Phi 6 Bacteriophage Inactivation by Metal Salts, Metal Powders, and Metal Surfaces
Abstract
:1. Introduction
2. Materials and Methods
2.1. Testing Virus Stability to Physicochemical Factors
2.2. Testing the Virucidal Effect of Metal Ion Salts
2.3. Testing the Virucidal Effect of Metal and Ceramic Powders
2.4. Testing the Virucidal Effect on Composite Disks Surfaces
2.5. Measurement of Surface Free Energy (SFE)
2.6. Statistical Analysis
3. Results
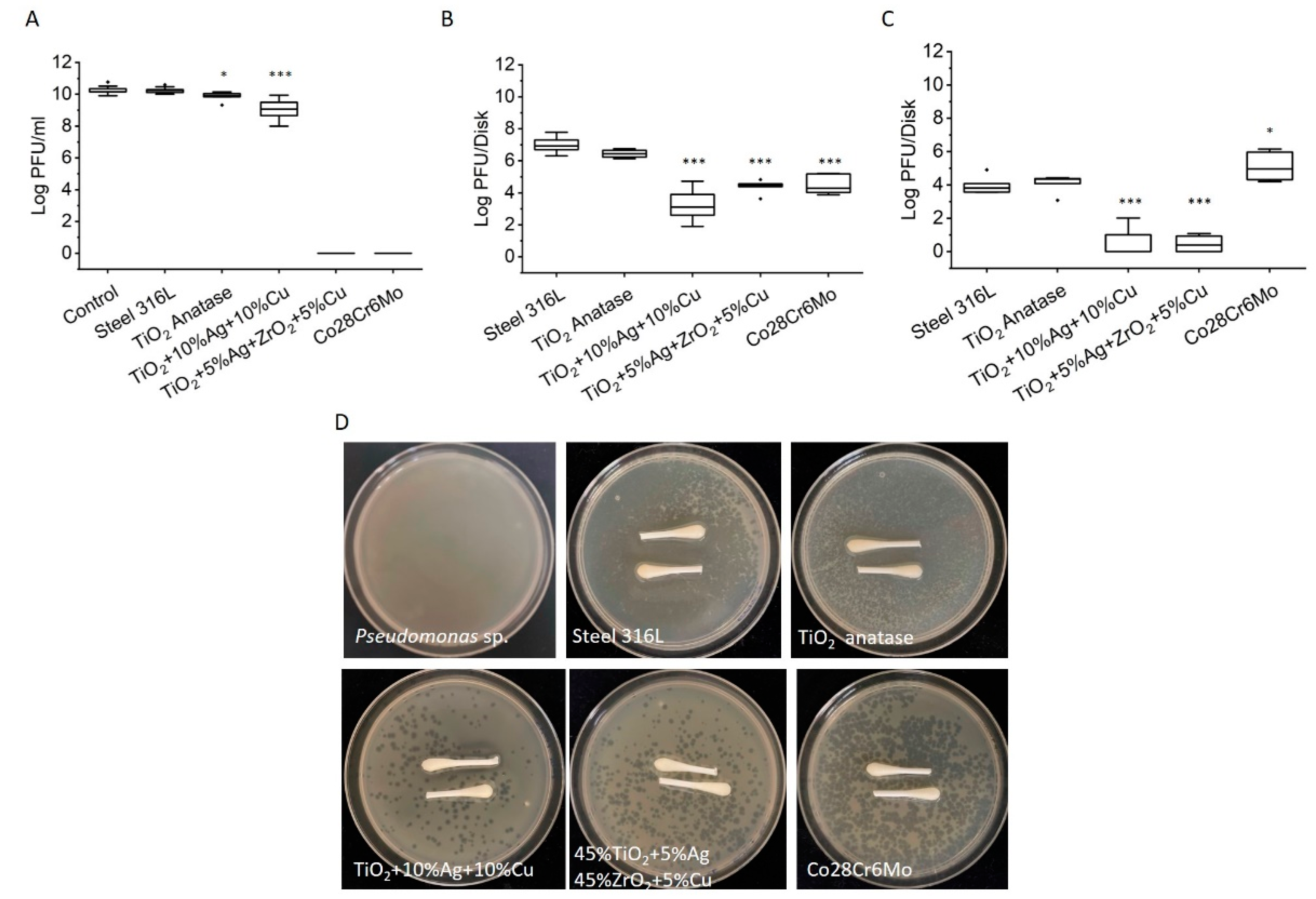
3.1. Virus Inactivation in Metal Ion Salt Solutions
3.2. Virus Inactivation in Contact with Ceramic and Metal Powder Suspensions
3.3. Virus Inactivation on Disks Surfaces
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vejerano, E.P.; Marr, L.C. Physico-chemical characteristics of evaporating respiratory fluid droplets. J. R. Soc. Interface 2018, 15, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Prussin, A.J.; Schwake, D.O.; Lin, K.; Gallagher, D.L.; Buttling, L.; Marr, L.C. Survival of the enveloped virus Phi6 in droplets as a function of relative humidity, absolute humidity, and temperature. Appl. Environ. Microbiol. 2018, 84, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turgeon, N.; Toulouse, M.-J.; Martel, B.; Moineau, S.; Duchaine, C. Comparison of five bacteriophages as models for viral aerosol studies. Appl. Environ. Microbiol. 2014, 80, 4242–4250. [Google Scholar] [CrossRef] [Green Version]
- Aquino de Carvalho, N.; Stachler, E.N.; Cimabue, N.; Bibby, K. Evaluation of Phi6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017, 51, 8692–8700. [Google Scholar] [CrossRef] [PubMed]
- Adcock, N.J.; Rice, E.W.; Sivaganesan, M.; Brown, J.D.; Stallknecht, D.E.; Swayne, D.E. The use of bacteriophages of the family Cystoviridae as surrogates for H5N1 highly pathogenic avian influenza viruses in persistence and inactivation studies. J. Environ. Sci. Health A 2009, 44, 1362–1366. [Google Scholar] [CrossRef]
- Whitworth, C.; Mu, Y.; Houston, H.; Martinez-Smith, M.; Noble-Wang, J.; Coulliette-Salmond, A.; Rose, L. Persistence of Bacteriophage Phi 6 on Porous and Nonporous Surfaces and the Potential for Its Use as an Ebola Virus or Coronavirus Surrogate. Appl. Environ. Microbiol. 2020, 86, 1–11. [Google Scholar] [CrossRef]
- Fedorenko, A.; Grinberg, M.; Orevi, T.; Kashtan, N. Survival of the enveloped bacteriophage Phi6 (a surrogate for SARS-CoV-2) in evaporated saliva microdroplets deposited on glass surfaces. Sci. Rep. 2020, 10, 22419. [Google Scholar] [CrossRef]
- Vidaver, A.K.; Koski, R.K.; Van Etten, J.L. Bacteriophage phi6: A Lipid-Containing Virus of Pseudomonas phaseolicola. J. Virol. 1973, 11, 799–805. [Google Scholar] [CrossRef] [Green Version]
