Molecular Epidemiology of Norovirus (NoV) Infection in Mie Prefecture: The Kinetics of Norovirus Antigenemia in Pediatric Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Norovirus Detection in Stool and Serum
2.3. Norovirus Genomic Amplification and Genotyping
2.4. Detection of Norovirus Antigen in Serum
2.5. Serum Antibody ELISA
2.6. Phylogenetic Analyses
2.7. Statistical Analyses
3. Results
3.1. Detection of Norovirus RNA and Antigen in Serum
Estimation of NoV-RNA
3.2. Clinical Features of NoV-Positive Cases
3.3. Responses Due to Pre-Existing NoV Antibodies
3.4. Correlation of Antigenemia with Acute Antibody Titers
3.5. Norovirus Genotypes and Phylogenetic Characteristics
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rha, B.; Lopman, B.A.; Alcala, A.N.; Riddle, M.S.; Porter, C.K. Incidence of Norovirus-Associated Medical Encounters among Active Duty United States Military Personnel and Their Dependents. PLoS ONE 2016, 11, e0148505. [Google Scholar] [CrossRef]
- Cannon, J.L.; Bonifacio, J.; Bucardo, F.; Buesa, J.; Bruggink, L.; Chan, M.C.; Fumian, T.M.; Giri, S.; Gonzalez, M.D.; Hewitt, J.; et al. Global Trends in Norovirus Genotype Distribution among Children with Acute Gastroenteritis. Emerg. Infect. Dis. 2021, 27, 1438–1445. [Google Scholar] [CrossRef]
- Glass, R.I.; Parashar, U.D.; Estes, M.K. Norovirus gastroenteritis. N. Engl. J. Med. 2009, 361, 1776–1785. [Google Scholar] [CrossRef]
