Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of A. hydrophila Specific Bacteriophages
2.1.1. Isolation of A. hydrophila Specific Bacteriophages
2.1.2. Phage Virion Morphology
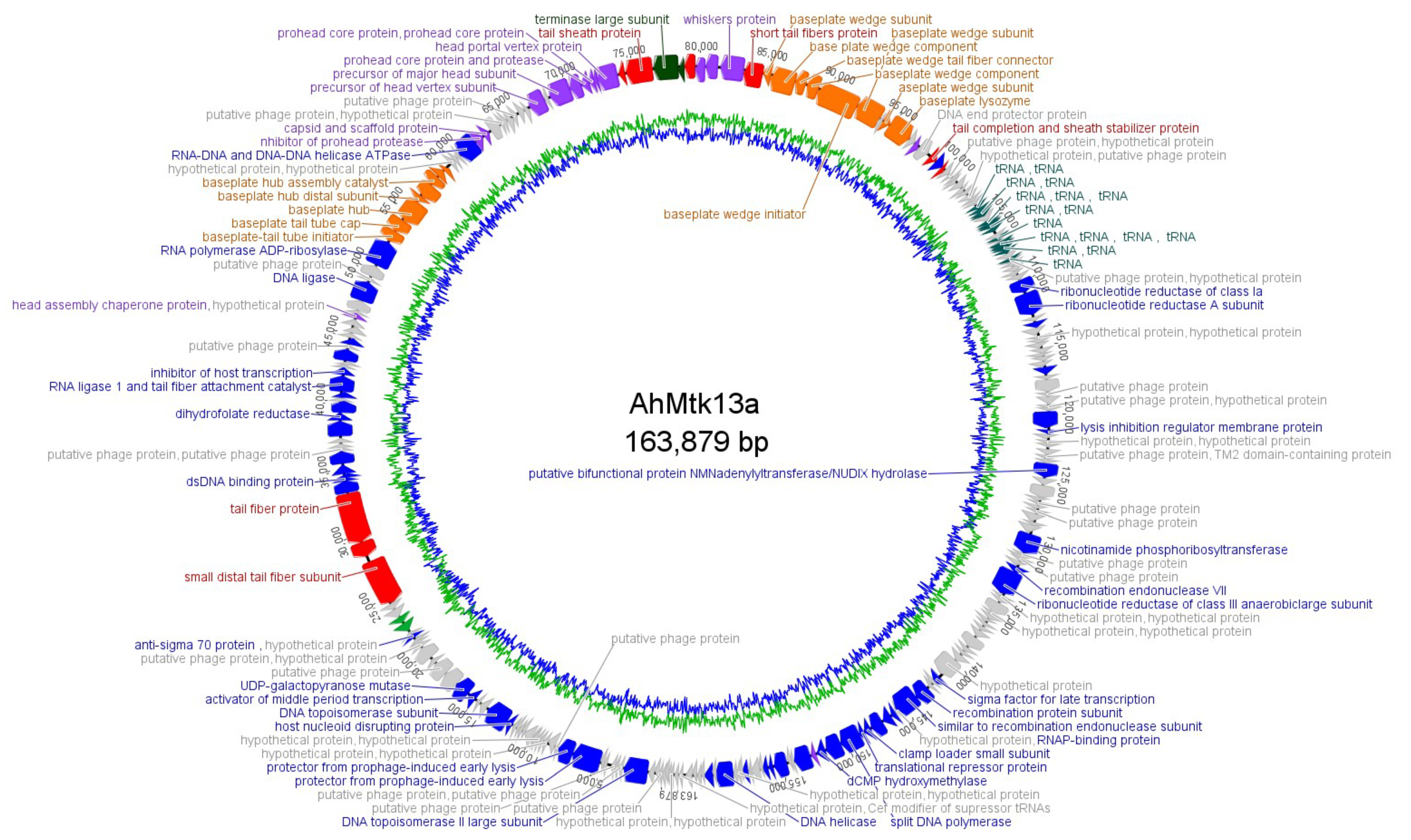
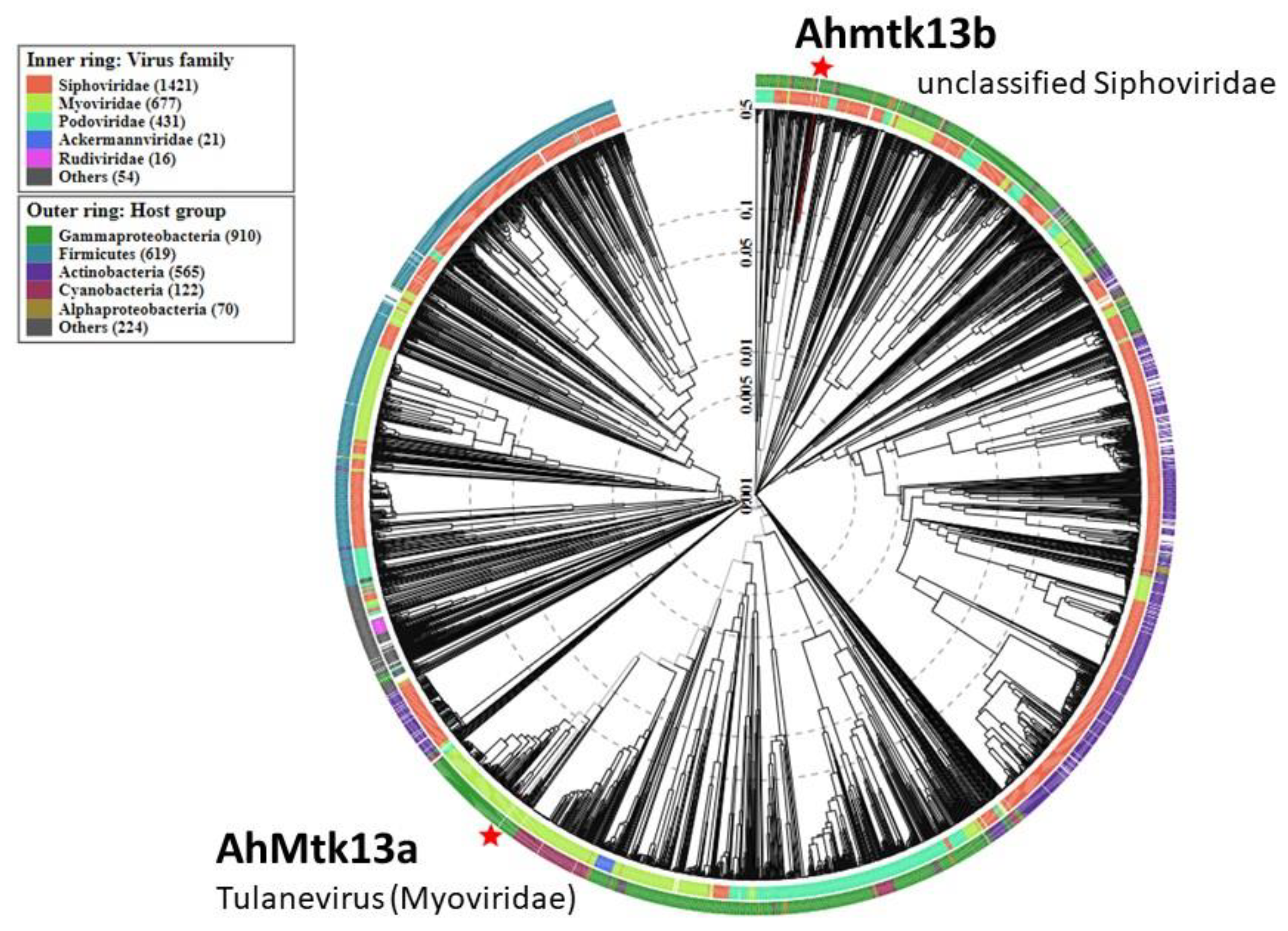
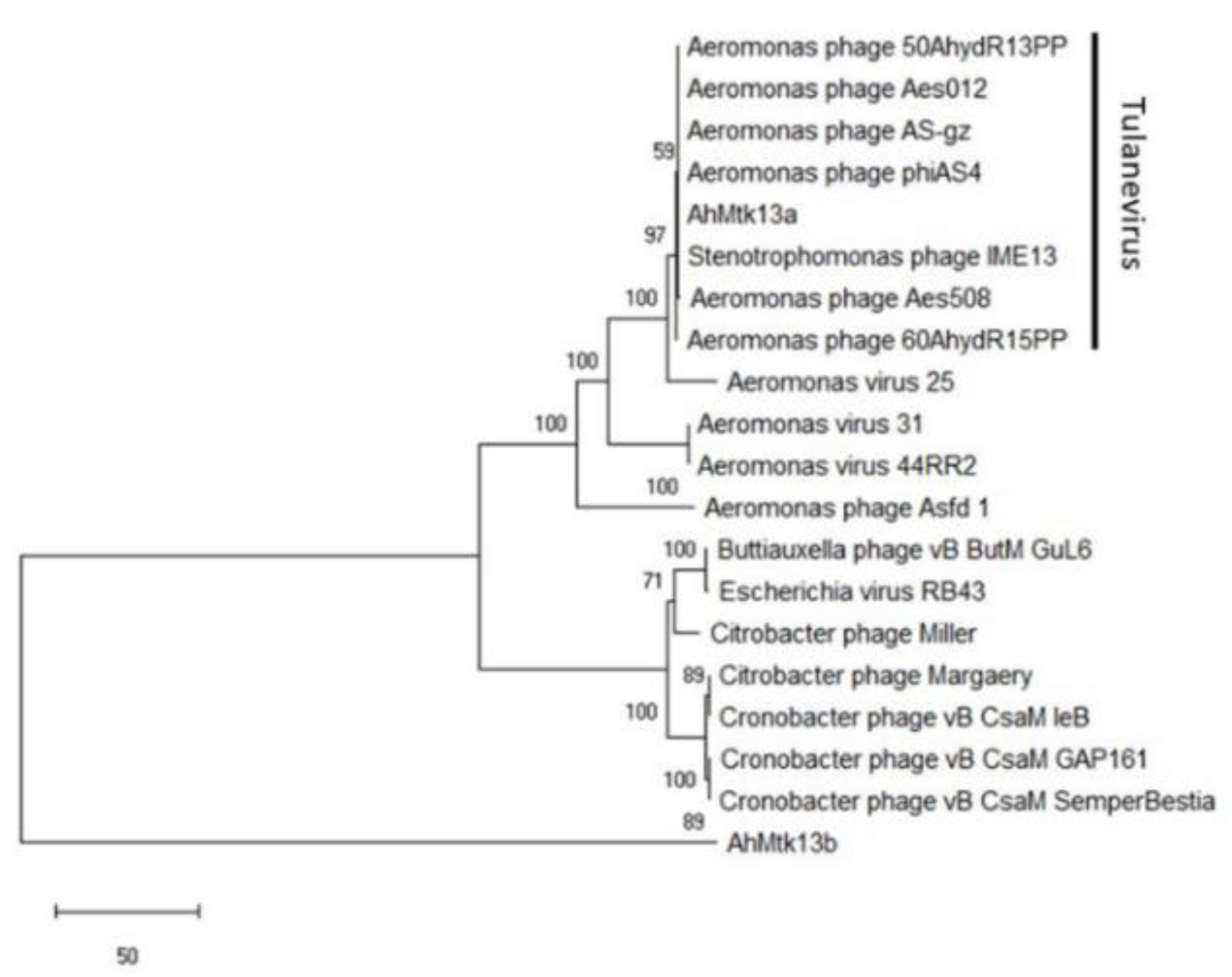
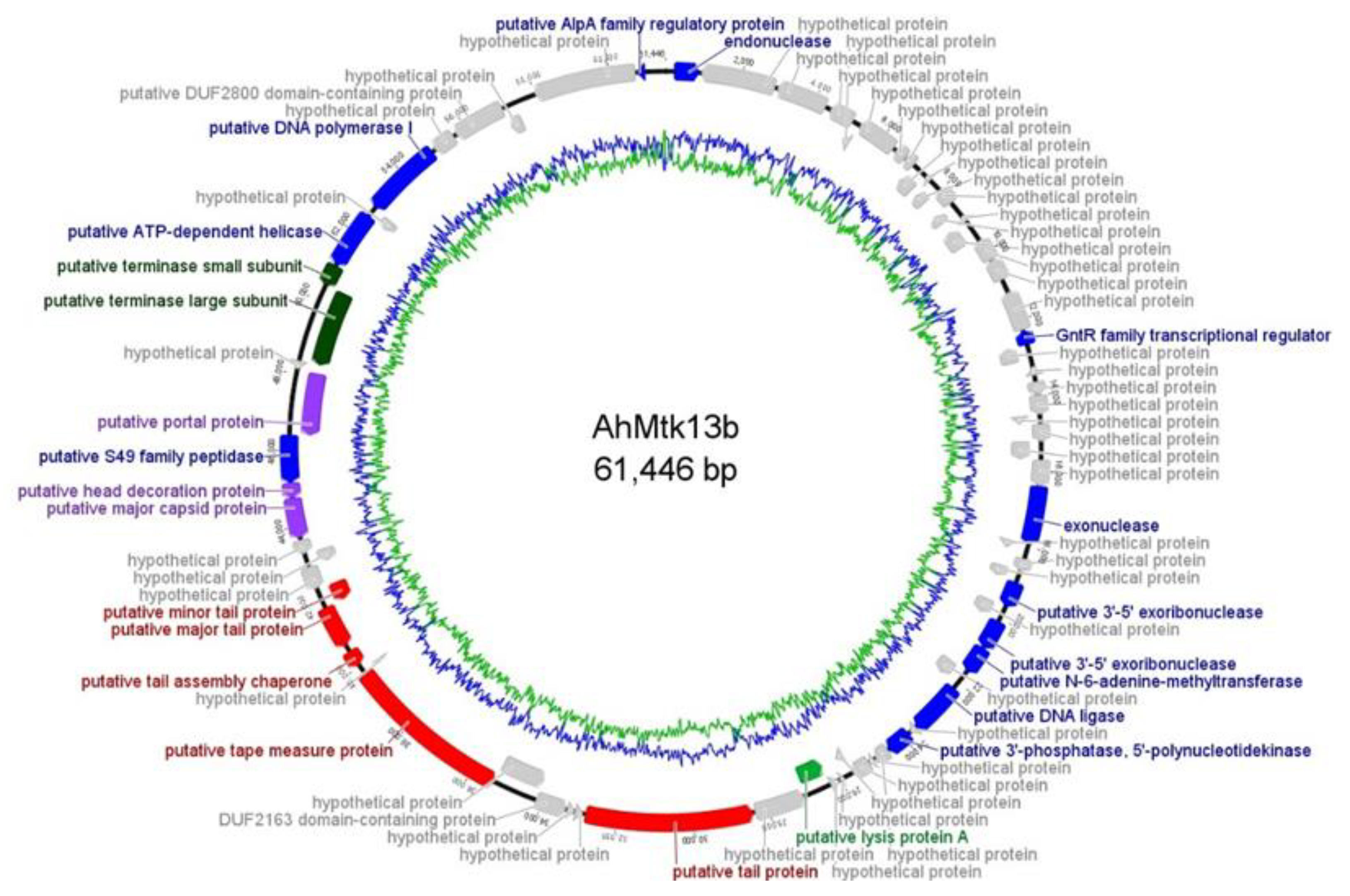
2.1.3. Whole Genome Sequencing of AhMtk13a and AhMtk13b Phages and In Silico Analysis
2.1.4. Phage Host Range
2.1.5. The Phage One-Step Growth Cycle
2.1.6. Lysis Stability of AhMtk13a in Liquid Culture
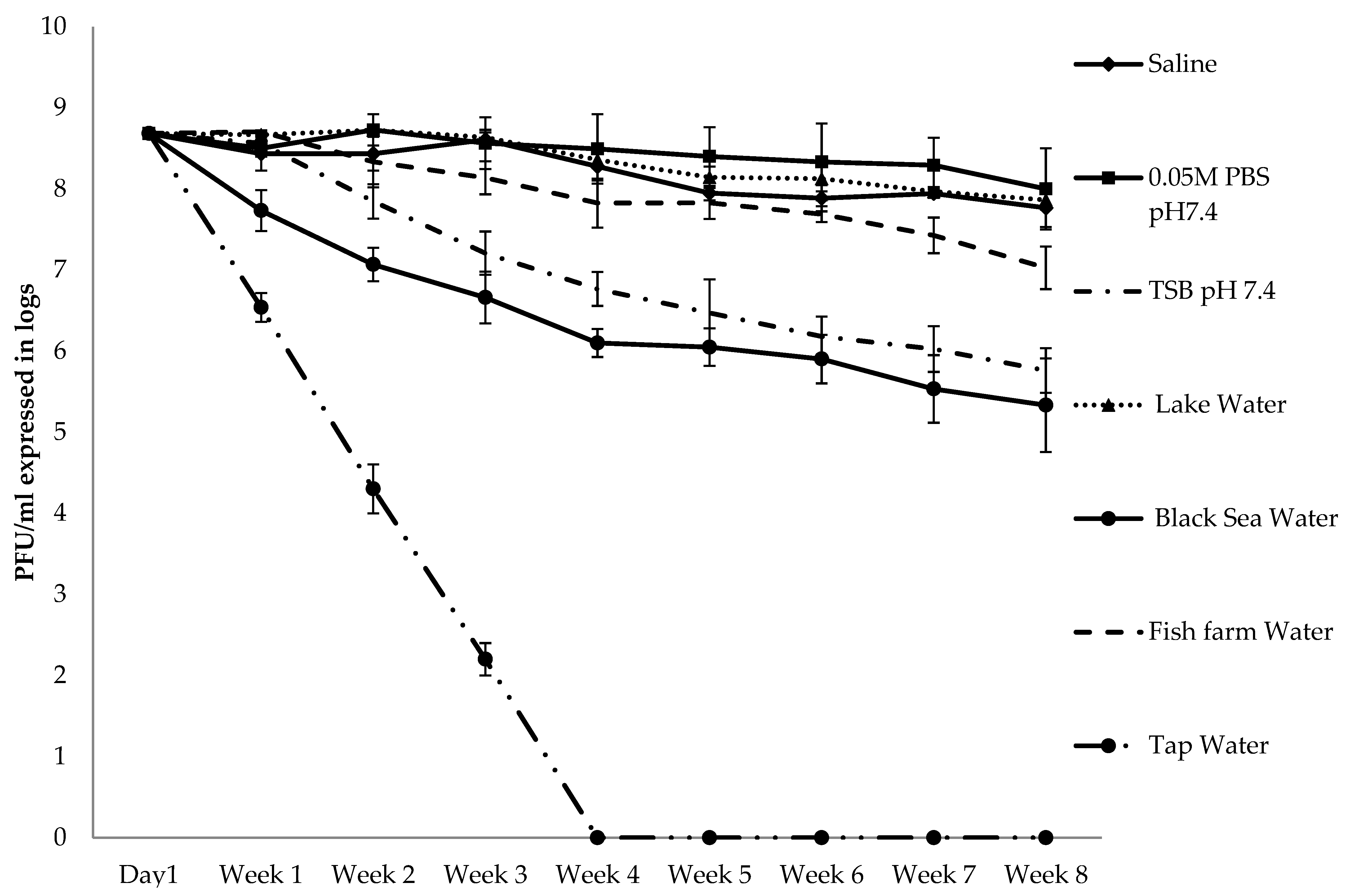
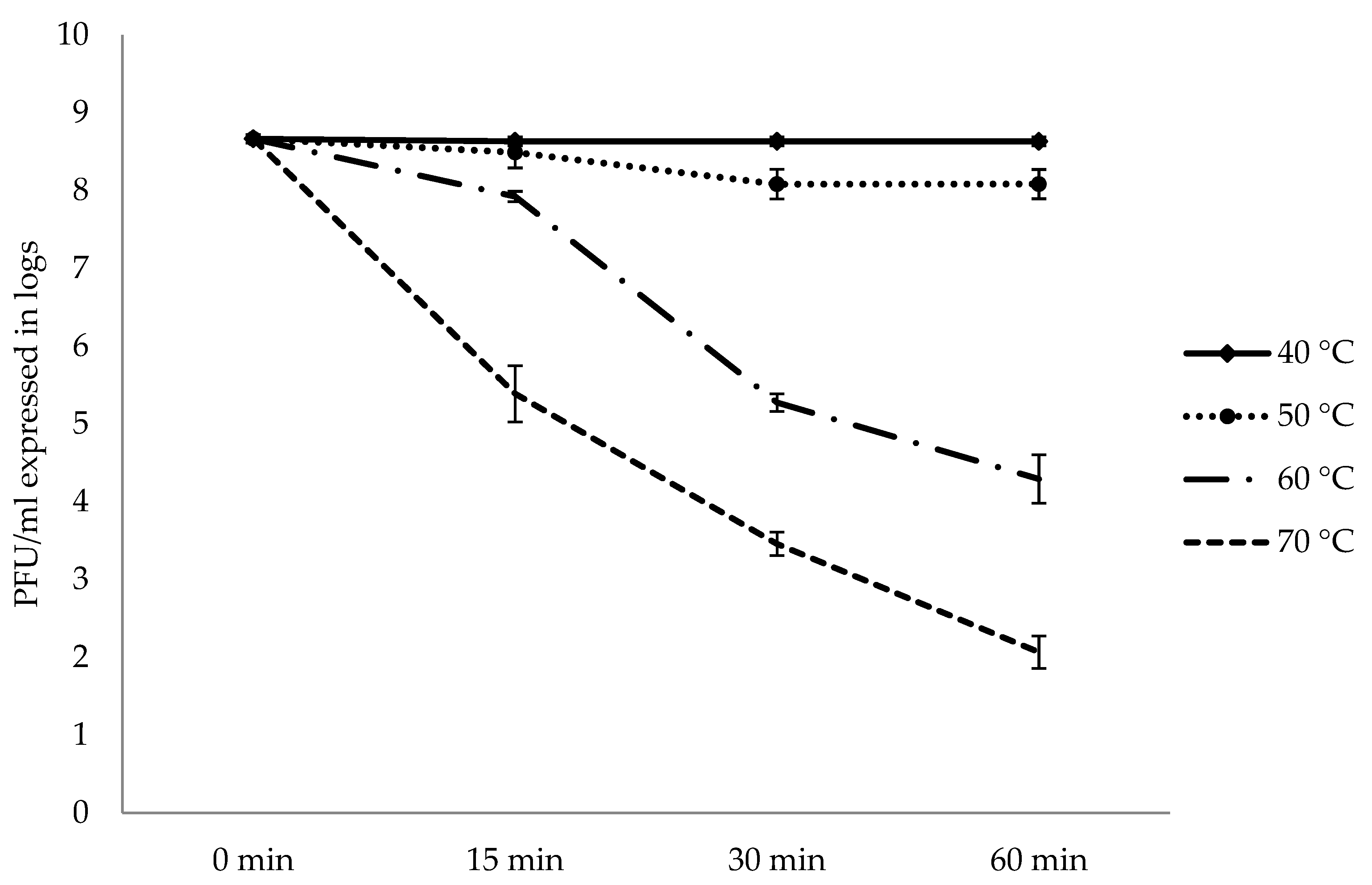
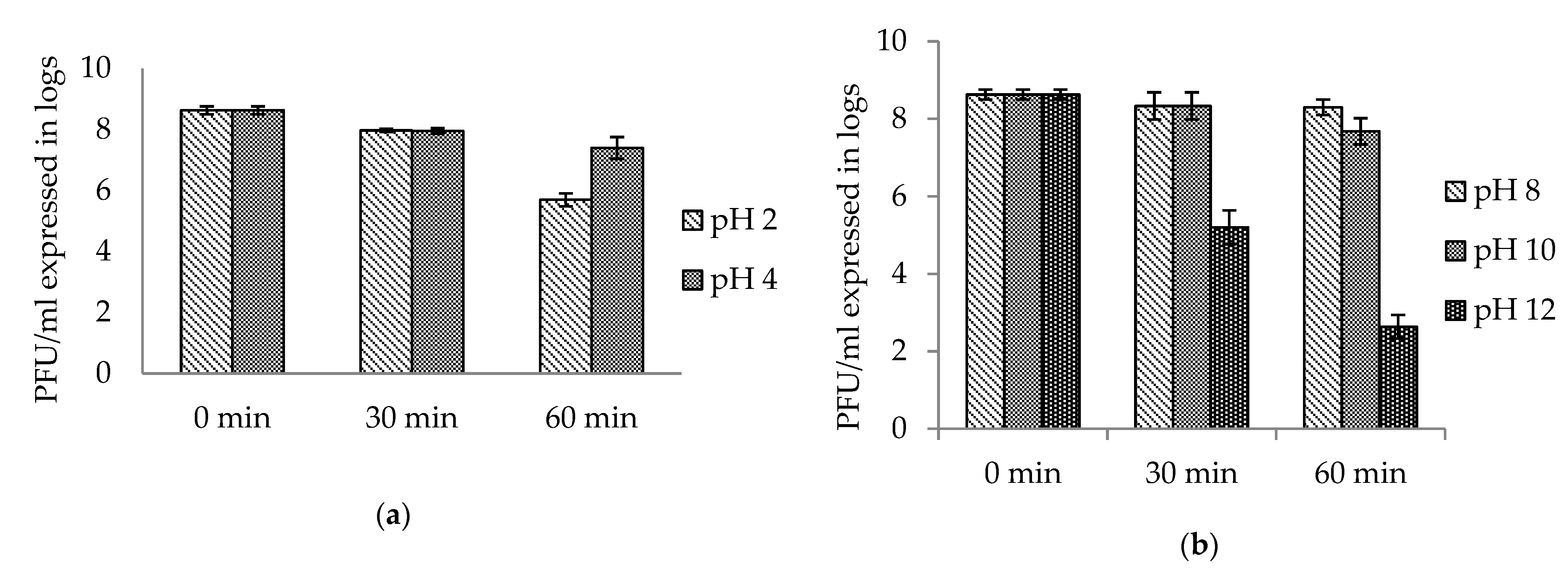
2.2. Stability and Infectivity of Bacteriophage AhMTK13a in Different Environmental Conditions
2.2.1. Survival in Different Liquid Environments
2.2.2. Influence of Temperature on AhMtk13a Phage Survival
2.2.3. Influence of Acidic and Alkaline Environment on Survival of AhMtk13a Phage
