The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications
Abstract
1. Introduction
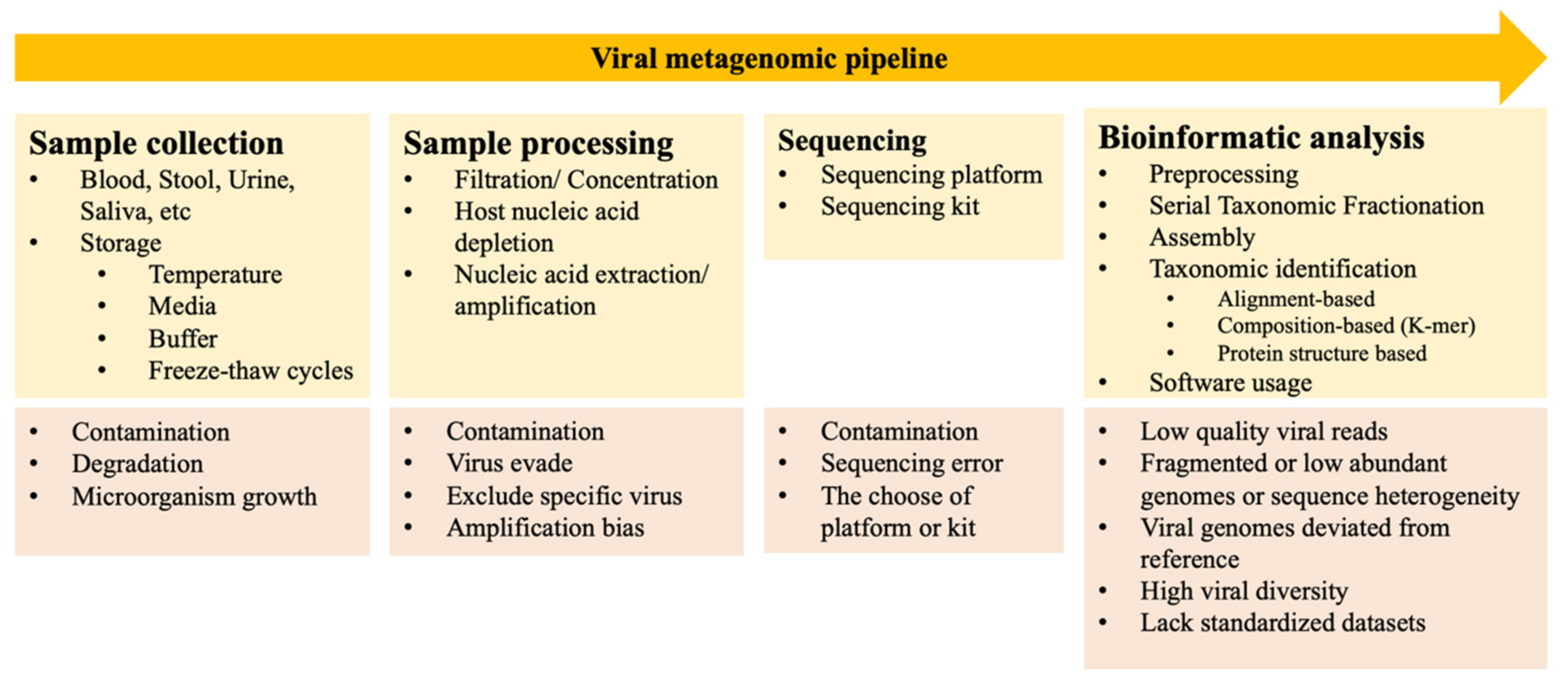
2. Metagenomic Approach
2.1. Viral Metagenomic Approach
2.2. Biases and Challenges Associated with Viral Metagenomics
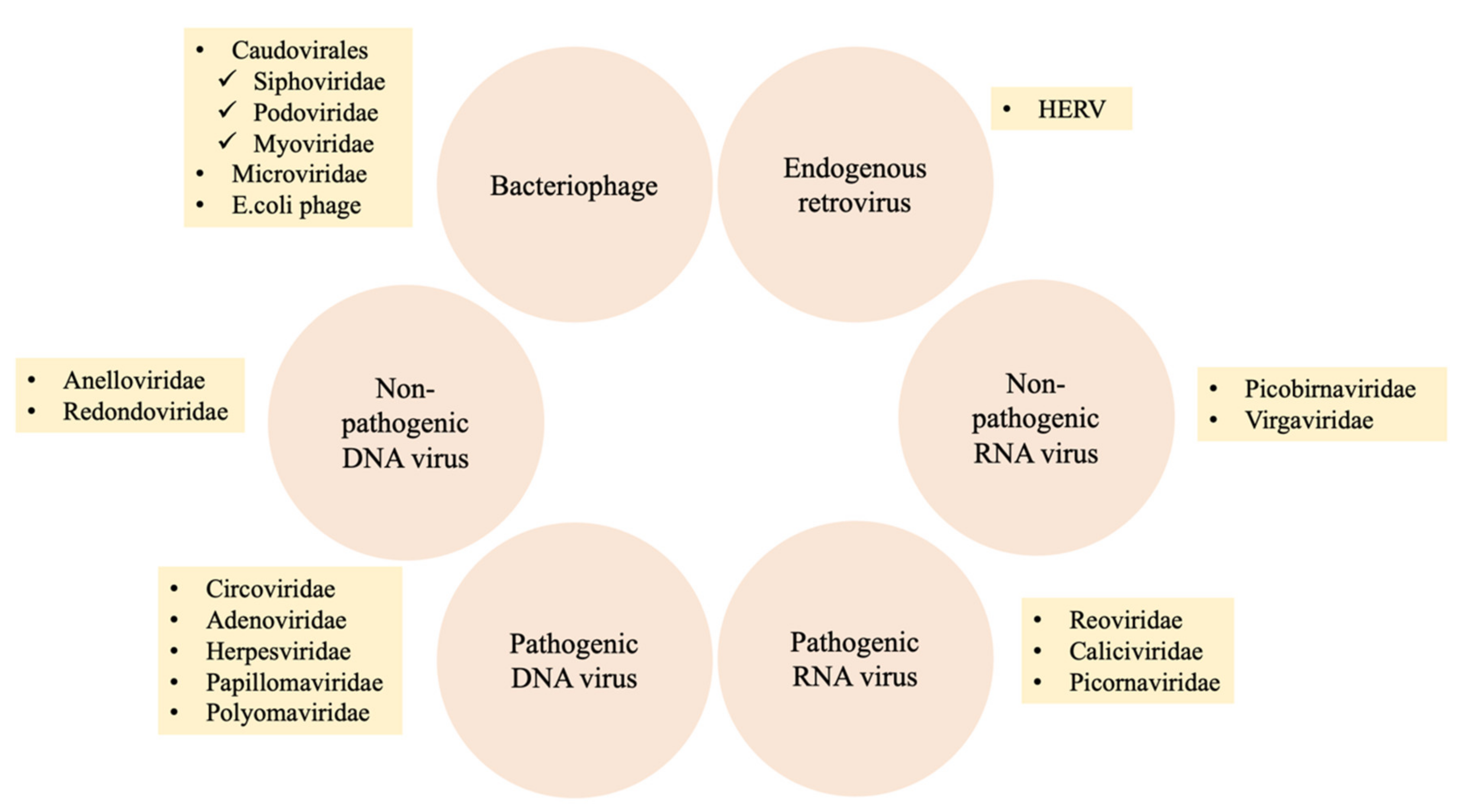
2.3. Composition of the Human Virome
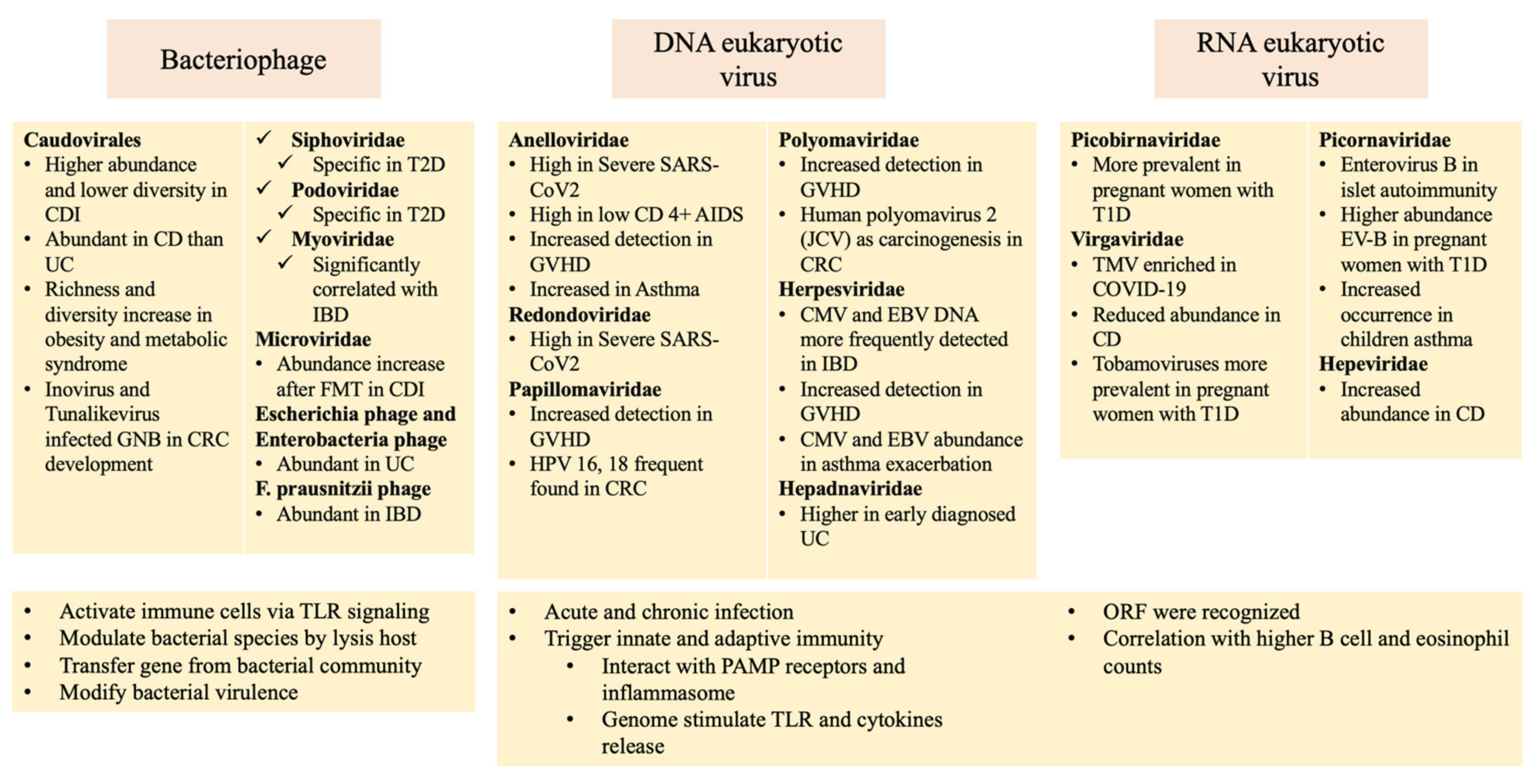
3. The Virome and Human Disease
3.1. Infectious and Inflammatory Diseases
3.1.1. Severe Acute Respiratory Syndrome Coronavirus 2
3.1.2. Human Immunodeficiency Virus
3.1.3. Clostridioides Difficile Infection
3.1.4. Inflammatory Bowel Disease
3.1.5. Graft-Versus-Host Disease
3.2. Chronic Diseases
3.2.1. Type 1 Diabetes
3.2.2. Type 2 Diabetes and Obesity
3.2.3. Hypertension
3.2.4. Asthma and Chronic Obstructive Pulmonary Disease
3.3. Cancer
Colorectal Cancer
3.4. Possible Pathogenic Relations between the Human Virome and Disease
4. Therapeutic Applications
4.1. Fecal Microbiota Transplantation
4.2. Phage-Based Therapy
4.3. Oncolytic Therapy
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chatterjee, A.; Duerkop, B.A. Beyond Bacteria: Bacteriophage-Eukaryotic Host Interactions Reveal Emerging Paradigms of Health and Disease. Front. Microbiol. 2018, 9, 1394. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Bushman, F.D. The human virome: Assembly, composition and host interactions. Nat. Rev. Microbiol. 2021, 19, 514–527. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, M.; Salamon, P.; Andresen, B.; Mahaffy, J.M.; Segall, A.M.; Mead, D.; Azam, F.; Rohwer, F. Genomic analysis of uncultured marine viral communities. Proc. Natl. Acad. Sci. USA 2002, 99, 14250–14255. [Google Scholar] [CrossRef] [PubMed]
- Cantalupo, P.G.; Pipas, J.M. Detecting viral sequences in NGS data. Curr. Opin. Virol. 2019, 39, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Viruses—Complete Genomes. Available online: https://www.ncbi.nlm.nih.gov/genomes/GenomesGroup.cgi?taxid=10239 (accessed on 15 December 2021).
- Koonin, E.V.; Dolja, V.V.; Krupovic, M. The healthy human virome: From virus–host symbiosis to disease. Curr. Opin. Virol. 2021, 47, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Friedland, R.P.; Haribabu, B. The role for the metagenome in the pathogenesis of COVID-19. EBioMedicine 2020, 61, 103019. [Google Scholar] [CrossRef] [PubMed]
- Kazemian, N.; Kao, D.; Pakpour, S. Fecal Microbiota Transplantation during and Post-COVID-19 Pandemic. Int. J. Mol. Sci. 2021, 22, 3004. [Google Scholar] [CrossRef]
- Miller, R.R.; Montoya, V.; Gardy, J.L.; Patrick, D.M.; Tang, P. Metagenomics for pathogen detection in public health. Genome Med. 2013, 5, 81. [Google Scholar] [CrossRef]
- Mokili, J.L.; Rohwer, F.; Dutilh, B.E. Metagenomics and future perspectives in virus discovery. Curr. Opin. Virol. 2012, 2, 63–77. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Potential Applications of Human Viral Metagenomics and Reference Materials: Considerations for Current and Future Viruses. Appl. Environ. Microbiol. 2020, 86, e01794-20. [Google Scholar] [CrossRef] [PubMed]
- Nooij, S.; Schmitz, D.; Vennema, H.; Kroneman, A.; Koopmans, M.P.G. Overview of Virus Metagenomic Classification Methods and Their Biological Applications. Front. Microbiol. 2018, 9, 749. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.; Constantinides, B.; Tapinos, A.; Robertson, D.L.; Prosperi, M. Challenges in the analysis of viral metagenomes. Virus Evol. 2016, 2, vew022. [Google Scholar] [CrossRef] [PubMed]
- Santiago-Rodriguez, T.M.; Hollister, E.B. Human Virome and Disease: High-Throughput Sequencing for Virus Discovery, Identification of Phage-Bacteria Dysbiosis and Development of Therapeutic Approaches with Emphasis on the Human Gut. Viruses 2019, 11, 656. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, K.; Liu, Y.; Huang, C.; Wu, M. Dynamic Impact of Virome on Colitis and Colorectal Cancer: Immunity, Inflammation, Prevention and Treatment, Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Chiu, C.Y.; Miller, S.A. Clinical metagenomics. Nat. Rev. Genet. 2019, 20, 341–355. [Google Scholar] [CrossRef]
- Thurber, R.V.; Haynes, M.; Breitbart, M.; Wegley, L.; Rohwer, F. Laboratory procedures to generate viral metagenomes. Nat. Protoc. 2009, 4, 470–483. [Google Scholar] [CrossRef]
- Aggarwala, V.; Liang, G.; Bushman, F.D. Viral communities of the human gut: Metagenomic analysis of composition and dynamics. Mob. DNA 2017, 8, 12. [Google Scholar] [CrossRef]
- McNaughton, A.L.; Roberts, H.E.; Bonsall, D.; de Cesare, M.; Mokaya, J.; Lumley, S.F.; Golubchik, T.; Piazza, P.; Martin, J.B.; de Lara, C.; et al. Illumina and Nanopore methods for whole genome sequencing of hepatitis B virus (HBV). Sci. Rep. 2019, 9, 7081. [Google Scholar] [CrossRef]
- Ren, J.; Song, K.; Deng, C.; Ahlgren, N.A.; Fuhrman, J.A.; Li, Y.; Xie, X.; Poplin, R.; Sun, F. Identifying viruses from metagenomic data using deep learning. Quant. Biol. 2020, 8, 64–77. [Google Scholar] [CrossRef]
- Zárate, S.; Taboada, B.; Yocupicio-Monroy, M.; Arias, C.F. Human virome. Arch. Med. Res. 2017, 48, 701–716. [Google Scholar] [CrossRef]