- Lun, J.H.; Hewitt, J.; Yan, G.J.H.; Enosi Tuipulotu, D.; Rawlinson, W.D.; White, P.A. Recombinant GII.P16/GII.4 Sydney 2012 Was the Dominant Norovirus Identified in Australia and New Zealand in 2017. Viruses 2018, 10, 548. [Google Scholar] [CrossRef]
- Siebenga, J.J.; Vennema, H.; Zheng, D.P.; Vinje, J.; Lee, B.E.; Pang, X.L.; Ho, E.C.; Lim, W.; Choudekar, A.; Broor, S.; et al. Norovirus illness is a global problem: Emergence and spread of norovirus GII.4 variants, 2001–2007. J. Infect. Dis. 2009, 200, 802–812. [Google Scholar] [CrossRef]
- Mattner, F.; Sohr, D.; Heim, A.; Gastmeier, P.; Vennema, H.; Koopmans, M. Risk groups for clinical complications of norovirus infections: An outbreak investigation. Clin. Microbiol. Infect. 2006, 12, 69–74. [Google Scholar] [CrossRef]
- Matsui, S.M.; Greenberg, H.B. Immunity to calicivirus infection. J. Infect. Dis. 2000, 181 (Suppl. S2), S331–S335. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Matson, D.O.; Ruiz-Palacios, G.M.; Hu, J.; Treanor, J.; Pickering, L.K. Expression, self-assembly, and antigenicity of a snow mountain agent-like calicivirus capsid protein. J. Clin. Microbiol. 1995, 33, 1452–1455. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Takeshita, S.; Nezu, A.; Aihara, Y.; Usuku, S.; Noguchi, Y.; Yokota, S. Norovirus-associated encephalopathy. Pediatr. Infect. Dis. J. 2006, 25, 651–652. [Google Scholar] [CrossRef]
- Desai, R.; Hembree, C.D.; Handel, A.; Matthews, J.E.; Dickey, B.W.; McDonald, S.; Hall, A.J.; Parashar, U.D.; Leon, J.S.; Lopman, B. Severe outcomes are associated with genogroup 2 genotype 4 norovirus outbreaks: A systematic literature review. Clin. Infect. Dis. 2012, 55, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.; Alansari, K.; Smatti, M.K.; Zaraket, H.; Al Thani, A.A.; Yassine, H.M. Epidemiological, Molecular, and Clinical Features of Norovirus Infections among Pediatric Patients in Qatar. Viruses 2019, 11, 400. [Google Scholar] [CrossRef] [PubMed]
- Long, S.S.; Prober, C.G.; Fisher, M. (Eds.) Principles and Practice of Pediatric Infectious Diseases, 5th ed.; Elsevier: Philadelphia, PA, USA, 2018; p. 1907. [Google Scholar]
- Takanashi, S.; Hashira, S.; Matsunaga, T.; Yoshida, A.; Shiota, T.; Tung, P.G.; Khamrin, P.; Okitsu, S.; Mizuguchi, M.; Igarashi, T.; et al. Detection, genetic characterization, and quantification of norovirus RNA from sera of children with gastroenteritis. J. Clin. Virol. 2009, 44, 161–163. [Google Scholar] [CrossRef]
- Barsoum, Z. Rotavirus and adenovirus detecting method: Sensitivity and specificity of rapid antigen testing: Prospective study in one region of Ireland. VirusDisease 2020, 31, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Nishio, T.; Yonetani, R.; Ito, E.; Yoneta, M.; Maruo, Y.; Yoshida, T.; Sugimoto, T. Development of rhabdomyolysis in a child after norovirus gastroenteritis. BMC Pediatr. 2016, 16, 176. [Google Scholar] [CrossRef]
- Huhti, L.; Hemming-Harlo, M.; Vesikari, T. Norovirus detection from sera of young children with acute norovirus gastroenteritis. J. Clin. Virol. 2016, 79, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Hansman, G.S.; Natori, K.; Shirato-Horikoshi, H.; Ogawa, S.; Oka, T.; Katayama, K.; Tanaka, T.; Miyoshi, T.; Sakae, K.; Kobayashi, S.; et al. Genetic and antigenic diversity among noroviruses. J. Gen. Virol. 2006, 87, 909–919. [Google Scholar] [CrossRef]
