A Highly Divergent Hepacivirus Identified in Domestic Ducks Further Reveals the Genetic Diversity of Hepaciviruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection, RNA Extraction, and Meta-Transcriptome Sequencing
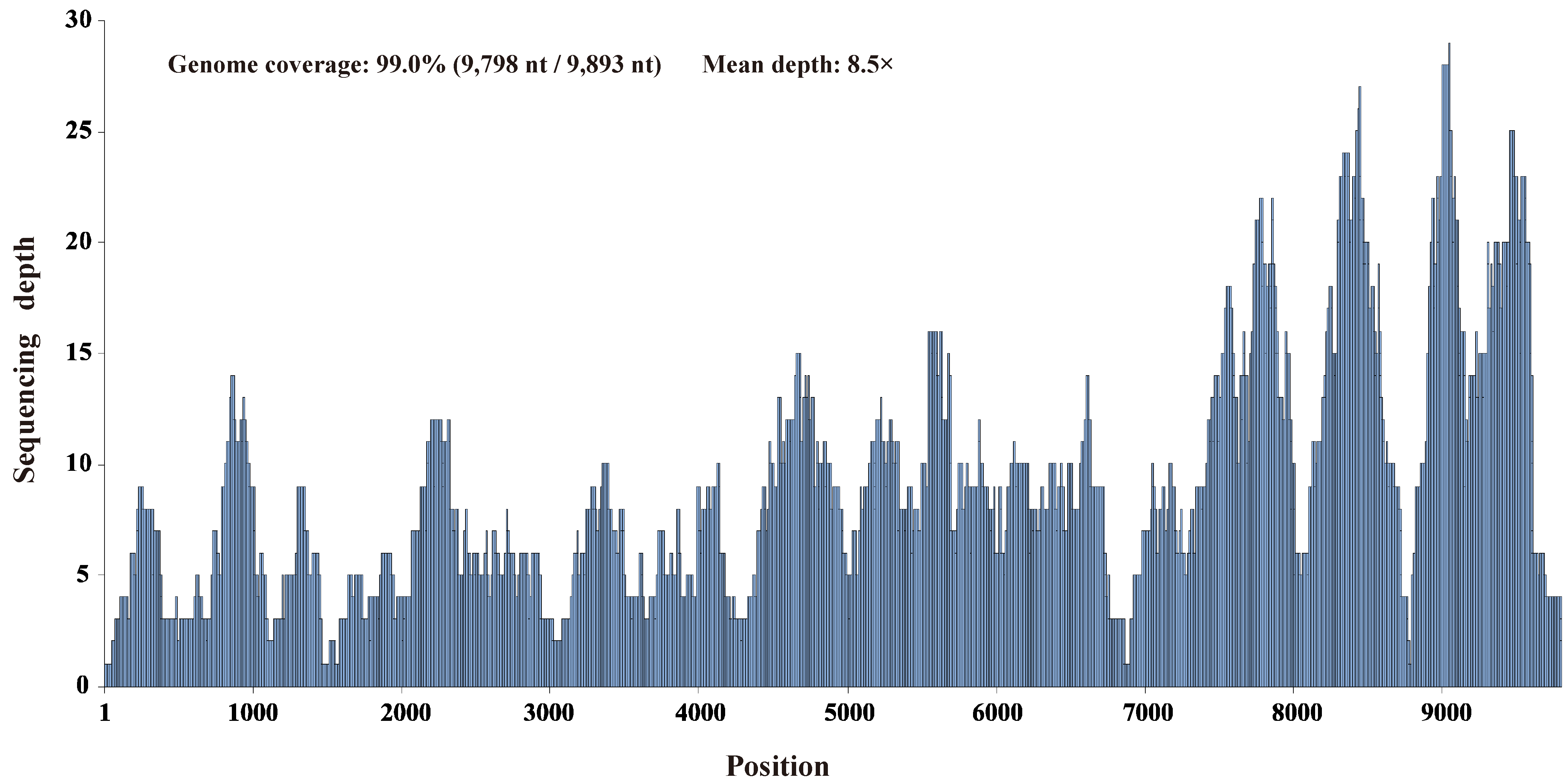
2.2. Bioinformatics Analyses and Genome Sequence Determination
2.3. PCR Screening for This Newly Identified Hepacivirus
2.4. Sequence Comparison and Phylogenetic Analyses
2.5. Genome Recombination Analysis
3. Results
3.1. Identification of a Novel Hepacivirus in Domestic Ducks
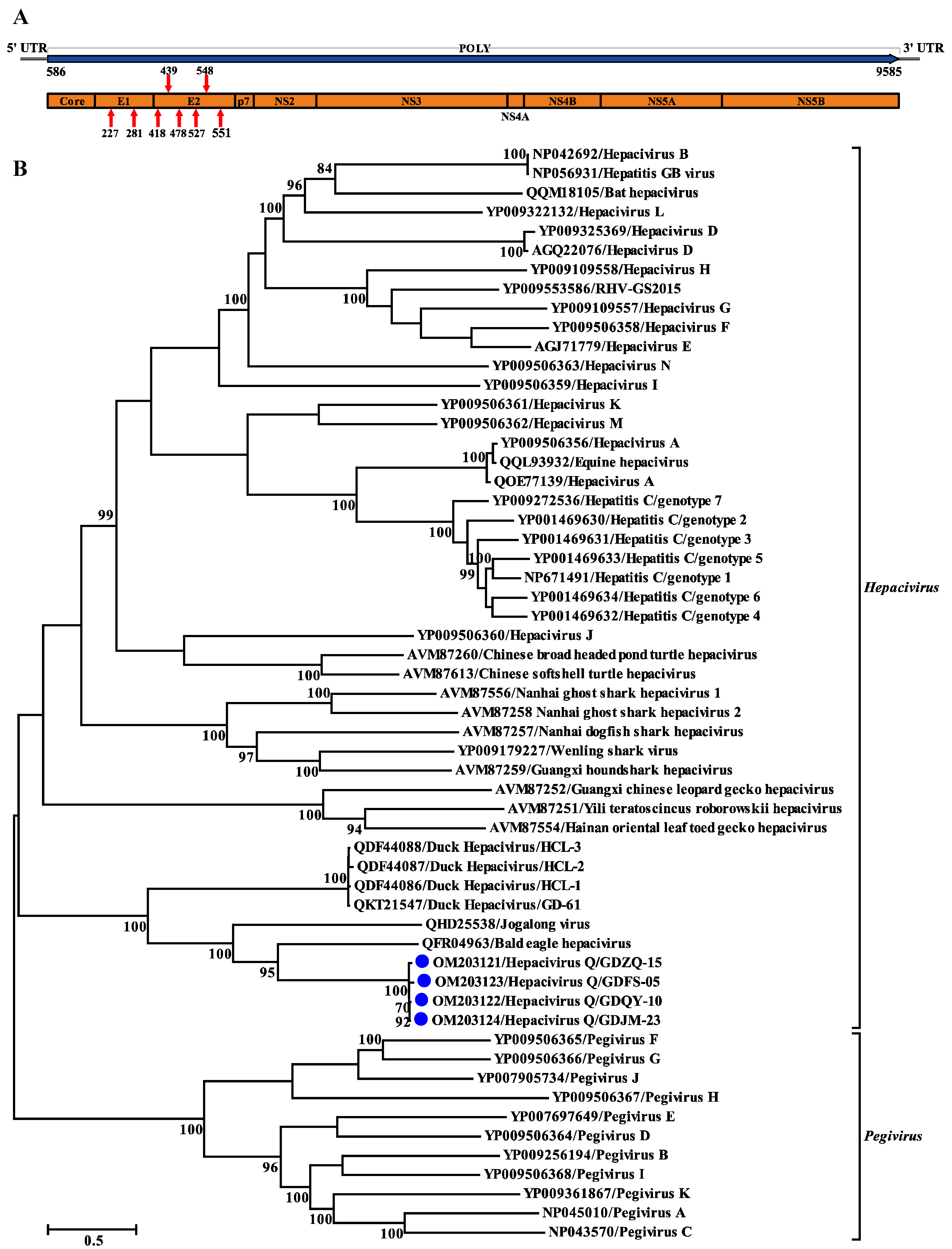
3.2. Genomic Characterization of Hepacivirus Q
3.3. Prevalence and Genetic Diversity of Hepacivirus Q in Guangdong
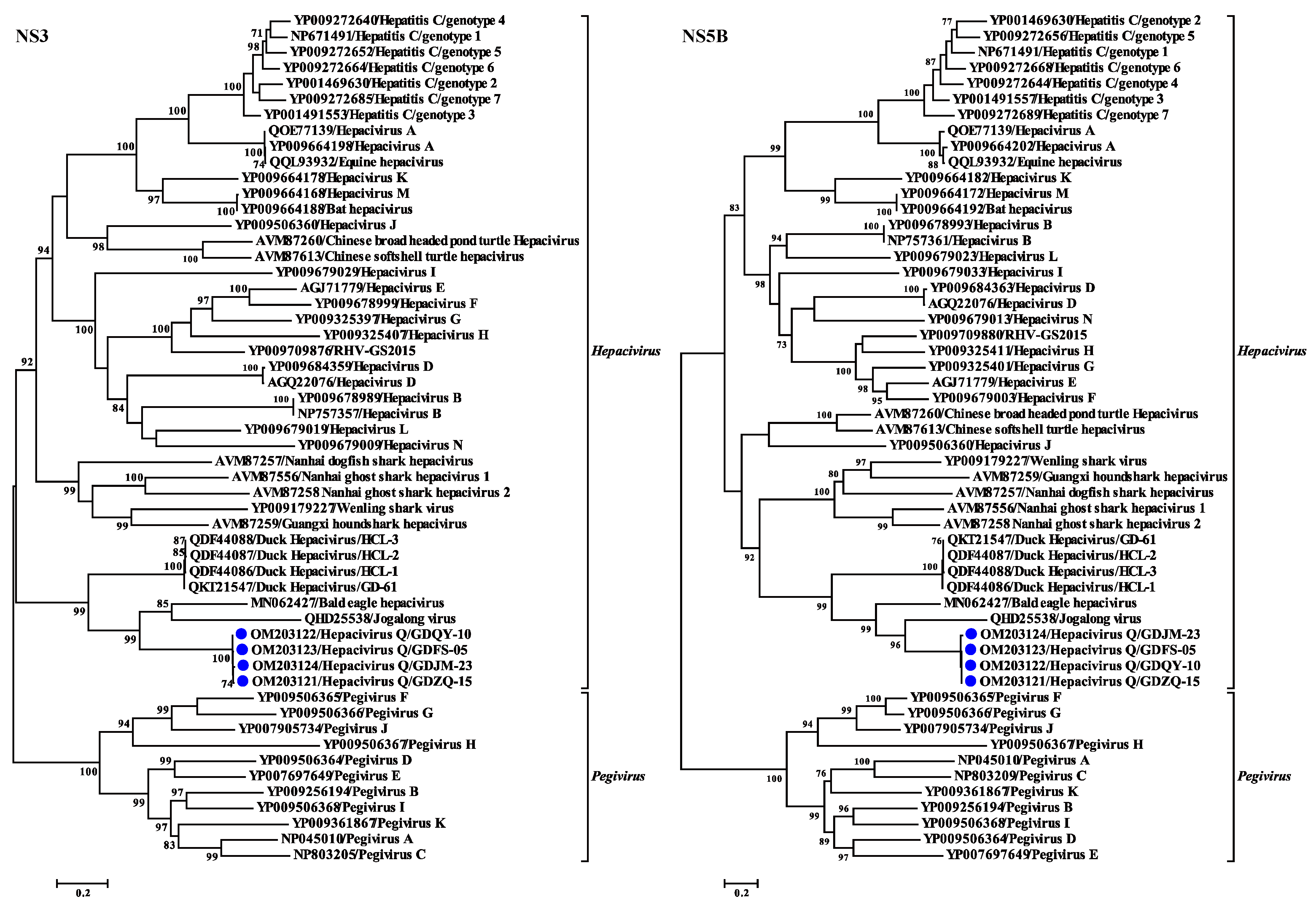
3.4. Phylogenetic and Recombination Analyses of Hepacivirus Q
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Simmonds, P.; Becher, P.; Bukh, J.; Gould, E.A.; Meyers, G.; Monath, T.; Muerhoff, S.; Pletnev, A.; Rico-Hesse, R.; Smith, D.B.; et al. ICTV virus taxonomy profile. J. Gen. Virol. 2017, 98, 2. [Google Scholar] [CrossRef] [PubMed]
- Moradpour, D.; Penin, F.; Rice, C.M. Replication of hepatitis C virus. Nat. Rev. Microbiol. 2007, 5, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Penin, F.; Dubuisson, J.; Rey, F.A.; Moradpour, D.; Pawlotsky, J.M. Structural biology of hepatitis C virus. Hepatology 2004, 39, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Boonstra, A.; van der Laan, L.J.; Vanwolleghem, T.; Janssen, H.L. Experimental models for hepatitis C viral infection. Hepatology 2009, 50, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Lyons, S.; Kapoor, A.; Sharp, C.; Schneider, B.S.; Wolfe, N.D.; Culshaw, G.; Corcoran, B.; McGorum, B.C.; Simmonds, P. Nonprimate hepaciviruses in domestic horses, United kingdom. Emerg. Infect. Dis. 2012, 18, 1976–1982. [Google Scholar] [CrossRef] [Green Version]