- Campbell, A. The future of bacteriophage biology. Nat. Rev. Genet. 2003, 4, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Erez, Z.; Steinberger-Levy, I.; Shamir, M.; Doron, S.; Stokar-Avihail, A.; Peleg, Y.; Melamed, S.; Leavitt, A.; Savidor, A.; Albeck, S.; et al. Communication between viruses guides lysis–lysogeny decisions. Nature 2017, 541, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Łoś, M.; Węgrzyn, G. Pseudolysogeny. Adv. Virus Res. 2012, 82, 339–349. [Google Scholar] [CrossRef] [PubMed]
- Rascovan, N.; Duraisamy, R.; Desnues, C. Metagenomics and the Human Virome in Asymptomatic Individuals. Annu. Rev. Microbiol. 2016, 70, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Fulci, V.; Stronati, L.; Cucchiara, S.; Laudadio, I.; Carissimi, C. Emerging Roles of Gut Virome in Pediatric Diseases. Int. J. Mol. Sci. 2021, 22, 4127. [Google Scholar] [CrossRef] [PubMed]
- Küry, P.; Nath, A.; Créange, A.; Dolei, A.; Marche, P.; Gold, J.; Giovannoni, G.; Hartung, H.P.; Perron, H. Human Endogenous Retroviruses in Neurological Diseases. Trends Mol. Med. 2018, 24, 379–394. [Google Scholar] [CrossRef]
- Zuo, T.; Liu, Q.; Zhang, F.; Yeoh, Y.K.; Wan, Y.; Zhan, H.; Lui, G.C.Y.; Chen, Z.; Li, A.Y.L.; Cheung, C.P.; et al. Temporal landscape of human gut RNA and DNA virome in SARS-CoV-2 infection and severity. Microbiome 2021, 9, 91. [Google Scholar] [CrossRef]
- Soffritti, I.; D’Accolti, M.; Fabbri, C.; Passaro, A.; Manfredini, R.; Zuliani, G.; Libanore, M.; Franchi, M.; Contini, C.; Caselli, E. Oral Microbiome Dysbiosis Is Associated with Symptoms Severity and Local Immune/Inflammatory Response in COVID-19 Patients: A Cross-Sectional Study. Front. Microbiol. 2021, 12, 687513. [Google Scholar] [CrossRef]
- Han, Y.; Jia, Z.; Shi, J.; Wang, W.; He, K. The active lung microbiota landscape of COVID-19 patients. medRxiv 2020. [Google Scholar] [CrossRef]
- Merenstein, C.; Liang, G.; Whiteside, S.A.; Cobián-Güemes, A.G.; Merlino, M.S.; Taylor, L.J.; Glascock, A.; Bittinger, K.; Tanes, C.; Graham-Wooten, J.; et al. Signatures of COVID-19 severity and immune response in the respiratory tract microbiome. medRxiv 2021. [Google Scholar] [CrossRef]
- Li, L.; Deng, X.; Linsuwanon, P.; Bangsberg, D.; Bwana, M.B.; Hunt, P.; Martin, J.N.; Deeks, S.G.; Delwart, E. AIDS Alters the Commensal Plasma Virome. J. Virol. 2013, 87, 10912–10915. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Deng, X.; Da Costa, A.C.; Bruhn, R.; Deeks, S.G.; Delwart, E. Virome analysis of antiretroviral-treated HIV patients shows no correlation between T-cell activation and anelloviruses levels. J. Clin. Virol. 2015, 72, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.D.; Curty, G.; Xutao, D.; Hofer, C.B.; Machado, E.S.; Seuánez, H.N.; Soares, M.A.; Delwart, E.; Soares, E.A. Composite Analysis of the Virome and Bacteriome of HIV/HPV Co-Infected Women Reveals Proxies for Immunodeficiency. Viruses 2019, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Altan, E.; Pilcher, C.; Hartogensis, W.; Hecht, F.M.; Deng, X.; Delwart, E. Semen virome of men with HIV on or off antiretroviral treatment. AIDS 2020, 34, 827–832. [Google Scholar] [CrossRef]
- Zuo, T.; Wong, S.H.; Lam, K.; Lui, R.; Cheung, K.; Tang, W.; Ching, J.Y.L.; Chan, P.K.S.; Chan, M.C.W.; Wu, J.C.Y.; et al. Bacteriophage transfer during faecal microbiota transplantation in Clostridium difficile infection is associated with treatment outcome. Gut 2018, 67, 634–643. [Google Scholar] [CrossRef]
- Fujimoto, K.; Kimura, Y.; Allegretti, J.R.; Yamamoto, M.; Zhang, Y.-Z.; Katayama, K.; Tremmel, G.; Kawaguchi, Y.; Shimohigoshi, M.; Hayashi, T.; et al. Functional Restoration of Bacteriomes and Viromes by Fecal Microbiota Transplantation. Gastroenterology 2021, 160, 2089–2102.e2012. [Google Scholar] [CrossRef]
- Wagner, J.; Maksimovic, J.; Farries, G.; Sim, W.H.; Bishop, R.F.; Cameron, D.J.; Catto-Smith, A.G.; Kirkwood, C.D. Bacteriophages in Gut Samples From Pediatric Crohn’s Disease Patients: Metagenomic Analysis Using 454 Pyrosequencing. Inflamm. Bowel Dis. 2013, 19, 1598–1608. [Google Scholar] [CrossRef]
- Norman, J.M.; Handley, S.A.; Baldridge, M.T.; Droit, L.; Liu, C.Y.; Keller, B.C.; Kambal, A.; Monaco, C.L.; Zhao, G.; Fleshner, P.; et al. Disease-specific alterations in the enteric virome in inflammatory bowel disease. Cell 2015, 160, 447–460. [Google Scholar] [CrossRef]
- Perez-Brocal, V.; Garcia-Lopez, R.; Nos, P.; Beltran, B.; Moret, I.; Moya, A. Metagenomic Analysis of Crohn’s Disease Patients Identifies Changes in the Virome and Microbiome Related to Disease Status and Therapy, and Detects Potential Interactions and Biomarkers. Inflamm. Bowel Dis. 2015, 21, 2515–2532. [Google Scholar] [CrossRef]
- Wang, W.; Jovel, J.; Halloran, B.; Wine, E.; Patterson, J.; Ford, G.; O’Keefe, S.; Meng, B.; Song, D.; Zhang, Y.; et al. Metagenomic Analysis of Microbiome in Colon Tissue from Subjects with Inflammatory Bowel Diseases Reveals Interplay of Viruses and Bacteria. Inflamm. Bowel Dis. 2015, 21, 1419–1427. [Google Scholar] [CrossRef]