- Bamford, D.H.; Romantschuk, M.; Somerharju, P.J. Membrane fusion in prokaryotes: Bacteriophage phi 6 membrane fuses with the Pseudomonas syringae outer membrane. EMBO J. 1987, 5, 1467–1473. [Google Scholar] [CrossRef]
- Kenney, J.M.; Hantula, J.; Fuller, S.D.; Mindich, L.; Ojala, P.M.; Bamford, D.H. Bacteriophage phi 6 envelope elucidated by chemical cross-linking, immunodetection, and cryoelectron microscopy. Virology 1992, 190, 635–644. [Google Scholar] [CrossRef]
- Li, T.; Bamford, D.H.; Bamford, J.K.; Thomas, G.J., Jr. Structural studies of the enveloped dsRNA bacteriophage phi 6 of Pseudomonas syringae by Raman spectroscopy. I. The virion and its membrane envelope. J. Mol. Biol. 1993, 230, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Laurinavicius, S.; Käkelä, R.; Bamford, D.H.; Somerharju, P. The origin of phospholipids of the enveloped bacteriophage phi6. Virology 2004, 326, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Burrell, C.J.; Howard, C.R.; Murphy, F.A. Virion Structure and Composition. In Fenner and White’s Medical Virology; Academic Press: Cambridge, MA, USA, 2017; pp. 27–37. [Google Scholar] [CrossRef]
- Etten, J.V.; Lane, L.; Gonzalez, C.; Partridge, J.; Vidaver, A. Comparative properties of bacteriophage phi6 and phi6 nucleocapsid. J. Virol. 1976, 18, 652–658. [Google Scholar] [CrossRef] [Green Version]
- Lin, K.; Schulte, C.R.; Marr, L.C. Survival of MS2 and Φ6 viruses in droplets as a function of relative humidity, pH, and salt, protein, and surfactant concentrations. PLoS ONE. 2020, 15, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Buhr, T.L.; Young, A.A.; Borgers-Klonkowski, E.; Kennihan, N.L.; Barnette, H.K.; Minter, Z.A.; Bohmke, M.D.; Osborn, E.B.; Hamilton, S.M.; Kimani, M.B.; et al. Hot, Humid Air Decontamination of Aircraft Confirmed That High Temperature and High Humidity Are Critical for Inactivation of Infectious, Enveloped Ribonucleic Acid (RNA) Virus. Front. Bioeng. Biotechnol. 2020, 8, 1–14. [Google Scholar] [CrossRef]
- Ma, B.; Linden, Y.S.; Gundy, P.M.; Gerba, C.P.; Sobsey, M.D.; Linden, K.G. Inactivation of Coronaviruses and Phage Phi6 from Irradiation across UVC Wavelengths. Environ. Sci. Technol. Lett. 2021, 8, 425–430. [Google Scholar] [CrossRef]
- Vatter, P.; Hoenes, K.; Hessling, M. Blue light inactivation of the enveloped RNA virus Phi6. BMC Res. Notes 2021, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Haller, W.; Hiatt, C.W. Mechanism of inactivation of bacteriophages by metals. Biochim. Biophys. Acta Spec. Sect. Nucleic Acids Relat. Subj. 1964, 91, 257–261. [Google Scholar] [CrossRef]
