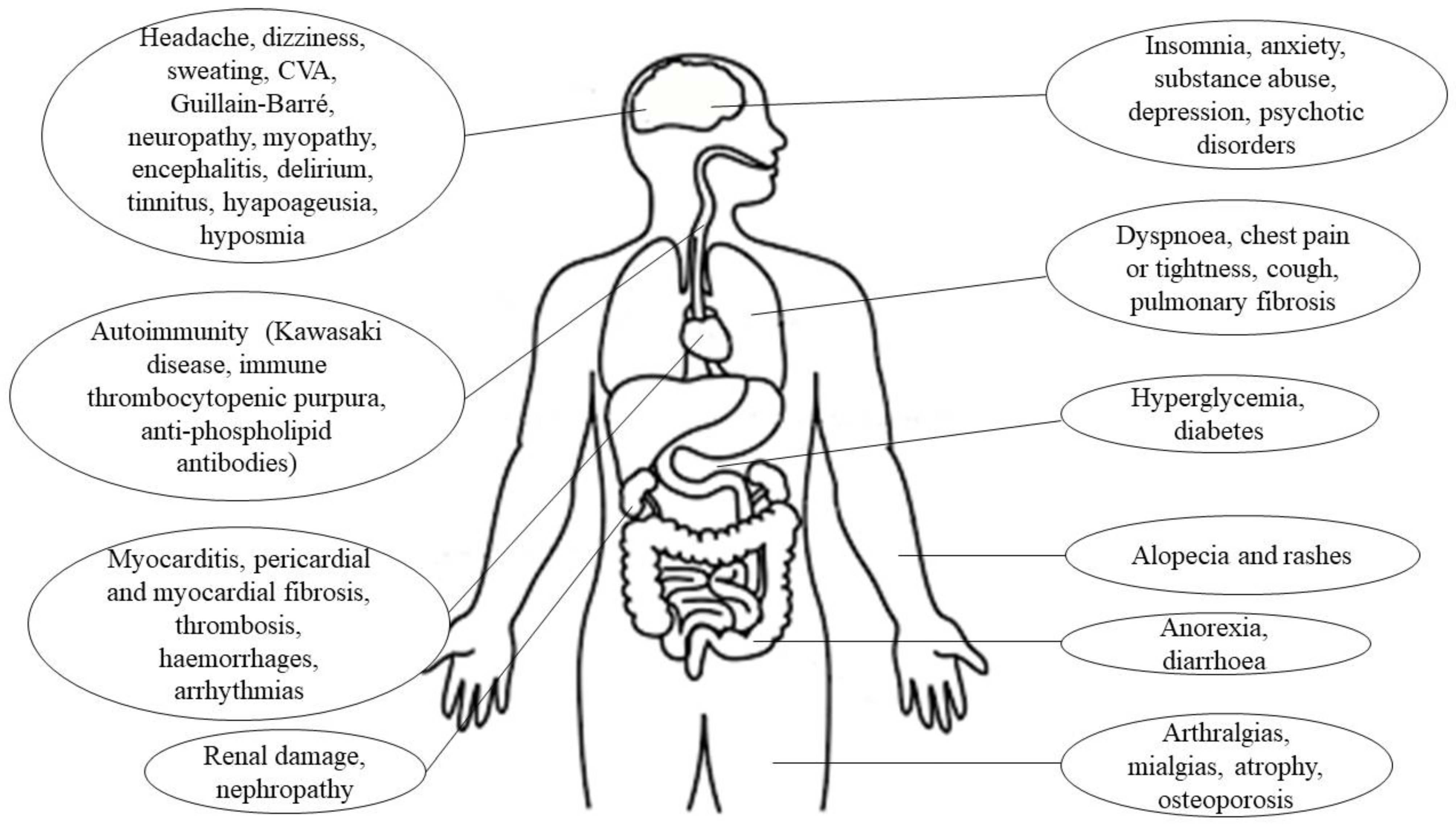



| Location | Clinical Manifestations |
|---|---|
| Respiratory System [6] | Cough, sore throat, dyspnea, pneumonia, bilateral interstitial inflammation, acute respiratory distress syndrome, rhinorrhea |
| Central nervous system [7] | Stroke, meningitis, encephalitis, headache, dizziness, ataxia, convulsions, confusion, hallucinations |
| Peripheral nervous system [7] | Hypoageusia, hiposmia/anosmia, neuralgia, Guillain–Barré syndrome, chemosensory dysfunction, hyporeflexia, stiffness |
| Endocrine system [8] | Hyperglycemia, ketoacidosis, adrenal insufficiency, thyrotoxicosis |
| Cardiovascular system [9,10] | Myocarditis, cardiac failure, acute myocardial infarction, cardiomyopathy, shock, arrhythmias, pulmonary thromboembolism, coagulation disorders, hypertension, palpitations |
| Digestive system [5,9] | Anorexia, nausea, vomiting, diarrhea, abdominal and epigastric pain, hepatic and pancreatic pathology |
| Excretory system [9,11] | Acute renal damage, tubular necrosis, nephropathy, proteinuria, hematuria |
| Locomotor system [12] | Rhabdomyolysis, mialgias, generalized weakness, fatigue, arthralgias, decreased bone density, osteonecrosis |
| Immune system [11] | Fever, lymphopenia, decreased CD4 and CD8, IL-10 and TNF- α, increased pro-inflamatory cytokines |
| Lymphatic system [9] | Mediastinal lymphadenopathy |
| Reproductive system [13] | Orchitis, scrotal discomfort, scrotal pain, infertility |
| Integumentary system [1] | Vesicular rash, maculopapular rash, urticarial rash, petechiae, acral lesions, livedoid lesions |
| First Author, Year of Publication and Country | Design | Participants (Size and Characteristics of the Initial Sample) |
|---|---|---|
| Liu et al. [40], 2020, China | Controlled randomized clinical trial | n: 72 Moderate COVID-19 (hospitalization) CG: n: 36; 25 ♂, 11 ♀ Age (mean ± SD): 68.9 ± 7.6 y BMI (mean ± SD): 22.9 ± 3.9 kg/m2 IG: n: 36; 24 ♂, 12 ♀ Age (mean ± SD): 69.4 ± 8.0 y BMI (mean ± SD): 23.1 ± 3.5 kg/m2 Post COVID-19 ≥ 6 months |
| Longobardi et al. [41], 2022, Brasil | Case report | n: 1 ♀ Critical COVID-19 (71 days of hospitalization, with 49 days in ICU and invasive mechanical ventilation) Age: 67 y BMI: 27.1 kg/m2 Post COVID-19 ≥ 3 months |
| Mayer et al. [42], 2021, USA | Case report | n: 1 ♀ Mild COVID-19 (No hospitalization and no oxygen therapy) Age: 37 y Post COVID-19 ≥ 6 wk |