- Malm, M.; Uusi-Kerttula, H.; Vesikari, T.; Blazevic, V. High serum levels of norovirus genotype-specific blocking antibodies correlate with protection from infection in children. J. Infect. Dis. 2014, 210, 1755–1762. [Google Scholar] [CrossRef]
- Blazevic, V.; Malm, M.; Honkanen, H.; Knip, M.; Hyöty, H.; Vesikari, T. Development and maturation of norovirus antibodies in childhood. Microbes Infect. 2016, 18, 263–269. [Google Scholar] [CrossRef]
- Tamura, K.; Peterson, D.; Peterson, N.; Stecher, G.; Nei, M.; Kumar, S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011, 28, 2731–2739. [Google Scholar] [CrossRef] [PubMed]
- Ramani, S.; Paul, A.; Saravanabavan, A.; Menon, V.K.; Arumugam, R.; Sowmyanarayanan, T.V.; Samuel, P.; Kang, G. Rotavirus antigenemia in Indian children with rotavirus gastroenteritis and asymptomatic infections. Clin. Infect. Dis. 2010, 51, 1284–1289. [Google Scholar] [CrossRef]
- Hemming, M.; Huhti, L.; Räsänen, S.; Salminen, M.; Vesikari, T. Rotavirus antigenemia in children is associated with more severe clinical manifestations of acute gastroenteritis. Pediatr. Infect. Dis. J. 2014, 33, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Justino, M.C.A.; Campos, E.A.; Mascarenhas, J.D.P.; Soares, L.S.; Guerra, S.F.S.; Furlaneto, I.P.; Pavão, M.J.C., Jr.; Maciel, T.S.; Farias, F.P.; Bezerra, O.M.; et al. Rotavirus antigenemia as a common event among children hospitalised for severe, acute gastroenteritis in Belém, northern Brazil. BMC Pediatr. 2019, 19, 193. [Google Scholar] [CrossRef] [PubMed]