- Sagripanti, J.L.; Routson, L.B.; Lytle, C.D. Virus inactivation by copper or iron ions alone or in the presence of peroxide. Appl. Environ. Microbiol. 1993, 59, 4374–4376. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Dennehy, J.J. Differential bacteriophage mortality on exposure to copper. Appl. Environ. Microbiol. 2011, 77, 6878–6883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortes, A.A.; Zuñiga, J.M. The use of copper to help prevent transmission of SARS-coronavirus and influenza viruses. A general review. Diagn. Microbiol. Infect. Dis. 2020, 98, 1–5. [Google Scholar] [CrossRef]
- Grass, G.; Rensing, C.; Solioz, M. Metallic copper as an antimicrobial surface. Appl. Environ. Microbiol. 2011, 77, 1541–1547. [Google Scholar] [CrossRef] [Green Version]
- Warnes, S.L.; Keevil, C.W. Inactivation of norovirus on dry copper alloy surfaces. PLoS ONE 2013, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Abraham, J.; Dowling, K.; Florentine, S. Can Copper Products and Surfaces Reduce the Spread of Infectious Microorganisms and Hospital-Acquired Infections? Materials 2021, 14, 3444. [Google Scholar] [CrossRef]
- Fujimori, Y.; Sato, T.; Hayata, T.; Nagao, T.; Nakayama, M.; Nakayama, T.; Sugamata, R.; Suzuki, K. Novel antiviral characteristics of nanosized copper(I) iodide particles showing inactivation activity against 2009 pandemic H1N1 influenza virus. Appl Environ. Microbiol. 2012, 78, 951–955. [Google Scholar] [CrossRef] [Green Version]
- Van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef]
- Bauer, D.J. The chemotherapeutic activity of compounds of copper, rhodium and certain other metals in mice infected with neurovaccinia and ectromelia viruses. Br. J. Exp. Pathol. 1958, 39, 480–489. [Google Scholar]
- Gaikwad, S.; Ingle, A.; Gade, A.; Rai, M.; Falanga, A.; Incoronato, N.; Russo, L.; Galdiero, S.; Galdiero, M. Antiviral activity of mycosynthesized silver nanoparticles against herpes simplex virus and human parainfluenza virus type 3. Int. J. Nanomed. 2013, 8, 4303–4314. [Google Scholar] [CrossRef] [Green Version]
- Trefry, J.C.; Wooley, D.P. Silver nanoparticles inhibit vaccinia virus infection by preventing viral entry through a macropinocytosis-dependent mechanism. J. Biomed. Nanotechnol. 2013, 9, 1624–1635. [Google Scholar] [CrossRef]
- Morris, D.; Ansar, M.; Speshock, J.; Ivanciuc, T.; Qu, Y.; Casola, A.; Garofalo, R. Antiviral and Immunomodulatory Activity of Silver Nanoparticles in Experimental RSV Infection. Viruses 2019, 8, 732. [Google Scholar] [CrossRef] [Green Version]