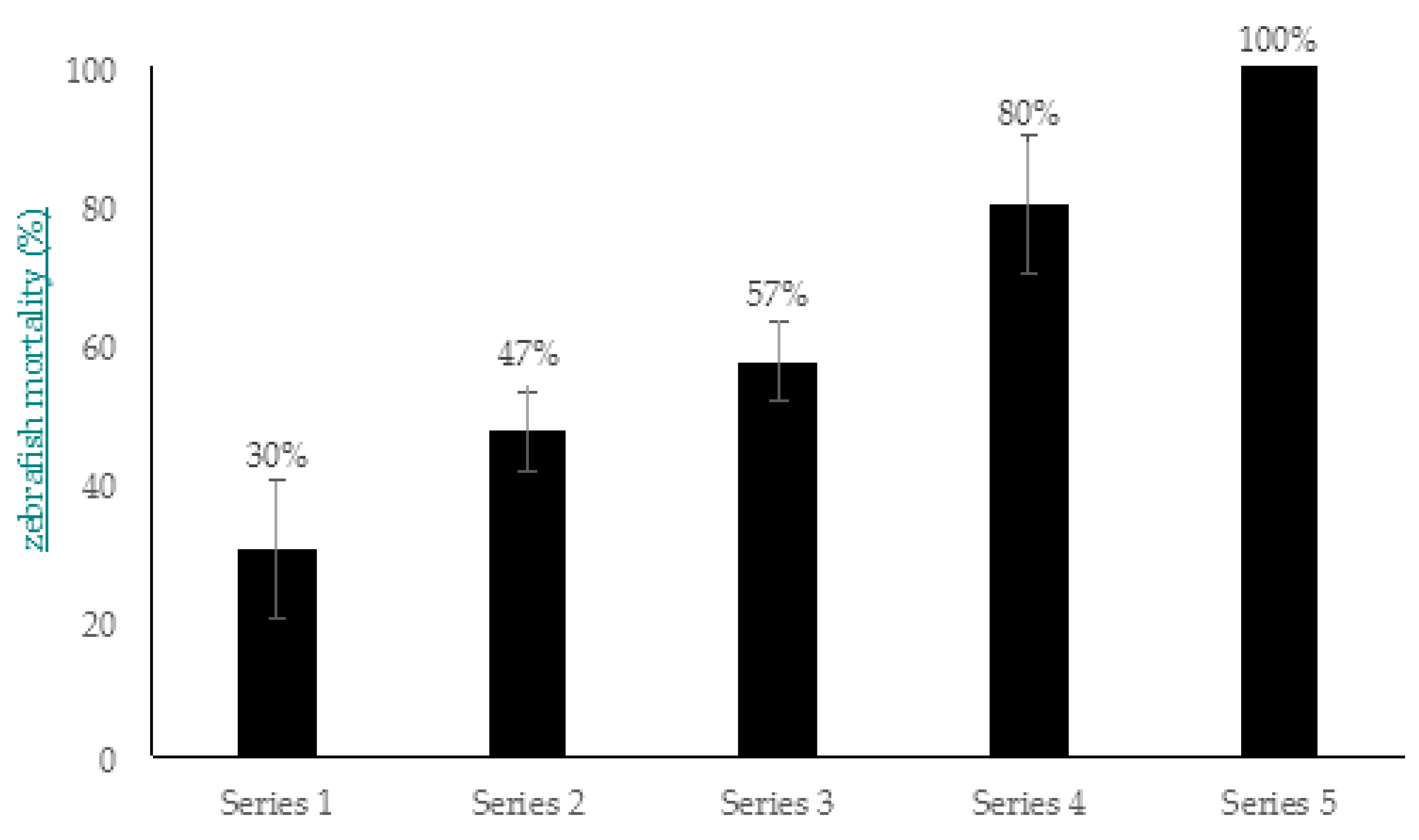
2.3. A. hydrophila GW3-10 Pathogenicity Experiments: Development of Infection Model
2.4. Estimation of Antibacterial Efficacy of AhMtk13A Phage in the Experimental A. hydrophila GW3-10 Infection in Zebrafish Model: Experimental Series 1
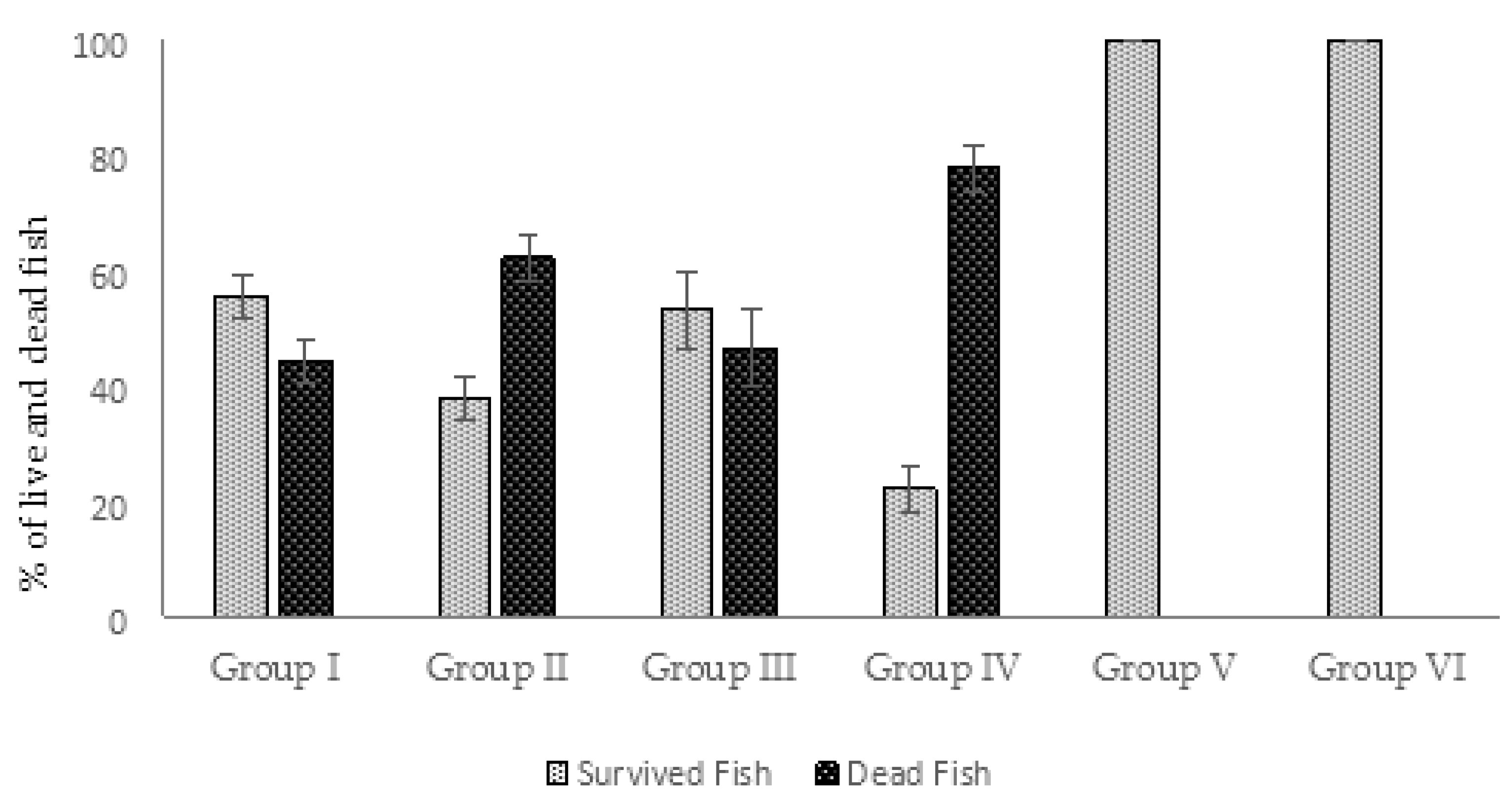
2.4.1. Protective Effect of AhMtk13a Phage Administration on Infected Zebrafish: Comparative Mortality Rates
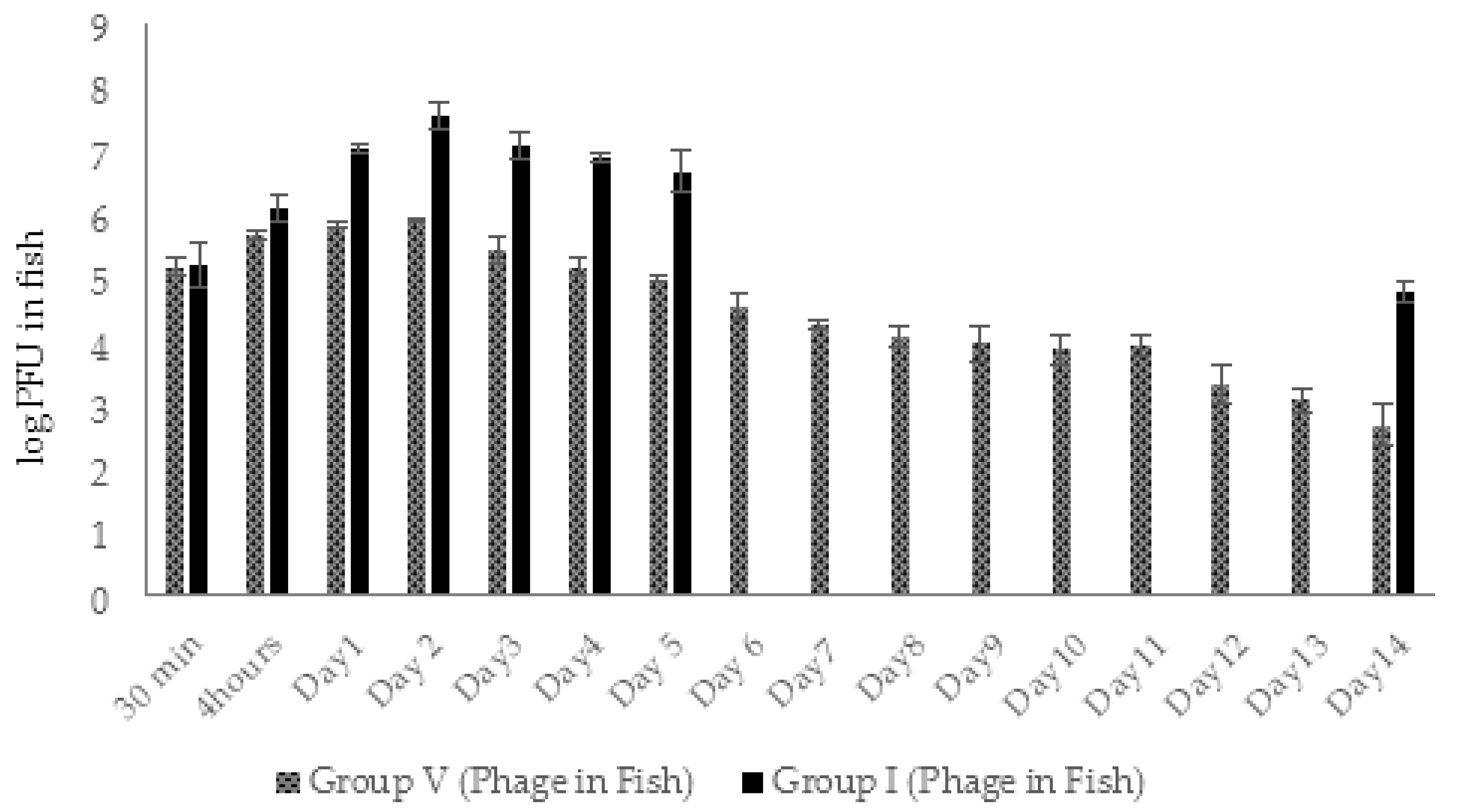
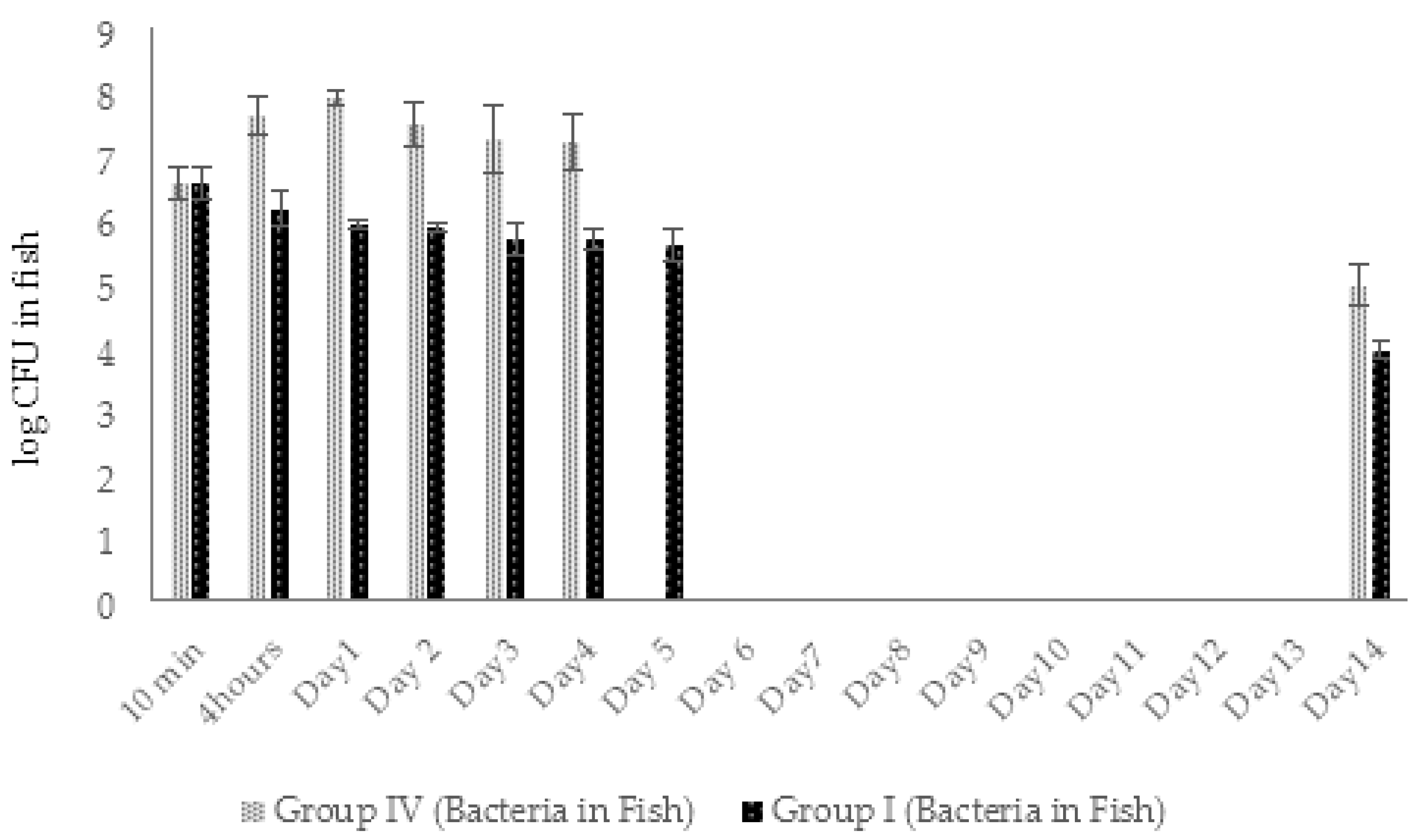
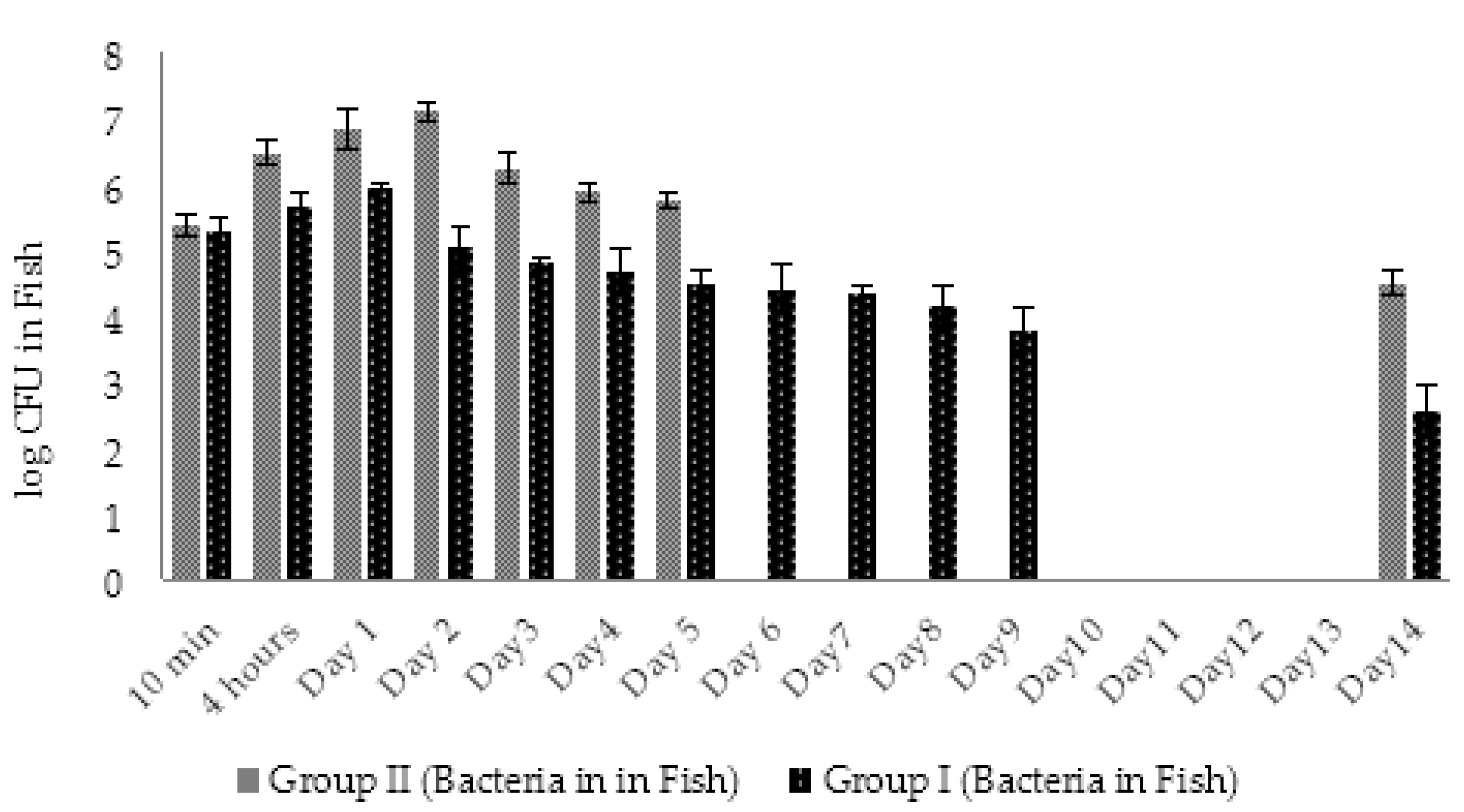
2.4.2. Dynamic Changes in Abundance of Phages and Bacteria in Zebrafish (Experimental Series 1)
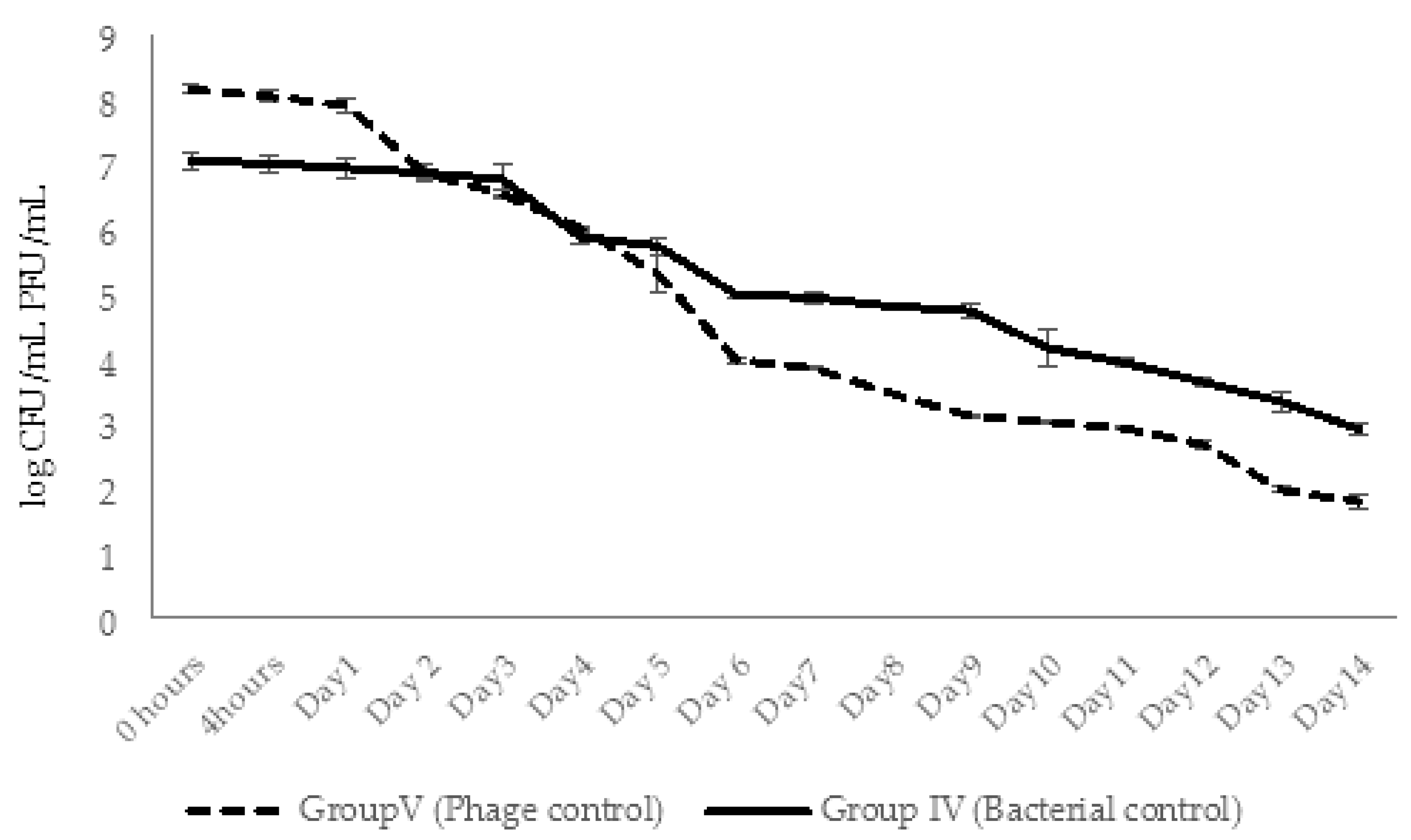
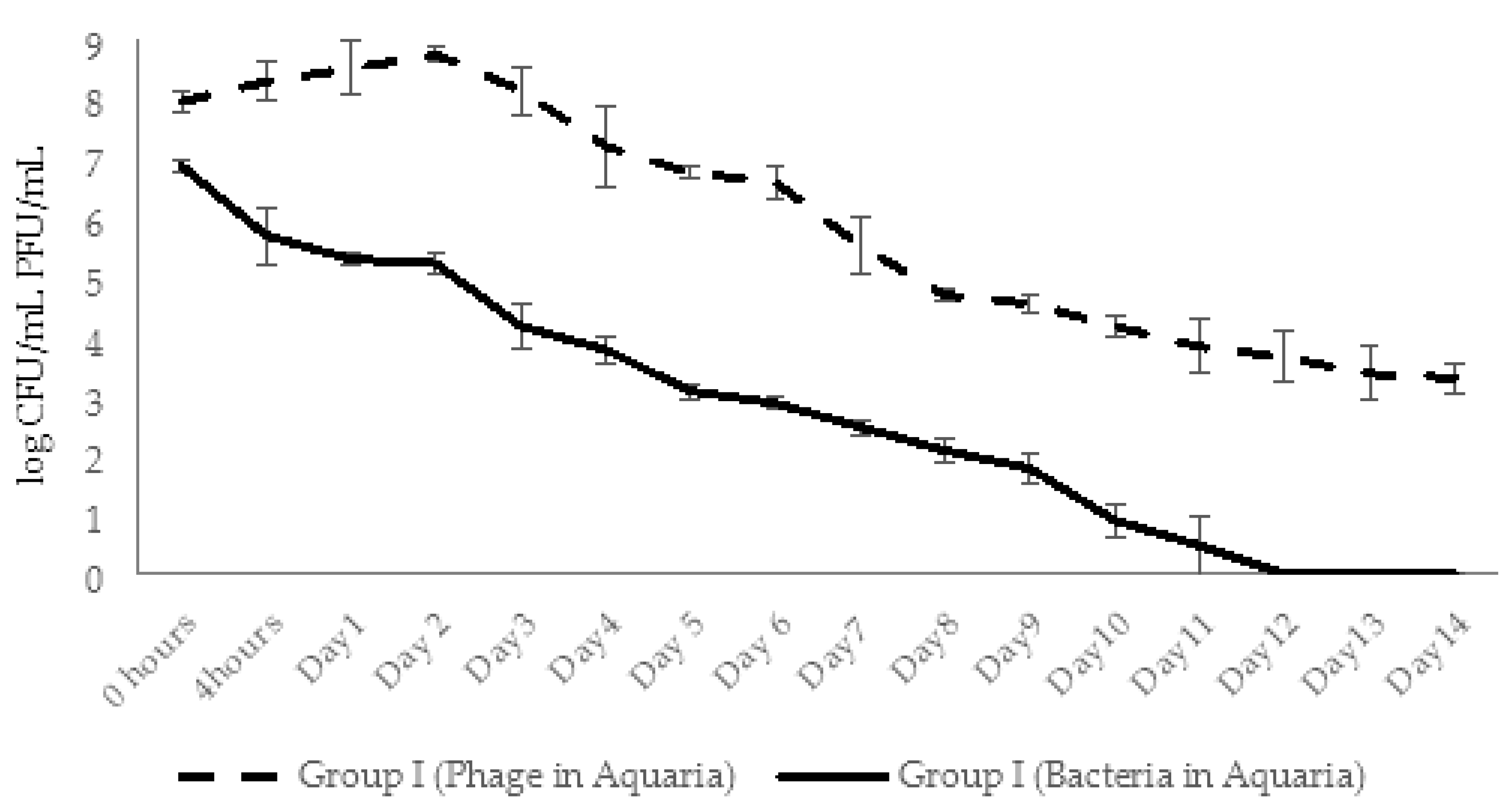
2.4.3. Dynamic Changes in Abundance of Phages and Bacteria in Aquaria Water (Experimental Series 1)
2.5. Estimation of Antibacterial Efficacy of AhMtk13A Phage in the Experimental Zebrafish Model of A. hydrophila GW3-10 Infection: Experimental Series 2