- Lauck, M.; Sibley, S.D.; Lara, J.; Purdy, M.A.; Khudyakov, Y.; Hyeroba, D.; Tumukunde, A.; Weny, G.; Switzer, W.M.; Chapman, C.A.; et al. A novel hepacivirus with an unusually long and intrinsically disordered NS5A protein in a wild Old World primate. J. Virol. 2013, 87, 8971–8981. [Google Scholar] [CrossRef] [Green Version]
- Quan, P.L.; Firth, C.; Conte, J.M.; Williams, S.H.; Zambrana-Torrelio, C.M.; Anthony, S.J.; Ellison, J.; Gilbert, A.T.; Kuzmin, I.V.; Niezgoda, M.; et al. Bats are a major natural reservoir for hepaciviruses and pegiviruses. Proc. Natl. Acad. Sci. USA 2013, 110, 8194–8199. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Lukashev, A.N.; Gmyl, A.; Coutard, B.; Adam, A.; Ritz, D.; Leijten, L.M.; Van Riel, D.; et al. Evidence for novel hepaciviruses in rodents. PLoS Pathog. 2013, 9, e1003438. [Google Scholar] [CrossRef] [Green Version]
- Baechlein, C.; Fischer, N.; Grundhoff, A.; Alawi, M.; Indenbirken, D.; Postel, A.; Baron, A.L.; Offinger, J.; Becker, K.; Beineke, A.; et al. Identification of a novel hepacivirus in domestic cattle from Germany. J. Virol. 2015, 89, 7007–7015. [Google Scholar] [CrossRef] [Green Version]
- Corman, V.M.; Grundhoff, A.; Baechlein, C.; Fischer, N.; Gmyl, A.; Wollny, R.; Dei, D.; Ritz, D.; Binger, T.; Adankwah, E.; et al. Highly divergent hepaciviruses from African cattle. J. Virol. 2015, 89, 5876–5882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walter, S.; Rasche, A.; Moreira-Soto, A.; Pfaender, S.; Bletsa, M.; Corman, V.M.; Aguilar-Setien, A.; García-Lacy, F.; Hans, A.; Todt, D.; et al. Differential infection patterns and recent evolutionary origins of equine hepaciviruses in donkeys. J. Virol. 