- Speshock, J.L.; Murdock, R.C.; Braydich-Stolle, L.K.; Schrand, A.M.; Hussain, S.M. Interaction of silver nanoparticles with Tacaribe virus. J. Nanobiotechnol. 2010, 8, 732. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Sun, R.W.; Chen, R.; Hui, C.K.; Ho, C.M.; Luk, J.M.; Lau, G.K.; Che, C.M. Silver nanoparticles inhibit hepatitis B virus replication. Antivir. Ther. 2008, 13, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.Y.M.; Medema, G.; Bonilla, B.E.; Brouns, S.J.J.; van Halem, D. Inactivation of RNA and DNA viruses in water by copper and silver ions and their synergistic effect. Water Res. X 2020, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lara, H.H.; Ayala-Nuñez, N.V.; Ixtepan-Turrent, L.; Rodriguez-Padilla, C. Mode of antiviral action of silver nanoparticles against HIV-1. J. Nanobiotechnol. 2010, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mehrbod, P.; Motamed, N.; Tabatabaian, M.; Soleimani Estyar, R.; Amini, E.; Shahidi, M.; Kheiri, M.T. In Vitro Antiviral Effect of “Nanosilver” on Influenza Virus. DARU J. Pharm. Sci. 2009, 17, 88–93. [Google Scholar]
- Minoshima, M.; Lu, Y.; Kimura, T.; Nakano, R.; Ishiguro, H.; Kubota, Y.; Hashimoto, K.; Sunada, K. Comparison of the antiviral effect of solid-state copper and silver compounds. J. Hazard. Mater. 2016, 15, 1–7. [Google Scholar] [CrossRef]
- Nefedova, E.; Koptev, V.; Bobikova, A.S.; Cherepushkina, V.; Mironova, T.; Afonyushkin, V.; Shkil, N.; Donchenko, N.; Kozlova, Y.; Sigareva, N.; et al. The Infectious Bronchitis Coronavirus Pneumonia Model Presenting a Novel Insight for the SARS-CoV-2 Dissemination Route. Vet. Sci. 2021, 8, 239. [Google Scholar] [CrossRef]
- Clegg, W.J.; Giuliani, F.; Long, Y.; Lloyd, S.J.; Molina-Aldareguia, J.M. Hardness of multilayered ceramics. Ceram.-Matrix Compos. 2006, 216–240. [Google Scholar] [CrossRef]
- Quinn, J.B.; Quinn, G.D. Indentation brittleness of ceramics: A fresh approach. J. Mater. Sci. 1997, 32, 4331–4346. [Google Scholar] [CrossRef]
- Rahmani, R.; Brojan, M.; Antonov, M. Lightweight 3D printed Ti6Al4V-AlSi10Mg hybrid composite for impact resistance and armor piercing shielding. J. Mater. Res. Technol. 2020, 9, 13842–13854. [Google Scholar] [CrossRef]
- Rahmani, R.; Brojan, M.; Antonov, M.; Prashanth, K.G. Perspectives of metal-diamond composites additive manufacturing using SLM-SPS and other techniques for increased wear-impact resistance. Int. J. Refract. Met. Hard Mater. 2020, 88, 1–13. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef]
- Rahmani, R.; Rosenberg, M.; Ivask, A.; Kollo, L. Comparison of mechanical and antibacterial properties of TiO2/Ag ceramics and Ti6Al4V-TiO2/Ag composite materials using combining SLM-SPS techniques. Metals 2019, 9, 874. [Google Scholar] [CrossRef] [Green Version]
- Yeargin, T.; Buckley, D.; Fraser, A.; Jiang, X. The survival and inactivation of enteric viruses on soft surfaces: A systematic review of the literature. Am. J. Infect. Control. 2016, 44, 1365–1373. [Google Scholar] [CrossRef]
- Lin, Q.; Lim, J.Y.C.; Xue, K.; Yew, P.Y.M.; Owh, C.; Chee, P.L.; Loh, X.J. Sanitizing agents for virus inactivation and disinfection. View 2020, 24, e16. [Google Scholar] [CrossRef]