2.5.1. Protective Effect of AhMtk13a Phage Administration on Infected Zebrafish: Comparative Mortality Rates (Experimental Series 2)
2.5.2. Dynamic Changes in Abundance of Phages and Bacteria in Zebrafish (Experimental Series 2)
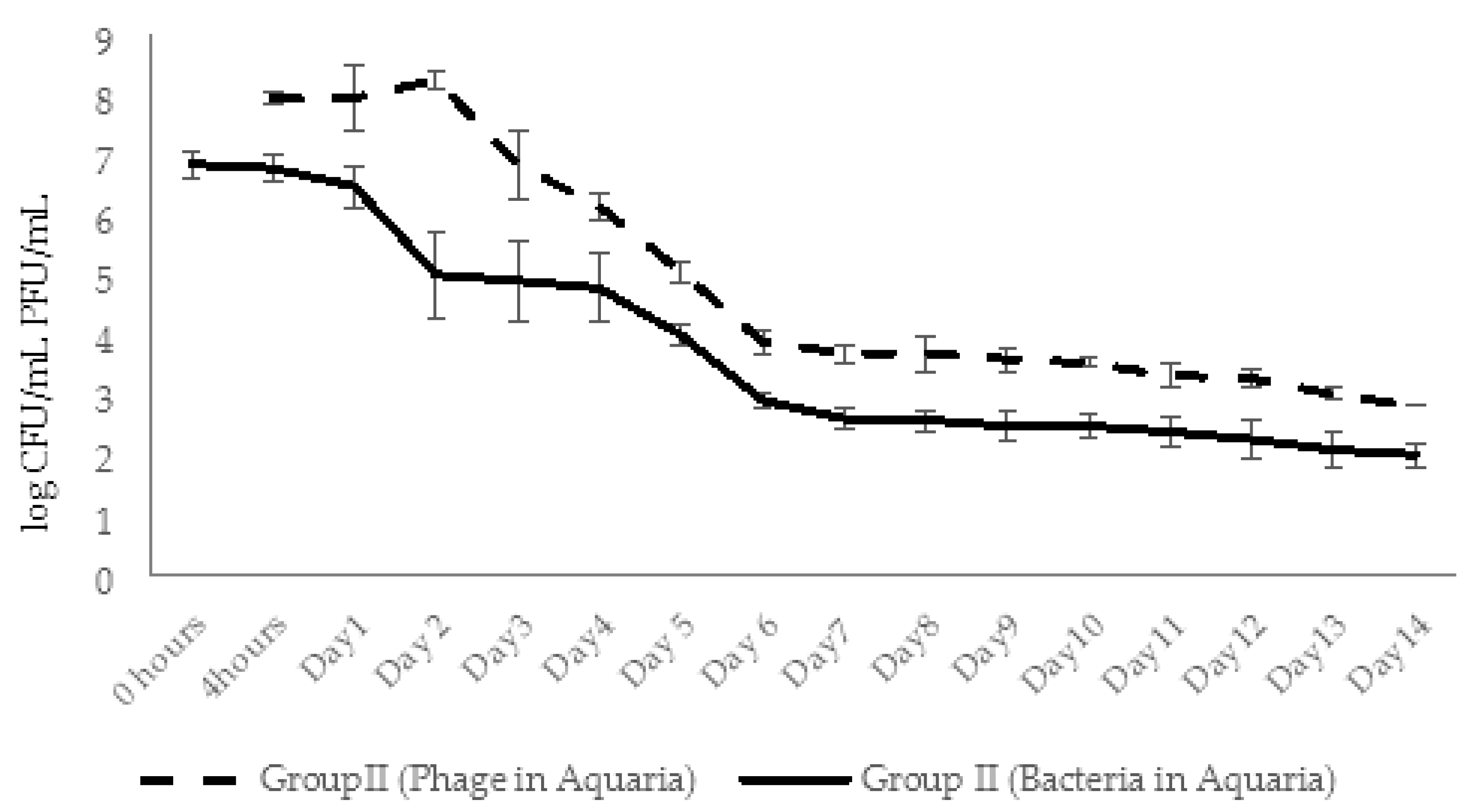
2.5.3. Dynamic Changes in Abundance of Phages and Bacteria in Aquaria Water (Experimental Series 2)
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Model Organisms
4.3. Isolation of A. hydrophila—Specific Bacteriophages
4.4. Purification and Propagation of Phages
4.5. Characterization of Bacteriophages
4.5.1. Phage Virion Morphology
4.5.2. Phage DNA Extraction
4.5.3. Bacteriophage Whole Genome Sequencing and In Silico Analysis
4.5.4. Phage Host Range
4.5.5. Phage Adsorption and One-Step Growth Cycle
4.5.6. Lysis Stability of AhMtk13a in Liquid Culture
4.6. Stability and Infectivity of Bacteriophage AhMTK13a in Different Environmental Conditions