- Lopes, S.; Andrade, P.; Conde, S.; Liberal, R.; Dias, C.C.; Fernandes, S.; Pinheiro, J.; Simoes, J.S.; Carneiro, F.; Magro, F.; et al. Looking into Enteric Virome in Patients with IBD: Defining Guilty or Innocence? Inflamm. Bowel Dis. 2017, 23, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Cornuault, J.K.; Petit, M.-A.; Mariadassou, M.; Benevides, L.; Moncaut, E.; Langella, P.; Sokol, H.; De Paepe, M. Phages infecting Faecalibacterium prausnitzii belong to novel viral genera that help to decipher intestinal viromes. Microbiome 2018, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Clooney, A.G.; Sutton, T.D.S.; Shkoporov, A.N.; Holohan, R.K.; Daly, K.M.; O’Regan, O.; Ryan, F.J.; Draper, L.A.; Plevy, S.E.; Ross, R.P.; et al. Whole-Virome Analysis Sheds Light on Viral Dark Matter in Inflammatory Bowel Disease. Cell Host Microbe 2019, 26, 764–778.e765. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.A.; Verstraete, S.G.; Phan, T.G.; Deng, X.; Stekol, E.; LaMere, B.; Lynch, S.V.; Heyman, M.B.; Delwart, E. Enteric Virome and Bacterial Microbiota in Children with Ulcerative Colitis and Crohn Disease. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ungaro, F.; Massimino, L.; Furfaro, F.; Rimoldi, V.; Peyrin-Biroulet, L.; D’Alessio, S.; Danese, S. Metagenomic analysis of intestinal mucosa revealed a specific eukaryotic gut virome signature in early-diagnosed inflammatory bowel disease. Gut Microbes 2019, 10, 149–158. [Google Scholar] [CrossRef]
- Zuo, T.; Lu, X.J.; Zhang, Y.; Cheung, C.P.; Lam, S.; Zhang, F.; Tang, W.; Ching, J.Y.L.; Zhao, R.; Chan, P.K.S.; et al. Gut mucosal virome alterations in ulcerative colitis. Gut 2019, 68, 1169–1179. [Google Scholar] [CrossRef]
- Liang, G.; Conrad, M.A.; Kelsen, J.R.; Kessler, L.R.; Breton, J.; Albenberg, L.G.; Marakos, S.; Galgano, A.; Devas, N.; Erlichman, J.; et al. Dynamics of the Stool Virome in Very Early-Onset Inflammatory Bowel Disease. J. Crohn’s Colitis 2020, 14, 1600–1610. [Google Scholar] [CrossRef]
- Legoff, J.; Resche-Rigon, M.; Bouquet, J.; Robin, M.; Naccache, S.N.; Mercier-Delarue, S.; Federman, S.; Samayoa, E.; Rousseau, C.; Piron, P.; et al. The eukaryotic gut virome in hematopoietic stem cell transplantation: New clues in enteric graft-versus-host disease. Nat. Med. 2017, 23, 1080–1085. [Google Scholar] [CrossRef]
- Zhao, G.; Vatanen, T.; Droit, L.; Park, A.; Kostic, A.D.; Poon, T.W.; Vlamakis, H.; Siljander, H.; Harkonen, T.; Hamalainen, A.M.; et al. Intestinal virome changes precede autoimmunity in type I diabetes-susceptible children. Proc. Natl. Acad. Sci. USA 2017, 114, E6166–E6175. [Google Scholar] [CrossRef]
- Tetz, G.; Brown, S.M.; Hao, Y.; Tetz, V. Type 1 Diabetes: An Association Between Autoimmunity, the Dynamics of Gut Amyloid-producing E. coli and Their Phages. Sci. Rep. 2019, 9, 9685. [Google Scholar] [CrossRef]
- Vehik, K.; Lynch, K.F.; Wong, M.C.; Tian, X.; Ross, M.C.; Gibbs, R.A.; Ajami, N.J.; Petrosino, J.F.; Rewers, M.; Toppari, J.; et al. Prospective virome analyses in young children at increased genetic risk for type 1 diabetes. Nat. Med. 2019, 25, 1865–1872. [Google Scholar] [CrossRef] [PubMed]
- Wook Kim, K.; Allen, D.W.; Briese, T.; Couper, J.J.; Barry, S.C.; Colman, P.G.; Cotterill, A.M.; Davis, E.A.; Giles, L.C.; Harrison, L.C.; et al. Distinct Gut Virome Profile of Pregnant Women with Type 1 Diabetes in the ENDIA Study. Open Forum Infect. Dis. 2019, 6, ofz025. [Google Scholar] [CrossRef] [PubMed]
- Cinek, O.; Kramna, L.; Odeh, R.; Alassaf, A.; Ibekwe, M.A.U.; Ahmadov, G.; Elmahi, B.M.E.; Mekki, H.; Lebl, J.; Abdullah, M.A. Eukaryotic viruses in the fecal virome at the onset of type 1 diabetes: A study from four geographically distant African and Asian countries. Pediatr. Diabetes 2021, 22, 558–566. [Google Scholar] [CrossRef]
- Ma, Y.; You, X.; Mai, G.; Tokuyasu, T.; Liu, C. A human gut phage catalog correlates the gut phageome with type 2 diabetes. Microbiome 2018, 6, 24. [Google Scholar] [CrossRef]
- Bikel, S.; Lopez-Leal, G.; Cornejo-Granados, F.; Gallardo-Becerra, L.; Garcia-Lopez, R.; Sanchez, F.; Equihua-Medina, E.; Ochoa-Romo, J.P.; Lopez-Contreras, B.E.; Canizales-Quinteros, S.; et al. Gut dsDNA virome shows diversity and richness alterations associated with childhood obesity and metabolic syndrome. iScience 2021, 24, 102900. [Google Scholar] [CrossRef]
- Hasan, M.R.; Rahman, M.; Khan, T.; Saeed, A.; Sundararaju, S.; Flores, A.; Hawken, P.; Rawat, A.; Elkum, N.; Hussain, K.; et al. Virome-wide serological profiling reveals association of herpesviruses with obesity. Sci. Rep. 2021, 11, 2562. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Vargas, D.; Concha-Rubio, N.D.; Navarrete, P.; Castro, M.; Medina, D.A. Short Communication: Obesity Intervention Resulting in Significant Changes in the Human Gut Viral Composition. Appl. Sci. 2021, 11, 10039. [Google Scholar] [CrossRef]
- Yang, K.; Niu, J.; Zuo, T.; Sun, Y.; Xu, Z.; Tang, W.; Liu, Q.; Zhang, J.; Ng, E.K.W.; Wong, S.K.H.; et al. Alterations in the Gut Virome in Obesity and Type 2 Diabetes Mellitus. Gastroenterology 2021, 161, 1257–1269.e1213. [Google Scholar] [CrossRef]
- Han, M.; Yang, P.; Zhong, C.; Ning, K. The Human Gut Virome in Hypertension. Front. Microbiol. 2018, 9, 3150. [Google Scholar] [CrossRef]
- Kim, S.; Rigatto, K.; Gazzana, M.B.; Knorst, M.M.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. Altered Gut Microbiome Profile in Patients with Pulmonary Arterial Hypertension. Hypertension 2020, 75, 1063–1071. [Google Scholar] [CrossRef]