- Hutasoit, N.; Kennedy, B.; Hamilton, S.; Luttick, A.; Rahman Rashid, R.A.; Palanisamy, S. SARS-CoV-2 (COVID-19) inactivation capability of copper-coated touch surface fabricated by cold-spray technology. Manuf. Lett. 2020, 25, 93–97. [Google Scholar] [CrossRef]
- Rai, M.; Deshmukh, S.D.; Ingle, A.P.; Gupta, I.R.; Galdiero, M.; Galdiero, S. Metal nanoparticles: The protective nanoshield against virus infection. Crit. Rev. Microbiol. 2016, 42, 46–56. [Google Scholar] [CrossRef]
- Lara, H.H.; Garza-Treviño, E.N.; Ixtepan-Turrent, L.; Singh, D.K. Silver nanoparticles are broad-spectrum bactericidal and virucidal compounds. J. Nanobiotechnol. 2011, 9, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinheiro, L.A.M.; Pereira, C.; Frazão, C.; Balcão, V.M.; Almeida, A. Efficiency of Phage φ6 for Biocontrol of Pseudomonas syringae pv. Syr. Vitr. Prelim. Study. Microorg. 2019, 23, 286. [Google Scholar] [CrossRef] [Green Version]
- Adams, M.H. Bacteriophages; Interscience Publishers, Inc.: New York, NY, USA, 1959; p. 164. [Google Scholar]
- Kaelble, D.H. Dispersion-Polar Surface Tension Properties of Organic Solids. J. Adhes. 1970, 2, 66–81. [Google Scholar] [CrossRef]
- Owens, D.; Wendt, R. Estimation of the Surface Free Energy of Polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rabel, W. Einige Aspekte der Benetzungstheorie und ihre Anwendung auf die Untersuchung und Veränderung der Oberflächene genschaften von Polymeren. Farbe Lack 1971, 77, 997–1005. [Google Scholar]
- Becker, B.; Henningsen, L.; Paulmann, D.; Bischoff, B.; Todt, D.; Steinmann, E.; Steinmann, J.; Brill, F.H.; Steinmann, J. Evaluation of the virucidal efficacy of disinfectant wipes with a test method simulating practical conditions. Antimicrob. Resist. Infect. Control 2019, 8, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rabenau, H.F.; Schwebke, I.; Blümel, J.; Eggers, M.; Glebe, D.; Rapp, I.; Sauerbrei, A.; Steinmann, E.; Steinmann, J.; Willkommen, H.; et al. Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine. Bekanntmachungen 2020, 63, 645–655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Tarabara, V.V. Virus adhesion to archetypal fomites: A study with human adenovirus and human respiratory syncytial virus. Chem. Eng. J. 2022, 429, 132085. [Google Scholar] [CrossRef]
- Henry, M.; Jolivet, J.P.; Livage, J. Aqueous Chemistry of Metal Cations: Hydrolysis, Condensation and Complexation. In Chemistry, Spectroscopy and Applications of Sol-Gel Glasses, Structure and Bonding; Reisfeld, R., JJørgensen, C.K., Eds.; Springer: Berlin/Heidelberg, Germany, 1992; Volume 77, pp. 153–206. [Google Scholar] [CrossRef]