| McNarry et al. [43], 2022, United Kingdom | Controlled randomized clinical trial | n: 148 Mild COVID-19 (dyspnea) CG: n: 37; 2 ♂, 35 ♀ Age (mean ± SD): 46.13 ± 12.73 y BMI (mean ± SD): 27.81 ± 5.83 kg/m2 Post COVID-19 (mean ± SD): 9.00 ± 3.67 months IG: n: 111; 16 ♂, 95 ♀ Age (mean ± SD): 46.76 ± 12.03 y BMI (mean ± SD): 27.64 ± 6.80 kg/m2 Post COVID-19 (mean ± SD): 9.04 ± 4.29 months |
| Nambi et al. [44], 2022, Egypt | Controlled randomized clinical trial | n: 76 Mild COVID-19 LI: n: 38 ♂; 3 withdrawals Age (mean ± SD): 63.2 ± 3.1 y BMI (mean ± SD): 23.1 ± 1.6 kg/m2 HI: 38 ♂; 4 withdrawals Age (mean ± SD): 64.1 ± 3.2 y BMI (mean ± SD): 22.8 ± 1.1 kg/m2 Post COVID-19 Sarcopenia |
| Pancera et al. [45], 2020, Italy | Case report | n: 1 ♂ Severe COVID-19 with ARDS (hospitalization in ICU and invasive mechanical ventilation) Age: 51 y BMI: 17.5 kg/m2 Active COVID-19, 10 days post hospital admission |
| Piquet, et al. [46], 2021, France | Cohort study | n: 100; 66 ♂, 34 ♀ Moderate or severe COVID-19 Age (median ± interquartile range): 66 ± 22 y BMI (mean ± SD): 26.0 ± 5.4 kg/m2 Post COVID-19 (mean ± SD): 20.4 ± 10.0 days |
| Santos et al. [47], 2021, Peru | Case report | n: 1 ♀ Moderate COVID-19 (no hospitalization, weight loss 8 kg, limitation of ADL) Age: 60 y Post COVID-19 28 days |
| Udina et al. [48], 2021, Spain | Cohort study | n: 33 Moderate or severe COVID-19 ICU: n: 20; 10 ♂,10♀ Age (mean ± SD): 58.2 ± 7.9 y No ICU: n: 13; 9 ♂, 4 ♀ Age (mean ± SD): 78.4 ± 8.1 y Post COVID |
| First Author, Year of Publication and Country | Intervention | Outcomes | Results |
|---|---|---|---|
| Liu et al. [40], 2020, China | 2 sess/wk; 6 wk; 10 min/sess RMT (Threshold PEP): 3 set * 10 breaths (60% MEP) Cough: 3 sets, 10 active coughs Diaphragm training: 30 breaths, ballast 1–3 kg Stretching Home RMT training: 30 reps/day breaths and coughs | FEV1, FVC, FEV1/FVC, DLCO 6MWT SF-36 ADL: FIM Scale Anxiety and depression: SAS scale, SDS scale | Changes from baseline (IG) ↑* FEV1, FVC, FEV1/FVC, DLCO ↑* 6MWT ↑* SF-36 ↔ FIM ↓* SAS ↓ SDS IG vs. CG ↑* FEV1, FVC, FEV1/FVC, DLCO ↑* 6MWT ↑* SF-36 ↔ FIM ↓* SAS ↓ SDS |
| Longobardi et al. [41], 2022, Brasil | 3 sess/wk; 10 wk Aerobic training: 20–45 min walk, Borg Scale [9,10,11,12,13,14,15,16] Strength training: 6 exercises, 3–4 sets, 10–15 reps, Borg Scale [9,10,11,12,13,14,15,16] Stretching | Handgrip test, 30-STS Modified Balke treadmill exercise protocol, Time-up and go FSS | Change from baseline ↑Handgrip test ↑ 30-STS ↑Balke protocol ↑ Time-up and go ↓ FSS |