2017, 91, e01711–e01716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Lin, X.D.; Vasilakis, N.; Tian, J.H.; Li, C.X.; Chen, L.J.; Eastwood, G.; Diao, X.-N.; Chen, M.-H.; Chen, X.; et al. Divergent viruses discovered in arthropods and vertebrates revise the evolutionary history of the flaviviridae and related viruses. J. Virol. 2016, 90, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, L.; Jin, M.; Feng, C.; Wang, X.; Zhang, D. A highly divergent hepacivirus-like flavivirus in domestic ducks. J. Gen. Virol. 2019, 100, 1234–1240. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, T.L.; Sibley, S.D.; Pinkerton, M.E.; Dunn, C.D.; Long, L.J.; White, L.C.; Strom, S.M. Multidecade mortality and a homolog of hepatitis C virus in Bald eagles (Haliaeetus leucocephalus), the national bird of the USA. Sci. Rep. 2019, 9, 14953. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.D.; Chen, X.; Tian, J.H.; Chen, L.J.; Li, K.; Wang, W.; Eden, J.S.; Shen, J.J.; Liu, L.; et al. The evolutionary history of vertebrate RNA viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef]
- Porter, A.F.; Pettersson, J.H.; Chang, W.S.; Harvey, E.; Rose, K.; Shi, M.; Eden, J.S.; Buchmann, J.; Moritz, C.; Holmes, E.C. Novel hepaci- and pegi-like viruses in native Australian wildlife and non-human primates. Virus Evol. 2020, 6, veaa064. [Google Scholar] [CrossRef]
- Chang, W.S.; Eden, J.S.; Hartley, W.J.; Shi, M.; Rose, K.; Holmes, E.C. Metagenomic discovery and co-infection of diverse wobbly possum disease viruses and a novel hepacivirus in Australian brushtail possums. One Health Outlook 2019, 1, 5. [Google Scholar] [CrossRef] [Green Version]