- Rahmani, R.; Antonov, M.; Prashanth, K.G. The impact resistance of highly densified metal alloys manufactured from gas-atomized pre-alloyed powders. Coatings 2021, 11, 216. [Google Scholar] [CrossRef]
- Xia, Y.; Mou, J.; Deng, G.; Wan, S.; Tieu, K.; Zhu, H.; Xue, Q. Sintered ZrO2–TiO2 ceramic composite and its mechanical appraisal. Ceram. Int. 2020, 46, 775–785. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, C.-S.; Chang, H.-K.; Kim, T.-O. Effects of ZrO2 addition on phase stability and photocatalytic activity of ZrO2/TiO2 nanoparticles. Adv. Powder Technol. 2010, 21, 141–144. [Google Scholar] [CrossRef]
- Rakowska, P.D.; Tiddia, M.; Faruqui, N.; Bankier, C.; Pei, Y.; Pollard, A.J.; Zhang, J.; Gilmore, I.S. Antiviral surfaces and coatings and their mechanisms of action. Commun. Mater. 2021, 2, 53. [Google Scholar] [CrossRef]
- Aydogdu, M.O.; Altun, E.; Chung, E.; Ren, G.; Homer-Vanniasinkam, S.; Chen, B.; Edirisinghe, M. Surface interactions and viability of coronaviruses. J. R. Soc. Interface 2021, 18, 2020079820200798. [Google Scholar] [CrossRef]
- Kolel-Veetil, M.; Sen, A.; Buehler, M.J. Surface adhesion of viruses and bacteria: Defend only and/or vibrationally extinguish also?! A perspective. MRS Adv. 2021, 6, 355–361. [Google Scholar] [CrossRef]
- Kuzyakov, Y.; Mason-Jones, K. Viruses in soil: Nano-scale undead drivers of microbial life, biogeochemical turnover and ecosystem functions. Soil Biol. Biochem. 2018, 127, 305–317. [Google Scholar] [CrossRef]



| SFE (mJ/m2) | Disperse (mJ/m2) | Polar (mJ/m2) | |
|---|---|---|---|
| Steel | 33.19 ± 1.66 | 30.44 ± 1.52 | 2.75 ± 0.14 |
| TiO2 anatase | 39.33 ± 1.97 | 32.26 ± 1.61 | 7.07 ± 0.35 |
| TiO2 + 10% Ag + 10% Cu | 40.47 ± 2.02 | 32.81 ± 1.64 | 7.66 ± 0.38 |
| 45%TiO2 + 5% Ag + 45%ZrO2 + 5% Cu | 33.77 ± 1.69 | 30.08 ± 1.50 | 3.69 ± 0.18 |
| Co28Cr6Mo | 55.20 ± 2.76 | 28.58 ± 1.43 | 26.62 ± 1.33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molan, K.; Rahmani, R.; Krklec, D.; Brojan, M.; Stopar, D. Phi 6 Bacteriophage Inactivation by Metal Salts, Metal Powders, and Metal Surfaces. Viruses 2022, 14, 204. https://doi.org/10.3390/v14020204
Molan K, Rahmani R, Krklec D, Brojan M, Stopar D. Phi 6 Bacteriophage Inactivation by Metal Salts, Metal Powders, and Metal Surfaces. Viruses. 2022; 14(2):204. https://doi.org/10.3390/v14020204
Chicago/Turabian StyleMolan, Katja, Ramin Rahmani, Daniel Krklec, Miha Brojan, and David Stopar. 2022. "Phi 6 Bacteriophage Inactivation by Metal Salts, Metal Powders, and Metal Surfaces" Viruses 14, no. 2: 204. https://doi.org/10.3390/v14020204
APA StyleMolan, K., Rahmani, R., Krklec, D., Brojan, M., & Stopar, D. (2022). Phi 6 Bacteriophage Inactivation by Metal Salts, Metal Powders, and Metal Surfaces. Viruses, 14(2), 204. https://doi.org/10.3390/v14020204