- Lang, S.; Demir, M.; Martin, A.; Jiang, L.; Zhang, X.; Duan, Y.; Gao, B.; Wisplinghoff, H.; Kasper, P.; Roderburg, C.; et al. Intestinal Virome Signature Associated with Severity of Nonalcoholic Fatty Liver Disease. Gastroenterology 2020, 159, 1839–1852. [Google Scholar] [CrossRef] [PubMed]
- Romero-Espinoza, J.A.; Moreno-Valencia, Y.; Coronel-Tellez, R.H.; Castillejos-Lopez, M.; Hernandez, A.; Dominguez, A.; Miliar-Garcia, A.; Barbachano-Guerrero, A.; Perez-Padilla, R.; Alejandre-Garcia, A.; et al. Virome and bacteriome characterization of children with pneumonia and asthma in Mexico City during winter seasons 2014 and 2015. PLoS ONE 2018, 13, e0192878. [Google Scholar] [CrossRef] [PubMed]
- Megremis, S.; Constantinides, B.; Xepapadaki, P.; Bachert, C.; Neurath-Finotto, S.; Jartti, T.; Kowalski, M.L.; Sotiropoulos, A.G.; Tapinos, A.; Vuorinen, T.; et al. Bacteriophage deficiency characterizes respiratory virome dysbiosis in childhood asthma. bioRxiv 2020, arXiv:2020.08.04.236067. [Google Scholar]
- Choi, S.; Sohn, K.H.; Jung, J.W.; Kang, M.G.; Yang, M.S.; Kim, S.; Choi, J.H.; Cho, S.H.; Kang, H.R.; Yi, H. Lung virome: New potential biomarkers for asthma severity and exacerbation. J. Allergy Clin. Immunol. 2021, 148, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Van Rijn, A.L.; van Boheemen, S.; Sidorov, I.; Carbo, E.C.; Pappas, N.; Mei, H.; Feltkamp, M.; Aanerud, M.; Bakke, P.; Claas, E.C.J.; et al. The respiratory virome and exacerbations in patients with chronic obstructive pulmonary disease. PLoS ONE 2019, 14, e0223952. [Google Scholar] [CrossRef]
- Hannigan, G.D.; Duhaime, M.B.; Ruffin, M.T.; Koumpouras, C.C.; Schloss, P.D. Viral and Bacterial Communities of Colorectal Cancer. bioRxiv 2017, 152868. [Google Scholar] [CrossRef]
- Nakatsu, G.; Zhou, H.; Wu, W.K.K.; Wong, S.H.; Coker, O.O.; Dai, Z.; Li, X.; Szeto, C.H.; Sugimura, N.; Lam, T.Y.; et al. Alterations in Enteric Virome Are Associated with Colorectal Cancer and Survival Outcomes. Gastroenterology 2018, 155, 529–541.e525. [Google Scholar] [CrossRef]
- Proal, A.D.; VanElzakker, M.B. Long COVID or Post-acute Sequelae of COVID-19 (PASC): An Overview of Biological Factors That May Contribute to Persistent Symptoms. Front. Microbiol. 2021, 12, 698169. [Google Scholar] [CrossRef]
- Segal, J.P.; Mak, J.W.Y.; Mullish, B.H.; Alexander, J.L.; Ng, S.C.; Marchesi, J.R. The gut microbiome: An under-recognised contributor to the COVID-19 pandemic? Ther. Adv. Gastroenterol. 2020, 13, 1756284820974914. [Google Scholar] [CrossRef]
- Ferreira, C.; Viana, S.D.; Reis, F. Gut Microbiota Dysbiosis-Immune Hyperresponse-Inflammation Triad in Coronavirus Disease 2019 (COVID-19): Impact of Pharmacological and Nutraceutical Approaches. Microorganisms 2020, 8, 1514. [Google Scholar] [CrossRef]
- Cao, J.; Wang, C.; Zhang, Y.; Lei, G.; Xu, K.; Zhao, N.; Lu, J.; Meng, F.; Yu, L.; Yan, J.; et al. Integrated gut virome and bacteriome dynamics in COVID-19 patients. Gut Microbes 2021, 13, 1887722. [Google Scholar] [CrossRef] [PubMed]
- Zuo, T.; Wu, X.; Wen, W.; Lan, P. Gut Microbiome Alterations in COVID-19. Genom. Proteom. Bioinform. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.W.; Deveson, I.W.; Pang, C.N.I.; Yeang, M.; Naing, Z.; Adikari, T.; Hammond, J.M.; Stevanovski, I.; Beukers, A.G.; Verich, A.; et al. Respiratory viral co-infections among SARS-CoV-2 cases confirmed by virome capture sequencing. Sci. Rep. 2021, 11, 3934. [Google Scholar] [CrossRef] [PubMed]
- Monaco, C.L.; Gootenberg, D.B.; Zhao, G.; Handley, S.A.; Ghebremichael, M.S.; Lim, E.S.; Lankowski, A.; Baldridge, M.T.; Wilen, C.B.; Flagg, M.; et al. Altered Virome and Bacterial Microbiome in Human Immunodeficiency Virus-Associated Acquired Immunodeficiency Syndrome. Cell Host Microbe 2016, 19, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Broecker, F.; Klumpp, J.; Moelling, K. Long-term microbiota and virome in a Zurich patient after fecal transplantation against Clostridium difficile infection. Ann. N. Y. Acad. Sci. 2016, 1372, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Lopetuso, L.R.; Ianiro, G.; Scaldaferri, F.; Cammarota, G.; Gasbarrini, A. Gut Virome and Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2016, 22, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, C.L.; Luo, Y.X.; Isaacs, S.; Rawlinson, W.D.; Craig, M.E.; Kim, K.W. The virome in early life and childhood and development of islet autoimmunity and type 1 diabetes: A systematic review and meta-analysis of observational studies. Rev. Med. Virol. 2021, 31, 1–14. [Google Scholar] [CrossRef]
- Needell, J.C.; Zipris, D. The Role of the Intestinal Microbiome in Type 1 Diabetes Pathogenesis. Curr. Diabetes Rep. 2016, 16, 89. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Filippi, C.M.; von Herrath, M.G. Viral trigger for type 1 diabetes: Pros and cons. Diabetes 2008, 57, 2863–2871. [Google Scholar] [CrossRef]