4.6.1. Survival in Different Liquid Environment
4.6.2. Influence of Temperature and Different pH on Survival AhMtk13a Phage
4.7. Assessment of Bacterial Pathogenicity In Vivo: Development of Infection Model for A. hydrophila GW3-10
4.7.1. Estimation of Zebrafish Mortality Depending on the Concentration of A. hydrophila GW3-10
4.7.2. Estimation of Safety of Treatment with Phage AhMtk13a on Zebrafish
4.8. Estimation of Antibacterial Efficacy of AhMtk13A Phage in the Laboratory Conditions in Aquaria Water and in the Zebrafish (Experimental Series 1)
4.8.1. Protective Effect of AhMtk13a Phage Administration on Infected Zebrafish
- Group I—fish injected with A. hydrophila GW3-10, placed in the aquaria with the same bacteria added, and immediately treated with the phage;
- Group II—fish injected with A. hydrophila GW3-10, placed in the aquaria with the same bacteria added, and treated with the phage in 4 h after infection;
- Group III—fish exposed to phage AhMtk13a containing aquaria 30 min before intraperitoneal injection with A. hydrophila GW3-10 and adding the same pathogen to the aquaria water;
- Group IV (bacterial control)—fish injected with the A. hydrophila GW3-10 and kept in the aquaria containing the same bacterial pathogen;
- Group V (phage control)—fish injected with saline and placed in the aquaria containing the experimental phage only;
- Group VI (vehicle control)—fish received saline only through intraperitoneal injection. Neither phage nor bacteria were added to these aquaria;
4.8.2. Enumeration of AhMtk13a Phage and A. hydrophila GW3-10 in Aquaria Water
4.9. Enumeration of AhMtk13a Phage and A. hydrophila GW3-10 in Zebrafish
4.10. Estimation of Antibacterial Efficacy of AhMtk13A Phage in the Laboratory Conditions in Aquaria Water and in the Zebrafish (Experimental Series 2)
- Group I—fish injected with A. hydrophila GW3-10, placed in the aquaria with the added same bacteria, and immediately treated with the phage;
- Group II (bacterial control)—fish injected with the A. hydrophila GW3-10 and kept in the aquaria containing the same bacterial pathogen;
- Group III (phage control)—fish injected with saline and placed in the aquaria containing the experimental phage only;
- Group IV (vehicle control)—fish received saline only through intraperitoneal injection. Neither phage nor bacteria were added to these aquaria.
4.11. Enumeration of AhMtk13a Phage and A. hydrophila GW3-10 in Zebrafish (Experimental Series 2)
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The State of World Fisheries and Aquaculture; Meeting the Sustainable Development Goals; FAO: Rome, Italy, 2018; ISBN 978-92-5-130562-1. [Google Scholar]
- EUGeorgia. Fisheries and Aquaculture in Georgia (Industry Research). 2020. Available online: http://eugeorgia.info/en/article/832/fisheries-and-aquaculture-in-georgia-industry-research (accessed on 13 December 2021).