- Williams, S.H.; Levy, A.; Yates, R.A.; Somaweera, N.; Neville, P.J.; Nicholson, J.; Lindsay, M.D.A.; Mackenzie, J.S.; Jain, K.; Imrie, A.; et al. Discovery of Jogalong virus, a novel hepacivirus identified in a Culex annulirostris (Skuse) mosquito from the Kimberley region of Western Australia. PLoS ONE 2020, 15, e0227114. [Google Scholar] [CrossRef] [Green Version]
- Harvey, E.; Rose, K.; Eden, J.S.; Lo, N.; Abeyasuriya, T.; Shi, M.; Doggett, S.L.; Holmes, E.C. Extensive diversity of RNA viruses in Australian Ticks. J. Virol. 2019, 93, e01358-18. [Google Scholar] [CrossRef] [Green Version]
- Shao, J.W.; Guo, L.Y.; Yuan, Y.X.; Ma, J.; Chen, J.M.; Liu, Q. A novel subtype of bovine hepacivirus identified in ticks reveals the genetic diversity and evolution of bovine hepacivirus. Viruses 2021, 13, 2206. [Google Scholar] [CrossRef]
- Li, L.L.; Liu, M.M.; Shen, S.; Zhang, Y.J.; Xu, Y.L.; Deng, H.Y.; Deng, F.; Duan, Z.J. Detection and characterization of a novel hepacivirus in long-tailed ground squirrels (Spermophilus undulatus) in China. Arch. Virol. 2019, 164, 2401–2410. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Zhao, J.; Ou, J.; Li, S. Novel HCV-Like virus detected in avian livers in Southern China and its implications for natural recombination events. Virol. Sin. 2021, 36, 149–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Li, W.F.; Yuan, S.; Guo, J.Y.; Li, Z.L.; Chi, S.H.; Huang, W.J.; Li, X.W.; Huang, S.J.; Shao, J.W. Meta-transcriptomic analysis reveals a new subtype of genotype 3 avian hepatitis E virus in chicken flocks with high mortality in Guangdong, China. BMC Vet. Res. 2019, 15, 131. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Li, D.; Liu, C.M.; Luo, R.; Sadakane, K.; Lam, T.W. MEGAHIT: An ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 2015, 31, 1674–1676. [Google Scholar] [CrossRef] [Green Version]