- Dedrick, S.; Sundaresh, B.; Huang, Q.; Brady, C.; Yoo, T.; Cronin, C.; Rudnicki, C.; Flood, M.; Momeni, B.; Ludvigsson, J.; et al. The Role of Gut Microbiota and Environmental Factors in Type 1 Diabetes Pathogenesis. Front. Endocrinol. 2020, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Larsen, N.; Vogensen, F.K.; van den Berg, F.W.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sorensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Jayasudha, R.; Chakravarthy, S.; Prashanthi, G.S.; Bhargava, A.; Tyagi, M.; Rani, P.K.; Pappuru, R.R.; Sharma, S.; Shivaji, S. Alterations in the gut bacterial microbiome in people with type 2 diabetes mellitus and diabetic retinopathy. Sci. Rep. 2021, 11, 2738. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.; Jain, S.; Nagpal, R.; Marotta, F. Increased fecal viral content associated with obesity in mice. World J. Diabetes 2016, 7, 316–320. [Google Scholar] [CrossRef]
- Guo, Y.; Li, X.; Wang, Z.; Yu, B. Gut Microbiota Dysbiosis in Human Hypertension: A Systematic Review of Observational Studies. Front. Cardiovasc. Med. 2021, 8, 650227. [Google Scholar] [CrossRef]
- Jankauskaite, L.; Miseviciene, V.; Vaideliene, L.; Kevalas, R. Lower Airway Virology in Health and Disease-From Invaders to Symbionts. Medicina 2018, 54, 72. [Google Scholar] [CrossRef]
- Hewson, C.A.; Haas, J.J.; Bartlett, N.W.; Message, S.D.; Laza-Stanca, V.; Kebadze, T.; Caramori, G.; Zhu, J.; Edbrooke, M.R.; Stanciu, L.A.; et al. Rhinovirus induces MUC5AC in a human infection model and in vitro via NF-kappaB and EGFR pathways. Eur. Respir. J. 2010, 36, 1425–1435. [Google Scholar] [CrossRef]
- Chen, Y.; Williams, V.; Filippova, M.; Filippov, V.; Duerksen-Hughes, P. Viral carcinogenesis: Factors inducing DNA damage and virus integration. Cancers 2014, 6, 2155–2186. [Google Scholar] [CrossRef]
- Zur Hausen, H. Oncogenic DNA viruses. Oncogene 2001, 20, 7820–7823. [Google Scholar] [CrossRef]
- Wong, S.H.; Yu, J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 690–704. [Google Scholar] [CrossRef]
- Damin, D.C.; Ziegelmann, P.K.; Damin, A.P. Human papillomavirus infection and colorectal cancer risk: A meta-analysis. Colorectal Dis. 2013, 15, e420–e428. [Google Scholar] [CrossRef] [PubMed]
- Emlet, C.; Ruffin, M.; Lamendella, R. Enteric Virome and Carcinogenesis in the Gut. Dig. Dis. Sci. 2020, 65, 852–864. [Google Scholar] [CrossRef] [PubMed]
- Damin, D.C.; Caetano, M.B.; Rosito, M.A.; Schwartsmann, G.; Damin, A.S.; Frazzon, A.P.; Ruppenthal, R.D.; Alexandre, C.O.P. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur. J. Surg. Oncol. 2007, 33, 569–574. [Google Scholar] [CrossRef]
- Ibragimova, M.K.; Tsyganov, M.M.; Litviakov, N.V. Human papillomavirus and colorectal cancer. Med. Oncol. 2018, 35, 140. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Li, Y.; Fan, Z.; Yao, F.; Shen, W.; Sun, J.; Yuan, Y.; Chen, J.; Cai, L.; Xie, Y.; et al. Gene Expression Analysis of Human Papillomavirus-Associated Colorectal Carcinoma. BioMed Res. Int. 2020, 2020, 5201587. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-P.; Jiang, J.-K.; Chen, C.-Y.; Chou, T.-Y.; Chen, Y.-C.; Chang, Y.-T.; Lin, S.-F.; Chan, C.-H.; Yang, C.-Y.; Lin, C.-H.; et al. Human cytomegalovirus preferentially infects the neoplastic epithelium of colorectal cancer: A quantitative and histological analysis. J. Clin. Virol. 2012, 54, 240–244. [Google Scholar] [CrossRef]
- Chen, H.P.; Jiang, J.K.; Lai, P.Y.; Chen, C.Y.; Chou, T.Y.; Chen, Y.C.; Chan, C.H.; Lin, S.F.; Yang, C.Y.; Chen, C.Y.; et al. Tumoral presence of human cytomegalovirus is associated with shorter disease-free survival in elderly patients with colorectal cancer and higher levels of intratumoral interleukin-17. Clin. Microbiol. Infect. 2014, 20, 664–671. [Google Scholar] [CrossRef]
- Chen, H.-P.; Jiang, J.-K.; Chan, C.-H.; Teo, W.-H.; Yang, C.-Y.; Chen, Y.-C.; Chou, T.-Y.; Lin, C.-H.; Chan, Y.-J. Genetic polymorphisms of the human cytomegalovirus UL144 gene in colorectal cancer and its association with clinical outcome. J. Gen. Virol. 2015, 96, 3613–3623. [Google Scholar] [CrossRef]
- Chen, H.-P.; Jiang, J.-K.; Chen, C.-Y.; Yang, C.-Y.; Chen, Y.-C.; Lin, C.-H.; Chou, T.-Y.; Cho, W.-L.; Chan, Y.-J. Identification of human cytomegalovirus in tumour tissues of colorectal cancer and its association with the outcome of non-elderly patients. J. Gen. Virol. 2016, 97, 2411–2420. [Google Scholar] [CrossRef][Green Version]
- Li, X.; Qian, D.; Ju, F.; Wang, B. Upregulation of Toll-like receptor 2 expression in colorectal cancer infected by human cytomegalovirus. Oncol. Lett. 2015, 9, 365–370. [Google Scholar] [CrossRef][Green Version]
- Teo, W.H.; Chen, H.-P.; Huang, J.C.; Chan, Y.-J. Human cytomegalovirus infection enhances cell proliferation, migration and upregulation of EMT markers in colorectal cancer-derived stem cell-like cells. Int. J. Oncol. 2017, 51, 1415–1426. [Google Scholar] [CrossRef] [PubMed]
- Harkins, L.; Volk, A.L.; Samanta, M.; Mikolaenko, I.; Britt, W.J.; Bland, K.I.; Cobbs, C.S. Specific localisation of human cytomegalovirus nucleic acids and proteins in human colorectal cancer. Lancet 2002, 360, 1557–1563. [Google Scholar] [CrossRef]