- Reymão, T.K.A.; Fumian, T.M.; Justino, M.C.A.; Hernandez, J.M.; Bandeira, R.S.; Lucena, M.S.S.; Teixeira, D.M.; Farias, F.P.; Silva, L.D.; Linhares, A.C.; et al. Norovirus RNA in serum associated with increased fecal viral load in children: Detection, quantification and molecular analysis. PLoS ONE 2018, 13, e0199763. [Google Scholar] [CrossRef]
- Ray, P.; Fenaux, M.; Sharma, S.; Malik, J.; Subodh, S.; Bhatnagar, S.; Greenberg, H.; Glass, R.I.; Gentsch, J.; Bhan, M.K. Quantitative evaluation of rotaviral antigenemia in children with acute rotaviral diarrhea. J. Infect. Dis. 2006, 194, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Morozov, V.; Hanisch, F.G.; Wegner, K.M.; Schroten, H. Pandemic GII.4 Sydney and Epidemic GII.17 Kawasaki308 Noroviruses Display Distinct Specificities for Histo-Blood Group Antigens Leading to Different Transmission Vector Dynamics in Pacific Oysters. Front Microbiol. 2018, 9, 2826. [Google Scholar] [CrossRef]
- Siegrist, C.A.; Aspinall, R. B-cell responses to vaccination at the extremes of age. Nat. Rev. Immunol. 2009, 9, 185–194. [Google Scholar] [CrossRef]
- Nurminen, K.; Blazevic, V.; Huhti, L.; Rasanen, S.; Koho, T.; Hytonen, V.P.; Vesikari, T. Prevalence of norovirus GII-4 antibodies in Finnish children. J. Med. Virol. 2011, 83, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Kumazaki, M.; Usuku, S. Genetic Analysis of Norovirus GII.4 Variant Strains Detected in Outbreaks of Gastroenteritis in Yokohama, Japan, from the 2006–2007 to the 2013–2014 Seasons. PLoS ONE 2015, 10, e0142568. [Google Scholar] [CrossRef]