- Aly, D.S.M. A Review of Fish Diseases in the Egyptian Aquaculture Sector; Working Report. 41; CGIAR: Montpellier, France, 2013. [Google Scholar]
- Chandrarathna, H.P.S.U.; Nikapitiya, C.; Dananjaya, S.H.S.; De Silva, B.C.J.; Heo, G.-J.; De Zoysa, M.; Lee, J. Isolation and Characterization of Phage AHP-1 and Its Combined Effect with Chloramphenicol to Control, Aeromonas hydrophila. Braz. J. Microbiol. 2020, 51, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Chandrarathna, H.P.S.U.; Nikapitiya, C.; Dananjaya, S.H.S.; Wijerathne, C.U.B.; Wimalasena, S.H.M.P.; Kwun, H.J.; Heo, G.-J.; Lee, J.; De Zoysa, M. Outcome of Co-Infection with Opportunistic and Multidrug Resistant Aeromonas hydrophila and A. veronii in Zebrafish: Identification, Characterization, Pathogenicity and Immune Responses. Fish Shellfish Immunol. 2018, 80, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.; Guo, G.; Yang, N.; Li, Q.; Yin, F.; Wang, P.; Zheng, J.; Zeng, J. Three Species of Aeromonas (A. dhakensis, A. hydrophila and A. jandaei) Isolated from Freshwater Crocodiles (Crocodylus siamensis) with Pneumonia and Septicemia. Lett. Appl. Microbiol. 2019, 68, 212–218. [Google Scholar] [CrossRef]
- Romero, J.; Feijoo, C.G.; Navarrete, P. Antibiotics in Aquaculture—Use, Abuse and Alternatives; IntechOpen: London, UK, 2012; ISBN 978-953-51-0497-1. [Google Scholar]
- Aly, S.; Albutti, A. Antimicrobials Use in Aquaculture and Their Public Health Impact. J. Aquac. Res. Dev. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Harikrishnan, R.; Balasundaram, C.; Heo, S.-M. Fish Health Aspects of Grouper Aquaculture. Aquaculture 2010, 309, 1–21. [Google Scholar] [CrossRef]
- Dadar, M.; Dhama, K.; Vakharia, V.N.; Hoseinifar, S.H.; Karthik, K.; Tiwari, R.; Khandia, R.; Munjal, A.; Salgado-Miranda, C.; Joshi, S.K. Advances in Aquaculture Vaccines Against Fish Pathogens: Global Status and Current Trends. Rev. Fish. Sci. Aquac. 2017, 25, 184–217. [Google Scholar] [CrossRef]
- Rodgers, C.; Furones, M. Antimicrobial Agents in Aquaculture: Practice, Needs and Issues. In The Use of Veterinary Drugs and Vaccines in Mediterranean Aquaculture; CIHEAM: Zaragoza, Spain, 2009; pp. 41–59. [Google Scholar]
- Vivas, J.; Riaño, J.; Carracedo, B.; Razquin, B.E.; López-Fierro, P.; Naharro, G.; Villena, A.J. The Auxotrophic AroA Mutant of Aeromonas hydrophila as a Live Attenuated Vaccine against A. salmonicida Infections in Rainbow Trout (Oncorhynchus mykiss). Fish Shellfish Immunol. 2004, 16, 193–206. [Google Scholar] [CrossRef]
- Assefa, A.; Abunna, F. Maintenance of Fish Health in Aquaculture: Review of Epidemiological Approaches for Prevention and Control of Infectious Disease of Fish. Vet. Med. Int. 2018, 2018, e5432497. [Google Scholar] [CrossRef]
- Ramos-Vivas, J.; Superio, J.; Galindo-Villegas, J.; Acosta, F. Phage Therapy as a Focused Management Strategy in Aquaculture. IJMS 2021, 22, 10436. [Google Scholar] [CrossRef]
- Maqsood, S.; Singh, P.; Samoon, M.H.; Munir, K. Emerging Role of Immunostimulants in Combating the Disease Outbreak in Aquaculture. Int. Aquat. Res. 2011, 3, 147–163. [Google Scholar]
- Wu, J.-L.; Lin, H.-M.; Jan, L.; Hsu, Y.-L.; Chang, L.-H. Biological Control of Fish Bacterial Pathogen, Aeromonas hydrophila, by Bacteriophage AH 1. Fish Pathol. 1981, 15, 271–276. [Google Scholar] [CrossRef]
- Nikapitiya, C.; Chandrarathna, H.P.S.U.; Dananjaya, S.H.S.; De Zoysa, M.; Lee, J. Isolation and Characterization of Phage (ETP-1) Specific to Multidrug Resistant Pathogenic Edwardsiella tarda and Its in Vivo Biocontrol Efficacy in Zebrafish (Danio rerio). Biologicals 2020, 63, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Matamp, N.; Bhat, S.G. Phage Endolysins as Potential Antimicrobials against Multidrug Resistant Vibrio alginolyticus and Vibrio parahaemolyticus: Current Status of Research and Challenges Ahead. Microorganisms 2019, 7, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le, T.S.; Nguyen, T.H.; Vo, H.P.; Doan, V.C.; Nguyen, H.L.; Tran, M.T.; Tran, T.T.; Southgate, P.C.; Kurtböke, D.İ. Protective Effects of Bacteriophages against Aeromonas hydrophila Species Causing Motile Aeromonas Septicemia (MAS) in Striped Catfish. Antibiotics 2018, 7, 16. [Google Scholar] [CrossRef] [Green Version]
- Akmal, M.; Rahimi-Midani, A.; Hafeez-Ur-Rehman, M.; Hussain, A.; Choi, T.-J. Isolation, Characterization, and Application of a Bacteriophage Infecting the Fish Pathogen Aeromonas hydrophila. Pathogens 2020, 9, 215. [Google Scholar] [CrossRef] [Green Version]
- Yun, S.; Jun, J.W.; Giri, S.S.; Kim, H.J.; Chi, C.; Kim, S.G.; Kim, S.W.; Kang, J.W.; Han, S.J.; Kwon, J.; et al. Immunostimulation of Cyprinus carpio Using Phage Lysate of Aeromonas hydrophila. Fish Shellfish Immunol. 2019, 86, 680–687. [Google Scholar] [CrossRef]
- Schulz, P.; Robak, S.; Dastych, J.; Siwicki, A.K. Influence of Bacteriophages Cocktail on European Eel (Anguilla anguilla) Immunity and Survival after Experimental Challenge. Fish Shellfish Immunol. 2019, 84, 28–37. [Google Scholar] [CrossRef]
- Adams, M.H. Bacteriophages; Interscience Publishers: New York, NY, USA, 1959. [Google Scholar]
- Nishimura, Y.; Yoshida, T.; Kuronishi, M.; Uehara, H.; Ogata, H.; Goto, S. ViPTree: The Viral Proteomic Tree Server. Bioinformatics 2017, 33, 2379–2380. [Google Scholar] [CrossRef]
- Adriaenssens, E.; Brister, J.R. How to Name and Classify Your Phage: An Informal Guide. Viruses 2017, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Appelmans, R. Le Dosage Du Bactériophage. C. R. Soc. Biol. 1921, 85, 701. [Google Scholar]
- Milligan-Myhre, K.; Charette, J.R.; Phennicie, R.T.; Stephens, W.Z.; Rawls, J.F.; Guillemin, K.; Kim, C.H. Study of Host-Microbe Interactions in Zebrafish. Methods Cell. Biol. 2011, 105, 87–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.; He, J.-B.; Shi, J.-W.; Xiao, Q.; Li, L.; Woo, P.C. An Adult Zebrafish Model for Laribacter hongkongensis Infection: Koch’s Postulates Fulfilled. Emerg. Microbes Infect. 2014, 3, e73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collymore, C.; Tolwani, A.; Lieggi, C.; Rasmussen, S. Efficacy and Safety of 5 Anesthetics in Adult Zebrafish (Danio rerio). J. Am. Assoc. Lab. Anim. Sci. 2014, 53, 198–203. [Google Scholar] [PubMed]
- Cafora, M.; Deflorian, G.; Forti, F.; Ferrari, L.; Binelli, G.; Briani, F.; Ghisotti, D.; Pistocchi, A. Phage Therapy against Pseudomonas aeruginosa Infections in a Cystic Fibrosis Zebrafish Model. Sci. Rep. 2019, 9, 1527. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2018—Meeting the Sustainable Development Goals; Licence: CC BY-NC-SA 3.0 IGO; FAO: Rome, Italy, 2018. [Google Scholar]
- Almeida, A.; Cunha, Â.; Gomes, N.C.M.; Alves, E.; Costa, L.; Faustino, M.A.F. Phage Therapy and Photodynamic Therapy: Low Environmental Impact Approaches to Inactivate Microorganisms in Fish Farming Plants. Mar. Drugs 2009, 7, 268–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, Z.J. Aquaculture Pharmaceuticals and Biologicals: Current Perspectives and Future Possibilities. Adv. Drug Deliv. Rev. 2001, 50, 229–243. [Google Scholar] [CrossRef]
- Wahli, T.; Knuesel, R.; Bernet, D.; Segner, H.; Pugovkin, D.; Burkhardt-Holm, P.; Escher, M.; Schmidt-Posthaus, H. Proliferative Kidney Disease in Switzerland: Current State of Knowledge. J. Fish Dis. 2002, 25, 491–500. [Google Scholar] [CrossRef] [Green Version]
- Dias, M.K.R.; Sampaio, L.S.; Proietti-Junior, A.A.; Yoshioka, E.T.O.; Rodrigues, D.P.; Rodriguez, A.F.R.; Ribeiro, R.A.; Faria, F.S.E.D.V.; Ozório, R.O.A.; Tavares-Dias, M. Lethal Dose and Clinical Signs of Aeromonas hydrophila in Arapaima gigas (Arapaimidae), the Giant Fish from Amazon. Vet. Microbiol. 2016, 188, 12–15. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, M.E.; Høi, L.; Schmidt, A.S.; Qian, D.; Shimada, T.; Shen, J.Y.; Larsen, J.L. Is Aeromonas hydrophila the Dominant Motile Aeromonas Species That Causes Disease Outbreaks in Aquaculture Production in the Zhejiang Province of China? Dis. Aquat. Organ. 2001, 46, 23–29. [Google Scholar] [CrossRef]
- Shayo, S.D.; Mwita, C.J.; Hosea, K.M. Virulence of Pseudomonas and Aeromonas Bacteria Recovered from Oreochromis niloticus (Perege) from Mtera Hydropower Dam; Tanzania. Ann. Biol. Res. 2012, 3, 5157–5161. [Google Scholar]
- Chanishvili, N. A Literature Review of the Practical Application of Bacteriophage Research; Nova Biomedical Books: New York, NY, USA, 2012; ISBN 978-1-62100-851-4. [Google Scholar]
- Higuera, G.; Bastías, R.; Tsertsvadze, G.; Romero, J.; Espejo, R.T. Recently Discovered Vibrio anguillarum Phages Can Protect against Experimentally Induced Vibriosis in Atlantic Salmon, Salmo salar. Aquaculture 2013, 392, 128–133. [Google Scholar] [CrossRef]
- Vinod, M.G.; Shivu, M.M.; Umesha, K.R.; Rajeeva, B.C.; Krohne, G.; Karunasagar, I.; Karunasagar, I. Isolation of Vibrio harveyi Bacteriophage with a Potential for Biocontrol of Luminous Vibriosis in Hatchery Environments. Aquaculture 2006, 255, 117–124. [Google Scholar] [CrossRef]
- Nakai, T.; Park, S.C. Bacteriophage Therapy of Infectious Diseases in Aquaculture. Res. Microbiol. 2002, 153, 13–18. [Google Scholar] [CrossRef]
- Elliott, L. Bacteriophage Therapy in Aquaculture-Friend or Foe; World Nutrition Forum: Munich, Germany, 2014. [Google Scholar]
- Ackermann, H.-W. Bacteriophage Observations and Evolution. Res. Microbiol. 2003, 154, 245–251. [Google Scholar] [CrossRef]
- Albers, S.; Czech, A. Exploiting TRNAs to Boost Virulence. Life 2016, 6, 4. [Google Scholar] [CrossRef] [Green Version]
- Steidel, S. The Role of Phage TRNAs in the Evolution of Codon Usage Biases in Giant Pseudomonas Phage PhiKZ and EL. Ph.D. Thesis, Loyola University Chicago, Chicago, IL, USA, 2017. [Google Scholar]
- Smith, M.C.M. Phage-Encoded Serine Integrases and Other Large Serine Recombinases. Microbiol. Spectr. 2015, 3, 3–4. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, K.; Van Duyne, G.D. The Ins and Outs of Serine Integrase Site-Specific Recombination. Curr. Opin. Struct. Biol. 2014, 24, 125–131. [Google Scholar] [CrossRef] [Green Version]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage Treatment of Human Infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [Green Version]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered Bacteriophages for Treatment of a Patient with a Disseminated Drug-Resistant Mycobacterium abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef]
- Pal, S. Phage Therapy an Alternate Disease Control in Aquaculture: A Review on Recent Advancements. J. Agric. Vet. Sci. 2015, 8, 68–81. [Google Scholar]
- Silva, Y.J.; Costa, L.; Pereira, C.; Mateus, C.; Cunha, A.; Calado, R.; Gomes, N.C.M.; Pardo, M.A.; Hernandez, I.; Almeida, A. Phage Therapy as an Approach to Prevent Vibrio anguillarum Infections in Fish Larvae Production. PLoS ONE 2014, 9, e114197. [Google Scholar] [CrossRef] [PubMed]
- Meipariani, A.; Alavidze, Z.; Dzidzishvili, L.; Chkonia, I. Method for Production of Mono and Combined Dry Bacteriophage Preparation. Georgian Patent 20,063,943 B, 11 June 2003. [Google Scholar]
- Voroshilova, N.; Kazakova, T.; Gorbatkova, G.; Bogovazova, G.; Afanasieva, E. Method of Production of Pyobacteriophage. Russian Patent RU2036232C1, 27 May 1995. [Google Scholar]
- Soviet Guidelines for Production of Combined Liquid Pyobacteriophage Preparation 242–82 (1982) Approved by the Ministry of Health of the USSR. Available online: https://link.springer.com/protocol/10.1007/978-1-4939-7395-8_9 (accessed on 6 January 2022). (In Russian).