- Jung, W.-T.; Li, M.-S.; Goel, A.; Boland, C.R. JC virus T-antigen expression in sporadic adenomatous polyps of the colon. Cancer 2008, 112, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- Shavaleh, R.; Kamandi, M.; Feiz Disfani, H.; Mansori, K.; Naseri, S.N.; Rahmani, K.; Ahmadi Kanrash, F. Association between JC virus and colorectal cancer: Systematic review and meta-analysis. Infect. Dis. 2020, 52, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Li, M.S.; Nagasaka, T.; Shin, S.K.; Fuerst, F.; Ricciardiello, L.; Wasserman, L.; Boland, C.R. Association of JC Virus T-Antigen Expression with the Methylator Phenotype in Sporadic Colorectal Cancers. Gastroenterology 2006, 130, 1950–1961. [Google Scholar] [CrossRef]
- Coelho, T.R.; Gaspar, R.; Figueiredo, P.; Mendonça, C.; Lazo, P.A.; Almeida, L. Human JC polyomavirus in normal colorectal mucosa, hyperplastic polyps, sporadic adenomas, and adenocarcinomas in Portugal. J. Med. Virol. 2013, 85, 2119–2127. [Google Scholar] [CrossRef]
- Enam, S.; Del Valle, L.; Lara, C.; Gan, D.D.; Ortiz-Hidalgo, C.; Palazzo, J.P.; Khalili, K. Association of human polyomavirus JCV with colon cancer: Evidence for interaction of viral T-antigen and beta-catenin. Cancer Res. 2002, 62, 7093–7101. [Google Scholar]
- Hannigan, G.D.; Duhaime, M.B.; Ruffin, M.T.; Koumpouras, C.C.; Schloss, P.D. Diagnostic Potential and Interactive Dynamics of the Colorectal Cancer Virome. mBio 2018, 9. [Google Scholar] [CrossRef]
- Duerkop, B.A.; Hooper, L.V. Resident viruses and their interactions with the immune system. Nat. Immunol. 2013, 14, 654–659. [Google Scholar] [CrossRef]
- Reyes, A.; Blanton, L.V.; Cao, S.; Zhao, G.; Manary, M.; Trehan, I.; Smith, M.I.; Wang, D.; Virgin, H.W.; Rohwer, F.; et al. Gut DNA viromes of Malawian twins discordant for severe acute malnutrition. Proc. Natl. Acad. Sci. USA 2015, 112, 11941–11946. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Dodi, G.; Attanasi, M.; Di Filippo, P.; Di Pillo, S.; Chiarelli, F. Virome in the Lungs: The Role of Anelloviruses in Childhood Respiratory Diseases. Microorganisms 2021, 9, 1357. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, J.; Ricci, V.; Albani, M.; Lanini, L.; Andreoli, E.; Macera, L.; Pistello, M.; Ceccherini-Nelli, L.; Bendinelli, M.; Maggi, F. Torquetenovirus DNA drives proinflammatory cytokines production and secretion by immune cells via toll-like receptor 9. Virology 2009, 394, 235–242. [Google Scholar] [CrossRef]
- Freer, G.; Maggi, F.; Pifferi, M.; Di Cicco, M.E.; Peroni, D.G.; Pistello, M. The Virome and Its Major Component, Anellovirus, a Convoluted System Molding Human Immune Defenses and Possibly Affecting the Development of Asthma and Respiratory Diseases in Childhood. Front. Microbiol. 2018, 9, 686. [Google Scholar] [CrossRef] [PubMed]
- Holt, P.G.; Sly, P.D. Viral infections and atopy in asthma pathogenesis: New rationales for asthma prevention and treatment. Nat. Med. 2012, 18, 726–735. [Google Scholar] [CrossRef]
- Reyes, A.; Wu, M.; McNulty, N.P.; Rohwer, F.L.; Gordon, J.I. Gnotobiotic mouse model of phage–bacterial host dynamics in the human gut. Proc. Natl. Acad. Sci. USA 2013, 110, 20236–20241. [Google Scholar] [CrossRef]
- Sweere, J.M.; Van Belleghem, J.D.; Ishak, H.; Bach, M.S.; Popescu, M.; Sunkari, V.; Kaber, G.; Manasherob, R.; Suh, G.A.; Cao, X.; et al. Bacteriophage trigger antiviral immunity and prevent clearance of bacterial infection. Science 2019, 363, eaat9691. [Google Scholar] [CrossRef]
- Gogokhia, L.; Buhrke, K.; Bell, R.; Hoffman, B.; Brown, D.G.; Hanke-Gogokhia, C.; Ajami, N.J.; Wong, M.C.; Ghazaryan, A.; Valentine, J.F.; et al. Expansion of Bacteriophages Is Linked to Aggravated Intestinal Inflammation and Colitis. Cell Host Microbe 2019, 25, 285–299.e8. [Google Scholar] [CrossRef]
- Robinson, C.M.; Jesudhasan, P.R.; Pfeiffer, J.K. Bacterial Lipopolysaccharide Binding Enhances Virion Stability and Promotes Environmental Fitness of an Enteric Virus. Cell Host Microbe 2014, 15, 36–46. [Google Scholar] [CrossRef]
- Wilks, J.; Lien, E.; Jacobson, A.N.; Fischbach, M.A.; Qureshi, N.; Chervonsky, A.V.; Golovkina, T.V. Mammalian Lipopolysaccharide Receptors Incorporated into the Retroviral Envelope Augment Virus Transmission. Cell Host Microbe 2015, 18, 456–462. [Google Scholar] [CrossRef]
- Broecker, F.; Russo, G.; Klumpp, J.; Moelling, K. Stable core virome despite variable microbiome after fecal transfer. Gut Microbes 2017, 8, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Ott, S.J.; Waetzig, G.H.; Rehman, A.; Moltzau-Anderson, J.; Bharti, R.; Grasis, J.A.; Cassidy, L.; Tholey, A.; Fickenscher, H.; Seegert, D.; et al. Efficacy of Sterile Fecal Filtrate Transfer for Treating Patients with Clostridium difficile Infection. Gastroenterology 2017, 152, 799–811.e7. [Google Scholar] [CrossRef] [PubMed]
- Narula, N.; Kassam, Z.; Yuan, Y.; Colombel, J.-F.; Ponsioen, C.; Reinisch, W.; Moayyedi, P. Systematic Review and Meta-analysis: Fecal Microbiota Transplantation for Treatment of Active Ulcerative Colitis. Inflamm. Bowel Dis. 2017, 23, 1702–1709. [Google Scholar] [CrossRef] [PubMed]