| Mayer et al. [42] 2021, USA | 2 sess/wk; 8 wk; 40–80 min/sess Aerobic training: 15–45 min, RPE [4,5,6] Strength training: 10–20 min, 10–15 rep, RPE [5,6] RMT: diaphragmatic reeducation | MRC-sum score, handgrip test, lower-extremity unilateral leg press MRC dyspnea scale Time-up and go, 6MWT Eq-5D-5L | Change from baseline ↑ MRC-sum score, hand-grip test, leg press ↓ MRC dyspnea scale ↑ Time-up and go ↑ 6MWT ↑ Eq-5D-5L |
| McNarry et al. [43], 2022, United Kingdom | 3 sess/wk; 8 wk; 20 min/sess RMT: 6 sets * 6 breaths (80% SMIP) | MIP, SMIP FITr, TDI Chester step Test Fitness K-BILD Daily activity: wrist accelerometer Mental health: TSRQ | Change from baseline (IG) ↑* MIP, SMIP ↑*FITr, TDI ↑* Chester step ↑*K-BILD ↑ Daily activity ↔ TSRQ IG vs. CG ↑* MIP, SMIP ↑FITr, ↑* TDI ↑ Chester step ↑* K-BILD ↑ Daily activity ↔ TSRQ |
| Nambi et al. [44], 2022, Egypt | 4 sess/wk; 8 wk Aerobic training: 30 min LI (40–60% HR max); HI (60–80% HR max) Strength training: 3 sets/group, 10 reps, 10 RM Stretching and diaphragmatic breathing: 15 min at the beginning and at the end of the training session | Handgrip test Muscle mass: Magnetic resonance SarQoL Kinesiophobia: Tampa Scale | Change from baseline (LI) ↑ Handgrip test ↑ Muscle mass ↑SarQoL ↓Kinesiophobia Change from baseline (HI) ↑ Handgrip test ↑ Muscle mass ↑SarQoL ↓Kinesiophobia LI vs. HI ↑* Handgrip test ↔ Muscle mass ↑* SarQoL ↓* Kinesiophobia |
| Pancera et al. [45], 2020, Italy | 1 sess/day; 25 days; 20–45 min/ sess Aerobic training: cycloergometer lower/upper extremity; 20–30 min Strength training: 3 set, 8–10 reps, 50–70% 1 RM RMT (Threshold PEP): 20 min, 10 cm H2O Neuromuscular electrical stimulation: 30 min quadriceps, 15–20 mA | MIP, MEP MRC-sum score BMI, quadriceps circumference SPPB BID EuroQoL ADL: BI | Change form baseline ↑ MIP, MEP ↑ MRC-sum score ↑ BMI, quadriceps circumference ↑ SPPB ↓ BID ↓EuroQoL ↑ BI |
| Piquet et al. [46], 2021, France | 2 sess/day; 5 days/wk; 20 min/sess Submaximal aerobic training: cycloergometer Strength training: strengthening with body weight exercises (sit-to-stand, tiptoe stands, squats), elastics, and weights, 3 sets, 10 reps for each exercise RMT: controlled diaphragmatic breathing, with work on the inspiratory and expiratory times | Handgrip test: left/right hand Strength upper extremity ADL: BI | Change from baseline ↑* Handgrip test left hand ↑*Handgrip test right hand ↑* Strength upper extremity ↑* BI |