- Wang, Y.; Hao, L.; Pan, L.; Xue, C.; Liu, Q.; Zhao, X.; Zhu, W. Age, primary symptoms, and genotype characteristics of norovirus outbreaks in Shanghai schools in 2017. Sci. Rep. 2018, 8, 15238. [Google Scholar] [CrossRef]
- Fu, J.; Bao, C.; Huo, X.; Hu, J.; Shi, C.; Lin, Q.; Zhang, J.; Ai, J.; Xing, Z. Increasing Recombinant Strains Emerged in Norovirus Outbreaks in Jiangsu, China: 2015–2018. Sci. Rep. 2019, 9, 20012. [Google Scholar] [CrossRef]
- Han, J.; Wu, X.; Chen, L.; Fu, Y.; Xu, D.; Zhang, P.; Ji, L. Emergence of norovirus GII.P16-GII.2 strains in patients with acute gastroenteritis in Huzhou, China, 2016–2017. BMC Infect. Dis. 2018, 18, 342. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.L.; Barclay, L.; Collins, N.R.; Wikswo, M.E.; Castro, C.J.; Magana, L.C.; Gregoricus, N.; Marine, R.L.; Chhabra, P.; Vinje, J. Genetic and Epidemiologic Trends of Norovirus Outbreaks in the United States from 2013 to 2016 Demonstrated Emergence of Novel GII.4 Recombinant Viruses. J. Clin. Microbiol. 2017, 55, 2208–2221. [Google Scholar] [CrossRef] [PubMed]
- Lindesmith, L.C.; Brewer-Jensen, P.D.; Mallory, M.L.; Debbink, K.; Swann, E.W.; Vinjé, J.; Baric, R.S. Antigenic Characterization of a Novel Recombinant GII.P16-GII.4 Sydney Norovirus Strain with Minor Sequence Variation Leading to Antibody Escape. J. Infect. Dis. 2018, 217, 1145–1152. [Google Scholar] [CrossRef] [PubMed]