- Buchfink, B.; Xie, C.; Huson, D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods 2015, 12, 59–60. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [Green Version]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [Green Version]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 2015, 4, e05378. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guindon, S.; Gascuel, O. A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. ProtTest 3: Fast selection of best-fit models of protein evolution. Bioinformatics 2011, 27, 1164–1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D.P.; Murrell, B.; Golden, M.; Khoosal, A.; Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 2015, 1, vev003. [Google Scholar] [CrossRef] [Green Version]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [CrossRef] [Green Version]
- Kapoor, A.; Simmonds, P.; Gerold, G.; Qaisar, N.; Jain, K.; Henriquez, J.A.; Firth, C.; Hirschberg, D.L.; Rice, C.M.; Shields, S.; et al. Characterization of a canine homolog of hepatitis C virus. Proc. Natl. Acad. Sci. USA 2011, 108, 11608–11613. [Google Scholar] [CrossRef] [Green Version]
- Galli, A.; Bukh, J. Comparative analysis of the molecular mechanisms of recombination in hepatitis C virus. Trends Microbiol. 2014, 22, 354–364. [Google Scholar] [CrossRef]
- Lu, G.; Ou, J.; Sun, Y.; Wu, L.; Xu, H.; Zhang, G.; Li, S. Natural recombination of equine hepacivirus subtype 1 within the NS5A and NS5B genes. Virology 2019, 533, 93–98. [Google Scholar] [CrossRef]
- Bletsa, M.; Vrancken, B.; Gryseels, S.; Boonen, I.; Fikatas, A.; Li, Y.; Laudisoit, A.; Lequime, S.; Bryja, J.; Makundi, R.; et al. Molecular detection and genomic characterization of diverse hepaciviruses in African rodents. Virus Evol. 2021, 7, veab036. [Google Scholar] [CrossRef]
- Hartlage, A.S.; Cullen, J.M.; Kapoor, A. The strange, expanding world of animal hepaciviruses. Annu. Rev. Virol. 2016, 3, 53–75. [Google Scholar] [CrossRef] [Green Version]