- Conceição-Neto, N.; Deboutte, W.; Dierckx, T.; Machiels, K.; Wang, J.; Yinda, K.C.; Maes, P.; Van Ranst, M.; Joossens, M.; Raes, J.; et al. Low eukaryotic viral richness is associated with faecal microbiota transplantation success in patients with UC. Gut 2018, 67, 1558–1559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zuo, T.; Yeoh, Y.K.; Cheng, F.W.T.; Liu, Q.; Tang, W.; Cheung, K.C.Y.; Yang, K.; Cheung, C.P.; Mo, C.C.; et al. Longitudinal dynamics of gut bacteriome, mycobiome and virome after fecal microbiota transplantation in graft-versus-host disease. Nat. Commun. 2021, 12, 65. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.S.; Mentzel, C.M.J.; Kot, W.; Castro-Mejía, J.L.; Zuffa, S.; Swann, J.R.; Hansen, L.H.; Vogensen, F.K.; Hansen, A.K.; Nielsen, D.S. Faecal virome transplantation decreases symptoms of type 2 diabetes and obesity in a murine model. Gut 2020, 69, 2122–2130. [Google Scholar] [CrossRef]
- Ng, S.C.; Xu, Z.; Mak, J.W.Y.; Yang, K.; Liu, Q.; Zuo, T.; Tang, W.; Lau, L.; Lui, R.N.; Wong, S.H.; et al. Microbiota engraftment after faecal microbiota transplantation in obese subjects with type 2 diabetes: A 24-week, double-blind, randomised controlled trial. Gut 2021, gutjnl-2020-323617. [Google Scholar] [CrossRef]
- McQuade, J.L.; Daniel, C.R.; Helmink, B.A.; Wargo, J.A. Modulating the microbiome to improve therapeutic response in cancer. Lancet Oncol. 2019, 20, e77–e91. [Google Scholar] [CrossRef]
- Park, R.; Umar, S.; Kasi, A. Immunotherapy in colorectal cancer: Potential of fecal transplant and microbiota-augmented clinical trials. Curr. Colorectal Cancer Rep. 2020, 16, 81–88. [Google Scholar] [CrossRef]
- Wang, Y.; Wiesnoski, D.H.; Helmink, B.A.; Gopalakrishnan, V.; Choi, K.; DuPont, H.L.; Jiang, Z.-D.; Abu-Sbeih, H.; Sanchez, C.A.; Chang, C.-C.; et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor-associated colitis. Nat. Med. 2018, 24, 1804–1808. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Carpentieri, C.; Sitchenko, K.L.; Kraft, C.S. Challenges in fecal donor selection and screening for fecal microbiota transplantation: A review. Gut Microbes 2017, 8, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, B.; Domingo-Calap, P. Phage Therapy in Gastrointestinal Diseases. Microorganisms 2020, 8, 1420. [Google Scholar] [CrossRef] [PubMed]
- Febvre, H.P.; Rao, S.; Gindin, M.; Goodwin, N.D.M.; Finer, E.; Vivanco, J.S.; Lu, S.; Manter, D.K.; Wallace, T.C.; Weir, T.L. PHAGE Study: Effects of Supplemental Bacteriophage Intake on Inflammation and Gut Microbiota in Healthy Adults. Nutrients 2019, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Galtier, M.; Sordi, L.D.; Sivignon, A.; de Vallée, A.; Maura, D.; Neut, C.; Rahmouni, O.; Wannerberger, K.; Darfeuille-Michaud, A.; Desreumaux, P.; et al. Bacteriophages Targeting Adherent Invasive Escherichia coli Strains as a Promising New Treatment for Crohn’s Disease. J. Crohn’s Colitis 2017, 11, 840–847. [Google Scholar] [CrossRef]
- Zheng, D.-W.; Dong, X.; Pan, P.; Chen, K.-W.; Fan, J.-X.; Cheng, S.-X.; Zhang, X.-Z. Phage-guided modulation of the gut microbiota of mouse models of colorectal cancer augments their responses to chemotherapy. Nat. Biomed. Eng. 2019, 3, 717–728. [Google Scholar] [CrossRef]
- Chang, R.Y.K.; Wallin, M.; Lin, Y.; Leung, S.S.Y.; Wang, H.; Morales, S.; Chan, H.-K. Phage therapy for respiratory infections. Adv. Drug Deliv. Rev. 2018, 133, 76–86. [Google Scholar] [CrossRef]
- Wu, N.; Chen, L.-K.; Zhu, T. Phage therapy for secondary bacterial infections with COVID-19. Curr. Opin. Virol. 2022, 52, 9–14. [Google Scholar] [CrossRef]
- Singh, A.K.; Gaur, V.; Kumar, A. Role of Phage Therapy in COVID-19 Infection: Future Prospects. In Bacteriophages; IntechOpen: London, UK, 2021. [Google Scholar]
- Maronek, M.; Link, R.; Ambro, L.; Gardlik, R. Phages and Their Role in Gastrointestinal Disease: Focus on Inflammatory Bowel Disease. Cells 2020, 9, 1013. [Google Scholar] [CrossRef]
- Turkington, C.J.R.; Varadan, A.C.; Grenier, S.F.; Grasis, J.A. The Viral Janus: Viruses as Aetiological Agents and Treatment Options in Colorectal Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 601573. [Google Scholar] [CrossRef]
- Santos Apolonio, J.; de Souza Gonçalves, V.L.; Cordeiro Santos, M.L.; Silva Luz, M.; Silva Souza, J.V.; Rocha Pinheiro, S.L.; de Souza, W.R.; Sande Loureiro, M.; de Melo, F.F. Oncolytic virus therapy in cancer: A current review. World J. Virol. 2021, 10, 229–255. [Google Scholar] [CrossRef]
| Human Disease | Samples | Case (n) | Control (n) | Virome Alternation | Reference |
|---|---|---|---|---|---|
| SARS-CoV-2 | Feces | 98 COVID-19 patients (3 asymptomatic, 53 mild, 34 moderate, 5 severe, 3 critical) | 78 non-COVID-19 controls matched for gender and co-morbidities |
| [29] |
| SARS-CoV-2 | Oral rinse samples | 39 COVID-19 patients | 36 healthy controls |
| [30] |
| SARS-CoV-2 | Bronchoalveolar lavage (BAL) fluid | 19 COVID-19 patients | 23 healthy controls |
| [31] |
| SARS-CoV-2 | OP, NP swabs, endotracheal aspirates (ETA) and BAL | 83 COVID-19 patients | 42 healthy controls |
| [32] |
| HIV | Plasma | 35 subjects were selected based on high or low CD4+ cell counts. |
| [33] | |
| HIV | Plasma | 19 subjects under ART-mediated viral suppression |
| [34] | |
| HIV | Cervical sample | 19 HIV/HPV co-infected women with multiple HPV infection |
| [35] | |
| HIV | Semen | 42 men with HIV |
| [36] | |
| C. difficile | Feces | 24 subjects with CDI | 20 healthy controls |