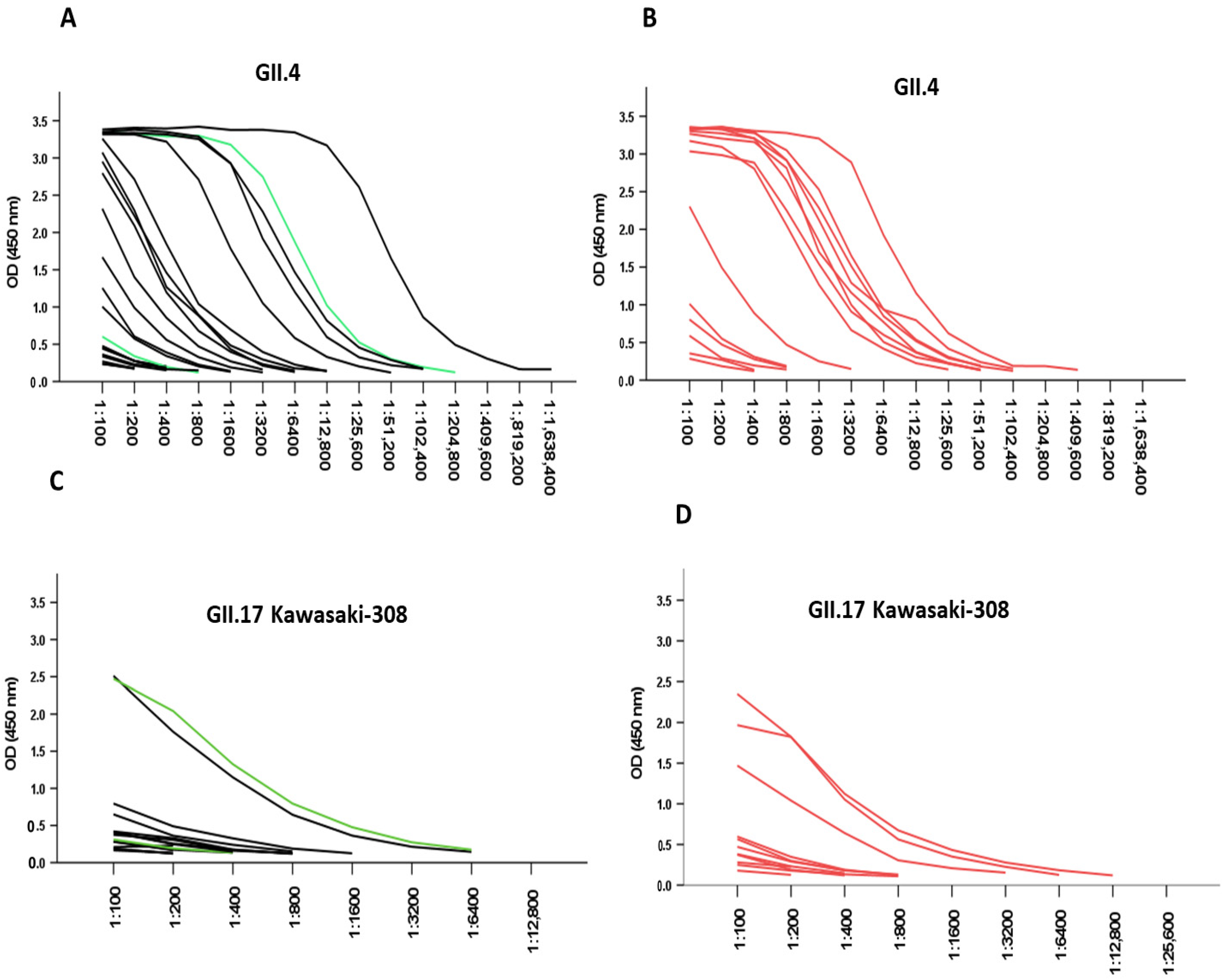
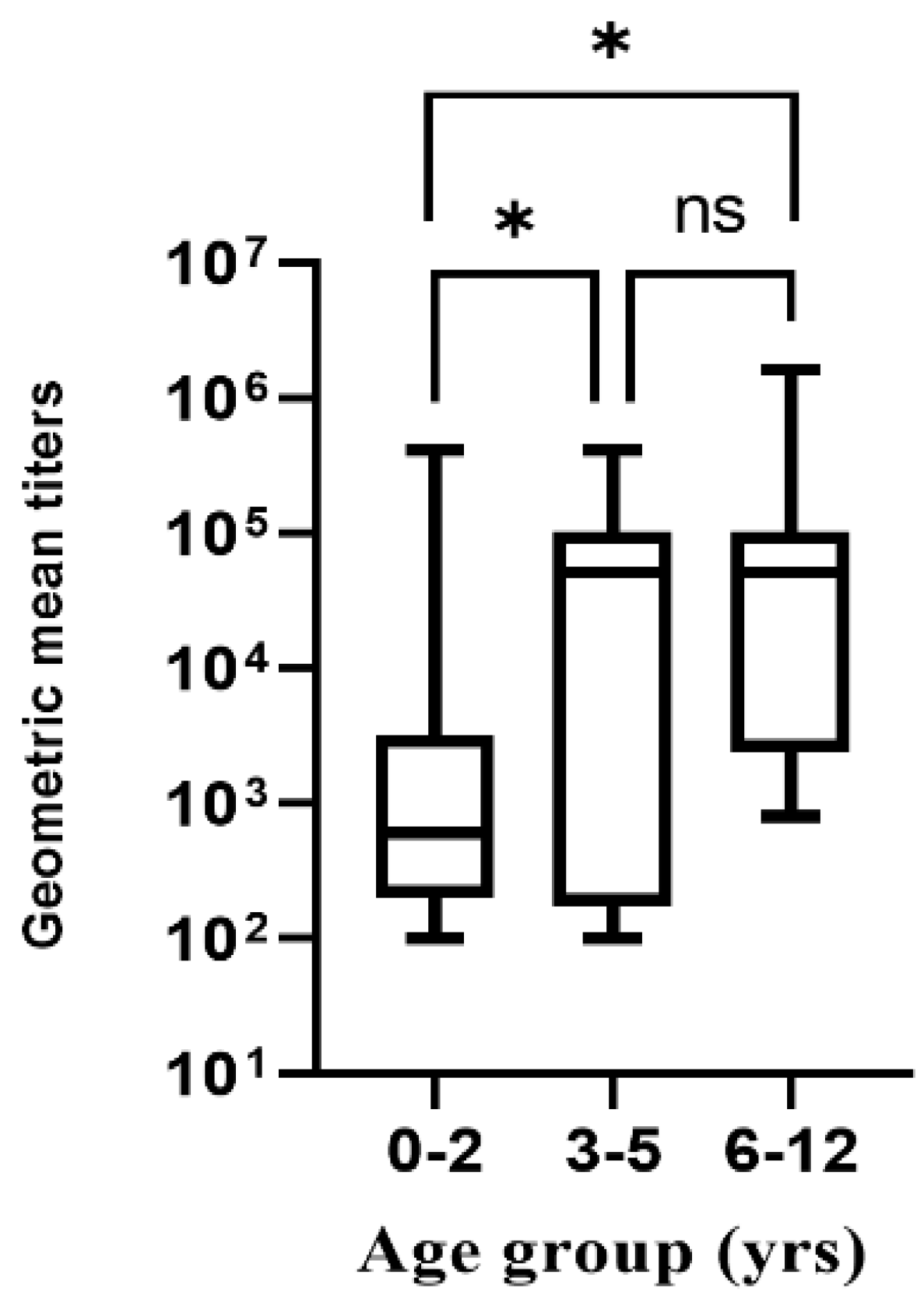
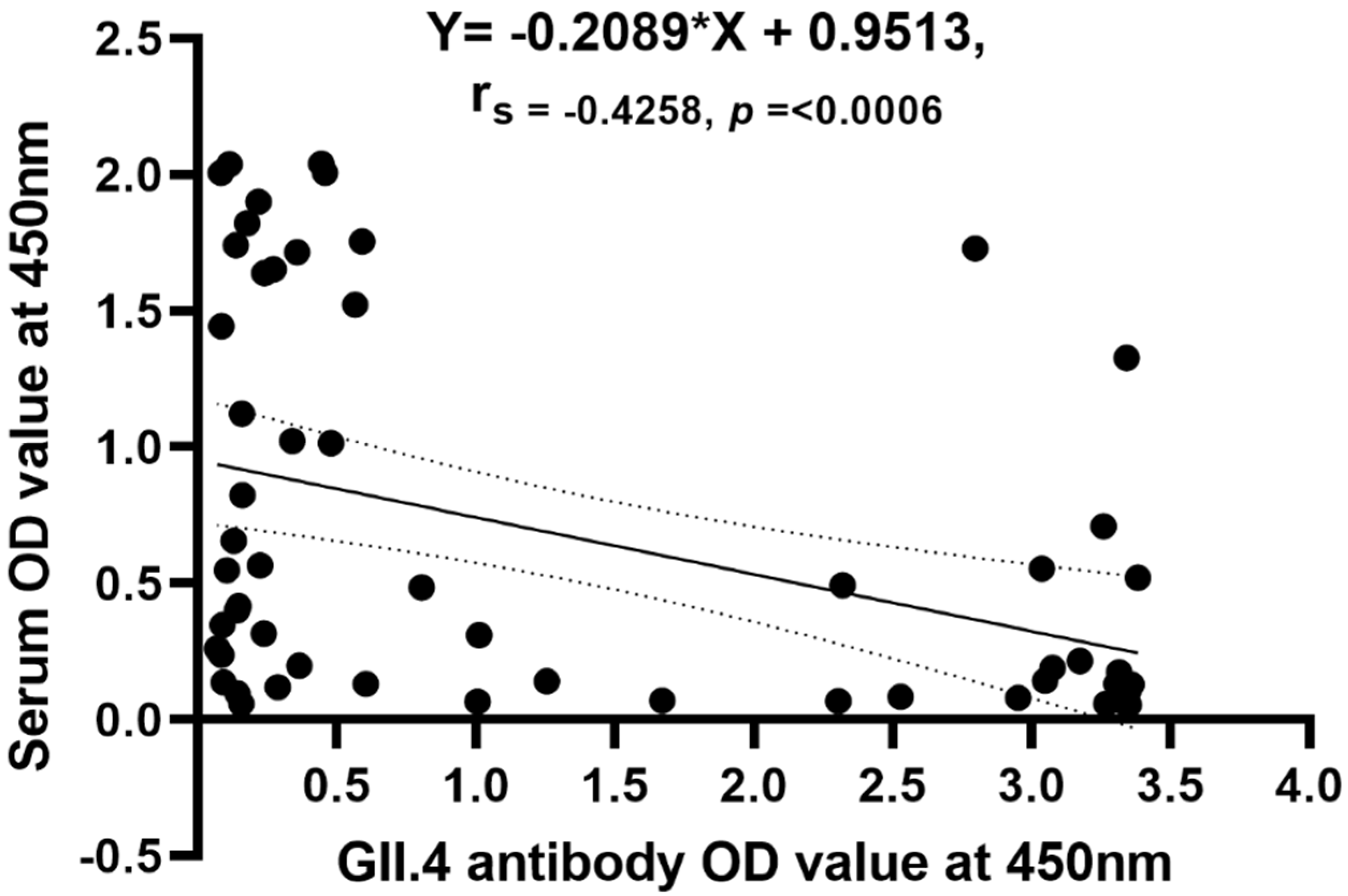
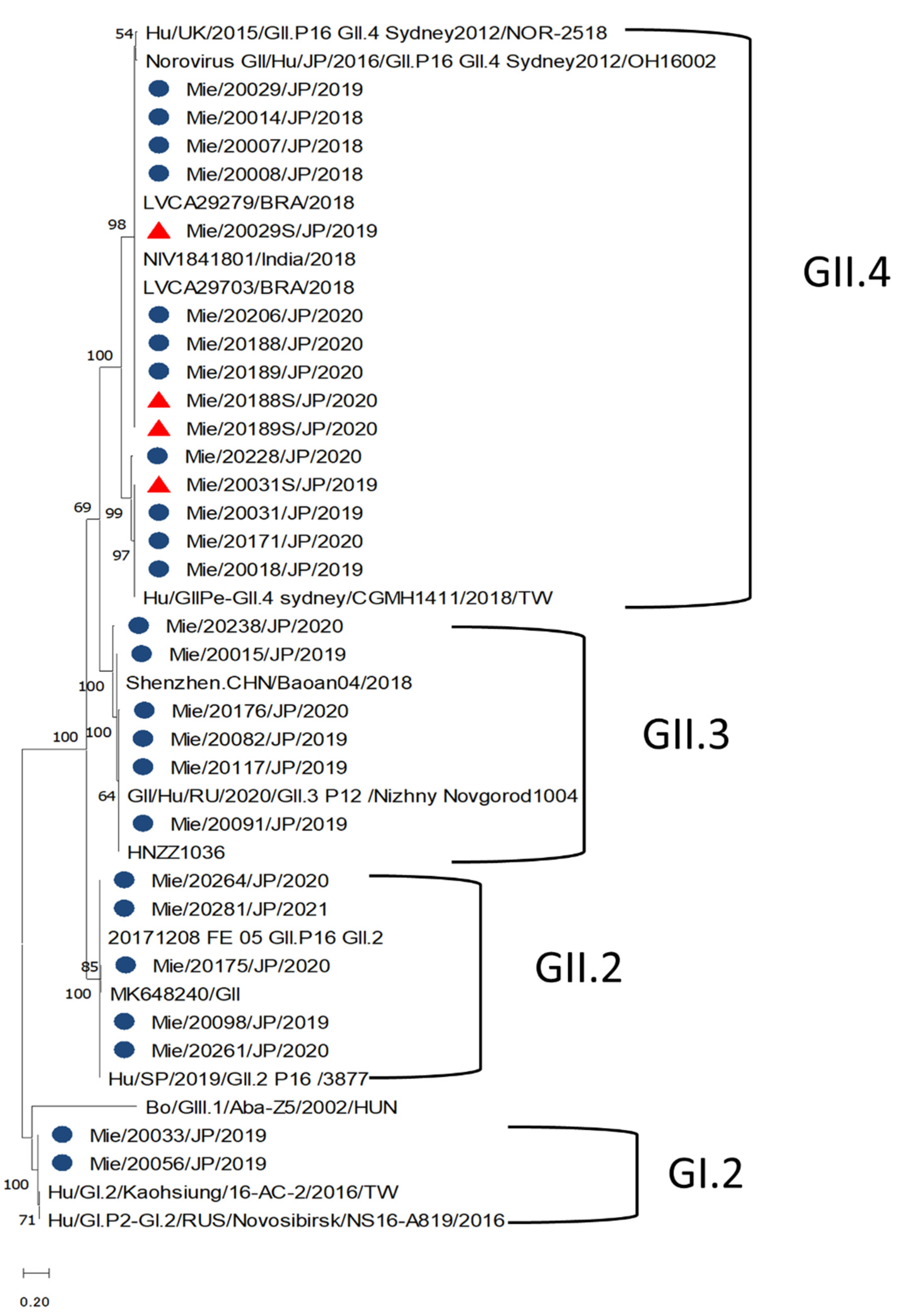
| Variable | NoV ELISA Positive Serum n = 34 | NoV ELISA Negative Serum n = 28 | p-Value |
|---|---|---|---|
| Age range, n (%) | <0.001 | ||
| 0−2 | 28 (82) | 9 (32) | |
| 3−5 | 5 (15) | 10 (36) | |
| 6−12 | 1 (3) | 9 (29) | |
| 13–18 | 0 (0) | 1 (4) | |
| Gender, n (%) | 0.072 | ||
| Male | 23 (68) | 12 (43) | |
| Female | 11 (32) | 16 (57) | |
| Clinical Profile | |||
| Max Number of stools/days, n (%) | 1.000 | ||
| ≤6 | 31 (91) | 26 (93) | |
| >6 | 3 (9) | 2 (7) | |
| Diarrhea duration, mean ± SD | 2 ± 1.89 | 1 ± 1.27 | 0.265 |
| Max Number of vomit/days, n (%) | 1.000 | ||
| ≤5 | 23 (68) | 19 (68) | |
| >5 | 11 (32) | 9 (32) | |
| Vomit duration, mean ± SD | 2 ± 1.11 | 1 ± 0.81 | 0.136 |
| Temperature °C, n (%) | 0.780 | ||
| ≤37.5 | 10 (29) | 7 (25) | |
| >37.5 | 24 (71) | 21 (75) | |
| Severity Score; mean ± SD | 11 ± 3.5 | 11 ± 2.5 | 0.615 |
| Clinical chemistry test | |||
| AST, IU/L; mean ± SD | 47 ± 25.8 | 40 ± 19.1 | 0.201 |
| ALT, IU/L; mean ± SD | 28 ± 23.2 | 29 ± 32.7 | 0.435 |
| CRP, mg/dL mean ± SD | 0.9 ± 2.3 | 1.8 ± 2.7 | 0.041 |
| Genotype | Number of Patients with NoV Positive in Stool (n = 63) | Number of Patients with NoV Positive in Stool and Serum (n = 9) |
|---|---|---|
| Capsid | ||
| GI.2 | 2 (3.2%) | |
| GII.4-Sydney/2012 | 32 (50.8%) | 4 (44.4%) |
| GII.3 | 15 (23.8%) | |
| GII.2 | 7 (11.1%) | |