- Reuter, G.; Maza, N.; Pankovics, P.; Boros, A. Non-primate hepacivirus infection with apparent hepatitis in a horse-Short communication. Acta Vet. Hung. 2014, 62, 422–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Value for the Indicated Virus in Relation to Hepacivirus Q | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Protein | A | B | C | D | E | F | G | H | I | J | K | L | M | N | RHV | JgV | HCL1 | GD61 | BeHV |
| Polyprotein | 23.9 | 24.6 | 23.7 | 24.6 | 24.8 | 24.0 | 24.0 | 24.0 | 24.1 | 24.7 | 24.6 | 25.5 | 25.4 | 24.1 | 25.0 | 39.1 | 35.5 | 35.6 | 46.6 |
| Core | 26.5 | 24.1 | 23.9 | 25.2 | 24.7 | 28.1 | 26.5 | 26.5 | 23.9 | 28.6 | 25.8 | 24.8 | 27.5 | 23.1 | 24.1 | 9.4 | 28.8 | 28.2 | 47.6 |
| E1 | 24.6 | 20.8 | 19.4 | 19.7 | 22.5 | 19.3 | 18.5 | 17.6 | 18.7 | 26.8 | 22.9 | 16.0 | 23.9 | 16.1 | 18.2 | 45.2 | 38.6 | 38.6 | 55.6 |
| E2 | 11.6 | 13.2 | 15.0 | 15.0 | 15.9 | 16.5 | 17.0 | 15.2 | 12.8 | 9.3 | 12.1 | 15.0 | 12.5 | 13.7 | 14.8 | 29.7 | 30.9 | 31.7 | 44.6 |
| p7 | 21.0 | 17.5 | 27.4 | 15.6 | 11.1 | 10.9 | 15.1 | 20.0 | 17.3 | 18.6 | 33.9 | 15.3 | 27.4 | 15.0 | 19.6 | 31.1 | 31.5 | 31.5 | 47.3 |
| NS2 | 15.6 | 15.7 | 17.0 | 16.1 | 15.8 | 15.8 | 16.8 | 17.9 | 17.8 | 17.8 | 17.5 | 18.8 | 17.5 | 15.3 | 14.3 | 32.2 | 29.9 | 29.9 | 41.9 |
| NS3 | 37.2 | 38.9 | 37.5 | 39.2 | 35.9 | 37.6 | 36.7 | 35.6 | 35.6 | 38.8 | 38.1 | 41.2 | 37.6 | 37.3 | 40.2 | 46.6 | 48.1 | 48.3 | 53.0 |
| NS4A | 17.6 | 25.5 | 21.6 | 26.9 | 23.5 | 25.5 | 19.6 | 17.3 | 21.2 | 15.4 | 21.2 | 27.5 | 25.0 | 23.5 | 29.4 | 42.3 | 28.8 | 28.8 | 46.2 |
| NS4B | 21.6 | 21.8 | 22.0 | 21.8 | 20.2 | 16.8 | 17.5 | 22.1 | 22.4 | 24.1 | 23.5 | 19.4 | 24.8 | 23.2 | 19.3 | 35.0 | 32.4 | 32.4 | 41.6 |