| [37] |
| Recurrent C. difficile | Feces | 9 patients with recurrent C. difficile infection (3 or more confirmed episodes of CDI) |
| [38] | |
| Crohn’s disease | Ileal and colonic biopsies Gut wash samples | 6 CD patients (ileal biopsy) 6 CD patients (colonic biopsy) 3 CD patients (gut washes) | 6 noninflammatory bowel disease patients (ileal biopsy) |
| [39] |
| Crohn’s disease Ulcerative colitis | Feces | 18 subjects with CD 42 subjects with UC | 12 household controls |
| [40] |
| Crohn’s disease | Feces and biopsy | 20 patients with CD | 20 healthy controls |
| [41] |
| Crohn’s disease Ulcerative colitis | Surgical samples or colonoscopic biopsy | 10 patients with IBD | 5 subjects undergoing colonoscopy for colon cancer surveillance |
| [42] |
| Crohn’s disease Ulcerative colitis | Blood and mucosal samples | 43 subjects with UC 52 subjects with CD | 50 healthy subjects |
| [43] |
| Crohn’s disease Ulcerative colitis | Feces | 52 IBD patients | 21 healthy controls |
| [44] |
| Crohn’s disease Ulcerative colitis | Feces | 27 subjects with CD 42 subjects with UC | 61 healthy controls |
| [45] |
| Crohn’s disease Ulcerative colitis | Feces | 7 children with CD 5 children with UC | 12 similar aged controls |
| [46] |
| Crohn’s disease Ulcerative colitis | Ileum biopsy | 55 colon-inflamed CD 161 ileum inflamed CD 61 colon-inflamed UC | 42 healthy controls |
| [47] |
| Ulcerative colitis | Rectal mucosa | 91 patients with UC | 76 healthy controls |
| [48] |
| Very early onset inflammatory bowel disease | Feces | 54 subjects with VEO-IBD | 23 healthy controls |
| [49] |
| GVHD | Feces | 44 adults who underwent allogeneic hematopoietic stem cell transplantation |
| [50] | |
| Human Diseases | Samples | Cases (n) | Controls (n) | Virome Alternation | References |
|---|---|---|---|---|---|
| Type 1 DM | Feces | 11 infants from Finland and Estonia based on HLA genotype | 11 matched controls |
| [51] |
| Type 1 DM | Feces | 10 children with autoantibodies (6 seroconverters and 4 children who developed T1D) | 8 non-seroconverted HLA-matched controls |
| [52] |
| Islet autoimmunity/ Type 1 DM | Feces | 323 islet autoimmunity and 95 T1D | 418 controls |
| [53] |
| Type 1 DM (Pregnant women) | Feces | 35 pregnant women with T1D | 26 pregnant women without T1D |
| [54] |
| Type 1 DM | Feces | 73 children and adolescents shortly after T1D onset (Azerbaijan 19, Jordan 20, Nigeria 14, Sudan 20) | 105 matched controls |
| [55] |
| Type 2 DM | Feces | 71 T2D patients | 74 non-diabetic Chinese adults |
| [56] |
| Obesity | Feces | 10 Children with obesity 8 Children of obesity with metabolic syndrome | 10 Children with healthy normal-weight |
| [57] |
| Obesity | Serum | 273 obese Qatari adults 120 obese Qatari children | 184 lean Qatari adults 111 lean Qatari children |
| [58] |
| Obesity | Feces | 21 pre-treated obese subjects | 28 post-obesity-treated subjects: 2 exercise and diet, 17 Roux-en-Y gastric bypasses, 2 sleeve gastrectomies, 7 verticals banded gastroplasties |
| [59] |
| Obesity and Type 2 DM | Feces | 128 obese subjects (BMI ≥ 28 kg/m2) | 101 lean controls (BMI ≥ 18.5 and <23 kg/m2) |
| [60] |
| Hypertension | Feces | 56 prehypertension 99 hypertension patients | 41 healthy controls |
| [61] |
| Pulmonary arterial hypertension | Feces | 18 type 1 PAH patients | 13 reference subjects |
| [62] |
| Fatty liver | Feces | 73 patients with NAFLD (29 with NAS score 0–4; 44 with NAS score 5–8) | 9 individuals without liver disease and 13 patients with mild primary biliary cholangitis |
| [63] |
| Asthma or Pneumonia | Nasopharyngeal swabs | 42 asthma children < 15 years old | 78 pneumonia children < 15 years old |
| [64] |
| Asthma | Nasopharyngeal swabs | 24 asthma children 11 asthma children | 10 healthy children 11 healthy children |
| [65] |
| Asthma | Nasopharyngeal swabs | 15 patients with non-severe asthma 15 patients with severe asthma | 12 healthy individuals |
| [66] |
| COPD | Nasopharyngeal swabs | 63 patients from the Bergen COPD Exacerbation Study |
| [67] | |
| Colorectal cancer | Feces | 30 had adenomas 30 had carcinomas | 30 had healthy colons |
| [68] |
| Colorectal cancer | Feces | 11 patients in Hong Kong 46 patients in Austria 91 patients in France and Germany | 112 controls in Hong Kong 63 controls in Australia 66 controls in France and Germany |
| [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, G.-H.; Lin, S.-C.; Hsu, Y.-H.; Chen, S.-Y. The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications. Viruses 2022, 14, 278. https://doi.org/10.3390/v14020278
Bai G-H, Lin S-C, Hsu Y-H, Chen S-Y. The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications. Viruses. 2022; 14(2):278. https://doi.org/10.3390/v14020278
Chicago/Turabian StyleBai, Geng-Hao, Sheng-Chieh Lin, Yi-Hsiang Hsu, and Shih-Yen Chen. 2022. "The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications" Viruses 14, no. 2: 278. https://doi.org/10.3390/v14020278
APA StyleBai, G.-H., Lin, S.-C., Hsu, Y.-H., & Chen, S.-Y. (2022). The Human Virome: Viral Metagenomics, Relations with Human Diseases, and Therapeutic Applications. Viruses, 14(2), 278. https://doi.org/10.3390/v14020278