| Santos et al. [47], 2021, Peru | 3 sess/wk; 5 wk; 11–75 min/sess Aerobic training: Coordination/balance exercises Strength training: resisted strength TENS CDTM Kinesitherapy: Passive/Active Stretching Manual therapy: Maitland Concept | Daniels strength test Balance: Unipodal Station Pain test: numeric pain scale Mobility: ROM | Change from baseline ↑ Daniel’s test ↑Unipodal Station test ↓ Numeric pain scale ↑ ROM |
| Udina et al. [48], 2021, Spain | 7 sess/wk; 10 days, 30 min/sess Aerobic training: cycloergometer, stairs and walking, 5–15 min, Modified Borg Scale [3,4,5] Strength training: 2–4 exercises, 2 sets, 10 reps, 30–80% 1 RM Balance: 2 exercises (static and dynamic) | Strength lower extremity SPPB 6MWT Walking speed FAC Balance ADL: BI | Change from baseline (ICU) ↑* Strength lower extremity ↑* SPPB ↑* 6MWT ↑* Walking speed ↑* FAC ↑* Balance ↑* BI Change from baseline (no-ICU) ↑* Strength lower extremity ↑* SPPB ↑* 6MWT ↑* Walking speed ↑* FAC ↑* Balance ↑* BI ICU vs. No-ICU ↑ Strength lower extremity ↑* SPPB ↑ 6MWT ↑ Walking speed ↔ FAC ↑ Balance ↔ BI |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Lázaro, D.; Santamaría, G.; Sánchez-Serrano, N.; Lantarón Caeiro, E.; Seco-Calvo, J. Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome. Viruses 2022, 14, 2797. https://doi.org/10.3390/v14122797
Fernández-Lázaro D, Santamaría G, Sánchez-Serrano N, Lantarón Caeiro E, Seco-Calvo J. Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome. Viruses. 2022; 14(12):2797. https://doi.org/10.3390/v14122797
Chicago/Turabian StyleFernández-Lázaro, Diego, Gema Santamaría, Nerea Sánchez-Serrano, Eva Lantarón Caeiro, and Jesús Seco-Calvo. 2022. "Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome" Viruses 14, no. 12: 2797. https://doi.org/10.3390/v14122797
APA StyleFernández-Lázaro, D., Santamaría, G., Sánchez-Serrano, N., Lantarón Caeiro, E., & Seco-Calvo, J. (2022). Efficacy of Therapeutic Exercise in Reversing Decreased Strength, Impaired Respiratory Function, Decreased Physical Fitness, and Decreased Quality of Life Caused by the Post-COVID-19 Syndrome. Viruses, 14(12), 2797. https://doi.org/10.3390/v14122797