| NS5A | 12.0 | 11.9 | 10.4 | 11.7 | 15.7 | 11.6 | 10.8 | 12.3 | 12.3 | 13.6 | 11.4 | 9.2 | 12.1 | 15.5 | 9.8 | 19.7 | 12.1 | 11.7 | 27.2 |
| NS5B | 31.6 | 31.6 | 31.1 | 34.0 | 31.4 | 30.9 | 32.1 | 32.1 | 33.0 | 34.4 | 34.2 | 34.6 | 35.1 | 32.0 | 32.2 | 56.3 | 43.1 | 43.3 | 54.8 |
| Cleavage Site at: | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Virus | C/E1 | E1/E2 | E2/p7 | P7/NS2 | NS2/NS3 | NS3/NS4A | NS4A/NS4B | NS4B/NS5A | NS5A/NS5B |
| Hepacivirus A | GEA/SVV | VSC/DNY | AEA/YLS | AWA/FDN | RLL/SPI | TQT/NAW | EEC/FDH | QNC/DFT | ESC/SLS |
| Hepacivirus B | CSG/ARV | IEA/TSG | MAA/GLP | ASA/FDT | AIT/APF | VNT/SGT | EEC/ASF | DDC/GLI | FSC/SMS |
| Hepacivirus C | ASA/YQV | VDA/ETH | AEA/ALE | AYA/LDT | RLL/API | VVT/STW | EEC/SQH | TPC/SGS | VCC/SMS |
| Hepacivirus D | GAS/CVV | VTS/TSL | AAA/AAM | AVG/FDD | MLN/PFS | NDC/SLV | EEC/SFG | AQC/DGG | AKC/ASW |
| Hepacivirus E | ATA/VSN | AAA/AAP | AYA/FTP | AYA/ISL | KYT/IPF | FFA/SGY | EEC/YNW | DLC/TPP | HSC/SMS |
| Hepacivirus F | GGA/VTN | VKA/LAL | AFA/FTP | SAY/SLN | ERT/APF | YFA/STT | EEC/YQW | EDC/SCR | HEC/SSW |
| Hepacivirus G | ASA/GIF | VAA/PVS | VGA/LEV | EAY/EGG | RFT/APF | YFA/ETV | EEC/STQ | DVC/TSP | TDC/SWS |
| Hepacivirus H | AEA/NLL | SAV/AVP | SEA/VPT | RAE/QFD | QLT/KPF | YYC/GLV | EEC/ANE | EIC/DGS | SSC/SKS |
| Hepacivirus I | VEP/KPL | SVA/APV | YAQ/PPL | VEA/FSS | QLS/SPV | ELA/STW | EEC/ALD | EPC/TDS | ETC/TYS |
| Hepacivirus J | AVS/HWC | AEG/LPF | ANA/LVL | AQG/GCL | RLT/APF | EEM/TDG | EEC/GFD | AEC/AGG | TSC/NYS |
| Hepacivirus K | GEA/SYA | AQA/NPI | ADA/ALT | AVG/GPY | RHC/SPI | DDT/STG | EEC/LSY | SEC/AFF | DEC/SAS |
| Hepacivirus L | AES/VPA | AAA/MPV | AWG/WPA | AQA/ASL | ERN/APM | YSA/GGL | EEC/MQT | AEC/DGM | SKM/SRS |