- Soviet Guidelines for Production of Liquid Streptococcal Phage Preparation 167–80 (1980) Approved by the Ministry of Health of the USSR. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0104853 (accessed on 6 January 2022). (In Russian).
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The Influence of External Factors on Bacteriophages—Review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Gomez, D.K.; Nakai, T.; Park, S.C. Isolation and Identification of Bacteriophages Infecting Ayu Plecoglossus altivelis altivelis Specific Flavobacterium psychrophilum. Vet. Microbiol. 2010, 140, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Pereira, C.; Silva, Y.J.; Santos, A.L.; Cunha, Â.; Gomes, N.C.M.; Almeida, A. Bacteriophages with Potential for Inactivation of Fish Pathogenic Bacteria: Survival, Host Specificity and Effect on Bacterial Community Structure. Mar. Drugs 2011, 9, 2236–2255. [Google Scholar] [CrossRef]
- Jepson, C.D.; March, J.B. Bacteriophage Lambda Is a Highly Stable DNA Vaccine Delivery Vehicle. Vaccine 2004, 22, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.R.; Axler, R.P.; Hicks, R.E. Effects of Freezing and Storage Temperature on MS2 Viability. J. Virol. Methods 2004, 122, 147–152. [Google Scholar] [CrossRef]
- Breitbart, M.; Wegley, L.; Leeds, S.; Schoenfeld, T.; Rohwer, F. Phage Community Dynamics in Hot Springs. Appl. Environ. Microbiol. 2004, 70, 1633–1640. [Google Scholar] [CrossRef] [Green Version]
- Kerby, G.P.; Gowdy, R.A.; Dillon, E.S.; Dillon, M.L.; Csâky, T.Z.; Sharp, D.G.; Beard, J.W. Purification, PH Stability and Sedimentation Properties of the T7 Bacteriophage of Escherichia coli. J. Immunol. 1949, 63, 93–107. [Google Scholar]
- Sharp, D.G.; Hook, A.E.; Taylor, A.R.; Beard, D.; Beard, J.W. Sedimentation Characters and PH Stability of the T2 Bacteriophage of Escherichia coli. J. Biol. Chem. 1946, 165, 259–270. [Google Scholar] [CrossRef]
- Park, S.; Nakai, T. Bacteriophage Control of Pseudomonas plecoglossicida Infection in Ayu, Plectoglossis altivelis. Dis. Aquat. Org. 2003, 53, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Choresca, C.H.; Shin, S.P.; Han, J.E.; Jun, J.W.; Park, S.C. Biological Control of Aeromonas salmonicida Subsp. salmonicida Infection in Rainbow Trout (Oncorhynchus mykiss) Using Aeromonas Phage PAS-1. Transbound. Emerg. Dis. 2015, 62, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Castillo, D.; Higuera, G.; Villa, M.; Middelboe, M.; Dalsgaard, I.; Madsen, L.; Espejo, R.T. Diversity of Flavobacterium psychrophilum and the Potential Use of Its Phages for Protection against Bacterial Cold Water Disease in Salmonids: Phage Therapy Assays for F. psychrophilum Infection. J. Fish Dis. 2012, 35, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Sugimoto, R.; Park, K.; Matsuoka, S.; Mori, K.; Nishioka, T.; Maruyama, K. Protective Effects of Bacteriophage on Experimental Lactococcus garvieae Infection in Yellowtail. Dis. Aquat. Org. 1999, 37, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laanto, E.; Bamford, J.K.H.; Ravantti, J.J.; Sundberg, L.-R. The Use of Phage FCL-2 as an Alternative to Chemotherapy against Columnaris Disease in Aquaculture. Front. Microbiol. 2015, 6, 829. [Google Scholar] [CrossRef] [Green Version]
- Imbeault, S.; Parent, S.; Lagacé, M.; Uhland, C.F.; Blais, J.-F. Using Bacteriophages to Prevent Furunculosis Caused by Aeromonas salmonicida in Farmed Brook Trout. J. Aquat. Anim. Health 2006, 18, 203–214. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Parys, L.V.; et al. Quality-Controlled Small-Scale Production of a Well-Defined Bacteriophage Cocktail for Use in Human Clinical Trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef]
- Clokie, M.R.J. Bacteriophages—Methods and Protocols; Humana: Totowa, NJ, USA, 2009. [Google Scholar]
- Carver, T.; Harris, S.R.; Berriman, M.; Parkhill, J.; McQuillan, J.A. Artemis: An Integrated Platform for Visualization and Analysis of High-Throughput Sequence-Based Experimental Data. Bioinformatics 2012, 28, 464–469. [Google Scholar] [CrossRef] [Green Version]
- Besemer, J.; Lomsadze, A.; Borodovsky, M. GeneMarkS: A Self-Training Method for Prediction of Gene Starts in Microbial Genomes. Implications for Finding Sequence Motifs in Regulatory Regions. Nucleic Acids Res. 2001, 29, 2607–2618. [Google Scholar] [CrossRef] [Green Version]
- El-Gebali, S.; Mistry, J.; Bateman, A.; Eddy, S.R.; Luciani, A.; Potter, S.C.; Qureshi, M.; Richardson, L.J.; Salazar, G.A.; Smart, A.; et al. The Pfam Protein Families Database in 2019. Nucleic Acids Res. 2019, 47, D427–D432. [Google Scholar] [CrossRef]
- Wheeler, T.J.; Eddy, S.R. Nhmmer: DNA Homology Search with Profile HMMs. Bioinformatics 2013, 29, 2487–2489. [Google Scholar] [CrossRef] [Green Version]
- Chan, P.P.; Lowe, T.M. TRNAscan-SE: Searching for TRNA Genes in Genomic Sequences. In Gene Prediction; Kollmar, M., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2019; Volume 1962, pp. 1–14. ISBN 978-1-4939-9172-3. [Google Scholar]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An Integrated and Extendable Desktop Software Platform for the Organization and Analysis of Sequence Data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Rohwer, F.; Edwards, R. The Phage Proteomic Tree: A Genome-Based Taxonomy for Phage. J. Bacteriol. 2002, 184, 4529–4535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, S.-H.; Ha, S.-M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A Taxonomically United Database of 16S RRNA Gene Sequences and Whole-Genome Assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Vandenheuvel, D.; Kropinski, A.M.; Mast, J.; De Vos, D.; Verbeken, G.; Noben, J.-P.; Lavigne, R.; Vaneechoutte, M.; Pirnay, J.-P. Characterization of Newly Isolated Lytic Bacteriophages Active against Acinetobacter baumannii. PLoS ONE 2014, 9, e104853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, M. Calculating Burst Size V1. Available online: https://www.protocols.io/view/Calculating-burst-size-ddm245 (accessed on 16 January 2022).
- Neely, M.N.; Pfeifer, J.D.; Caparon, M. Streptococcus-Zebrafish Model of Bacterial Pathogenesis. Infect. Immun. 2002, 70, 3904–3914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pressley, M.E.; Phelan, P.E.; Witten, P.E.; Mellon, M.T.; Kim, C.H. Pathogenesis and Inflammatory Response to Edwardsiella tarda Infection in the Zebrafish. Dev. Comp. Immunol. 2005, 29, 501–513. [Google Scholar] [CrossRef]
- Phelan, P.E.; Pressley, M.E.; Witten, P.E.; Mellon, M.T.; Blake, S.; Kim, C.H. Characterization of Snakehead Rhabdovirus Infection in Zebrafish (Danio rerio). J. Virol. 2005, 79, 1842–1852. [Google Scholar] [CrossRef] [Green Version]
- National Institutes of Health. Guidelines for Use of Zebrafish in the NIH Intramural Research Program Euthanasia Guidelines; National Institutes of Health: Bethesda, MD, USA, 2009.








| Bacterial Strains | Total Number of Isolates | Susceptible to Phage AhMtk13a | Susceptible to Phage AhMtk13b |
|---|---|---|---|
| A. hydrophila | 39 | 28/39 | 13/39 |
| A. hydrophila CIP103770 | 1 | 1/1 | 0/1 |
| A. caviae | 5 | 0/5 | 0/5 |
| A. sobria | 2 | 0/2 | 0/2 |
| A. salmonicida CIP 104001T; CIP 103209T | 2 | 0/2 | 0/2 |
| Control A. hydrophila GW3-10 in TSB | MOI of AhMtk13a and A. hydrophila GW3-10 in TSB | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.001 | 0.01 | 0.1 | 1 | 10 | 100 | 1000 | ||
| 0.5 MFTS * | _ | _ | _ | _ | _ | _ | _ | 6 h |
| 2 MFTS * | 1 MFTS * | 0.5 MFTS * | _ | _ | _ | _ | _ | 24 h |
| 3 MFTS * | 2 MFTS * | 1 MFTS * | 0.5 MFTS * | _ | _ | _ | _ | 48 h |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janelidze, N.; Jaiani, E.; Didebulidze, E.; Kusradze, I.; Kotorashvili, A.; Chalidze, K.; Porchkhidze, K.; Khukhunashvili, T.; Tsertsvadze, G.; Jgenti, D.; et al. Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio. Viruses 2022, 14, 412. https://doi.org/10.3390/v14020412
Janelidze N, Jaiani E, Didebulidze E, Kusradze I, Kotorashvili A, Chalidze K, Porchkhidze K, Khukhunashvili T, Tsertsvadze G, Jgenti D, et al. Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio. Viruses. 2022; 14(2):412. https://doi.org/10.3390/v14020412
Chicago/Turabian StyleJanelidze, Nino, Ekaterine Jaiani, Elene Didebulidze, Ia Kusradze, Adam Kotorashvili, Kristine Chalidze, Ketevan Porchkhidze, Tinatin Khukhunashvili, George Tsertsvadze, Dodo Jgenti, and et al. 2022. "Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio" Viruses 14, no. 2: 412. https://doi.org/10.3390/v14020412
APA StyleJanelidze, N., Jaiani, E., Didebulidze, E., Kusradze, I., Kotorashvili, A., Chalidze, K., Porchkhidze, K., Khukhunashvili, T., Tsertsvadze, G., Jgenti, D., Bajashvili, T., & Tediashvili, M. (2022). Phenotypic and Genetic Characterization of Aeromonas hydrophila Phage AhMtk13a and Evaluation of Its Therapeutic Potential on Simulated Aeromonas Infection in Danio rerio. Viruses, 14(2), 412. https://doi.org/10.3390/v14020412