| GII.NS | 7 (11.1%) | 5 (55.6%) |
| Capsid/Polymerase, n = 44 | ||
| GII.4 Sydney[P16] | 12 (27.3%) | |
| GII.4 Sydney[P31] | 11 (25%) | |
| GII.3[P12] | 9 (20.5%) | |
| GII.2[P16] | 6 (13.6%) | |
| GII.NS | 6 (13.6%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amexo, J.X.; Negoro, M.; Kuurdor, E.D.-M.; Lartey, B.L.; Sokejima, S.; Sugata, K.; Tonto, P.B.; Taniguchi, K. Molecular Epidemiology of Norovirus (NoV) Infection in Mie Prefecture: The Kinetics of Norovirus Antigenemia in Pediatric Patients. Viruses 2022, 14, 173. https://doi.org/10.3390/v14020173
Amexo JX, Negoro M, Kuurdor ED-M, Lartey BL, Sokejima S, Sugata K, Tonto PB, Taniguchi K. Molecular Epidemiology of Norovirus (NoV) Infection in Mie Prefecture: The Kinetics of Norovirus Antigenemia in Pediatric Patients. Viruses. 2022; 14(2):173. https://doi.org/10.3390/v14020173
Chicago/Turabian StyleAmexo, Jennifer X., Manami Negoro, Elijah Deku-Mwin Kuurdor, Belinda L. Lartey, Shigeru Sokejima, Ken Sugata, Prince Baffour Tonto, and Kiyosu Taniguchi. 2022. "Molecular Epidemiology of Norovirus (NoV) Infection in Mie Prefecture: The Kinetics of Norovirus Antigenemia in Pediatric Patients" Viruses 14, no. 2: 173. https://doi.org/10.3390/v14020173
APA StyleAmexo, J. X., Negoro, M., Kuurdor, E. D.-M., Lartey, B. L., Sokejima, S., Sugata, K., Tonto, P. B., & Taniguchi, K. (2022). Molecular Epidemiology of Norovirus (NoV) Infection in Mie Prefecture: The Kinetics of Norovirus Antigenemia in Pediatric Patients. Viruses, 14(2), 173. https://doi.org/10.3390/v14020173