| Hepacivirus M | VDA/SFA | SQA/AEH | AEG/AME | VGG/GPV | RHC/SPI | TPT/SAW | EEC/ADY | RNC/SCS | SPC/SAS |
| Hepacivirus N | VSG/YRQ | VEA/TTT | ATA/ALL | VTA/LDF | APC/SPI | LDV/WGA | EEC/WGF | VPC/GFN | KEC/SYS |
| JgV | AVA/FSD | AQA/GTH | IEG/AVN | VAG/FWF | KLA/API | SAG/LTV | EEC/AST | TNC/TSP | VCC/GES |
| DuHV-HCL1 | ASA/DHI | GMA/DRS | AEG/MLS | VLG/ASV | QYT/API | NCS/AAY | EEC/SAE | YEC/NSE | ESC/SFS |
| DuHV-GD61 | ASA/DHI | GMA/DRS | AEG/MLS | VLG/ASV | QYT/API | NCS/AAY | EEC/SAE | YEC/NSE | ESC/SFS |
| BeHV | ADS/SHD | VEG/GLQ | VTA/AVN | VSG/TEV | NWS/APL | VSC/SLY | EEC/AGN | YDC/ANS | HSC/SAS |
| Hepacivirus Q | EPA/THL | VEG/GLV | VGA/AIN | VAG/SEI | RWS/APF | VNC/SML | EEC/SSS | QLC/SSN | CSC/SMS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.-L.; Yao, X.-Y.; Zhang, Y.-Q.; Lv, Z.-H.; Liu, H.; Sun, J.; Shao, J.-W. A Highly Divergent Hepacivirus Identified in Domestic Ducks Further Reveals the Genetic Diversity of Hepaciviruses. Viruses 2022, 14, 371. https://doi.org/10.3390/v14020371
Zhang X-L, Yao X-Y, Zhang Y-Q, Lv Z-H, Liu H, Sun J, Shao J-W. A Highly Divergent Hepacivirus Identified in Domestic Ducks Further Reveals the Genetic Diversity of Hepaciviruses. Viruses. 2022; 14(2):371. https://doi.org/10.3390/v14020371
Chicago/Turabian StyleZhang, Xue-Lian, Xin-Yan Yao, Yu-Qian Zhang, Zhi-Hang Lv, Hong Liu, Jing Sun, and Jian-Wei Shao. 2022. "A Highly Divergent Hepacivirus Identified in Domestic Ducks Further Reveals the Genetic Diversity of Hepaciviruses" Viruses 14, no. 2: 371. https://doi.org/10.3390/v14020371
APA StyleZhang, X.-L., Yao, X.-Y., Zhang, Y.-Q., Lv, Z.-H., Liu, H., Sun, J., & Shao, J.-W. (2022). A Highly Divergent Hepacivirus Identified in Domestic Ducks Further Reveals the Genetic Diversity of Hepaciviruses. Viruses, 14(2), 371. https://doi.org/10.3390/v14020371






