Densovirus Oil Suspension Significantly Improves the Efficacy and Duration of Larvicidal Activity against Aedes albopictus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Mosquito Maintenance
2.3. Study Design
2.4. AalDV-5 Formulation Testing
2.5. Semi-Field Bioassay
2.6. Open-Field Bioassay
2.7. Water Sampling and AalDV-5 Quantification
2.8. Viral Detection in Dead Larvae
2.9. Pathogenicity Assessment in Non-Target Species
2.9.1. Toxicity in Cultured Cells In Vitro
2.9.2. Toxicity in Carp (Koi Carp)
2.9.3. Toxicity in Poultry
2.9.4. Toxicity in Mice
2.9.5. Acute Toxicity Assays with Mice (Acute Respiratory/Injection Pathogenicity Test)
2.10. Statistical Analysis
3. Results
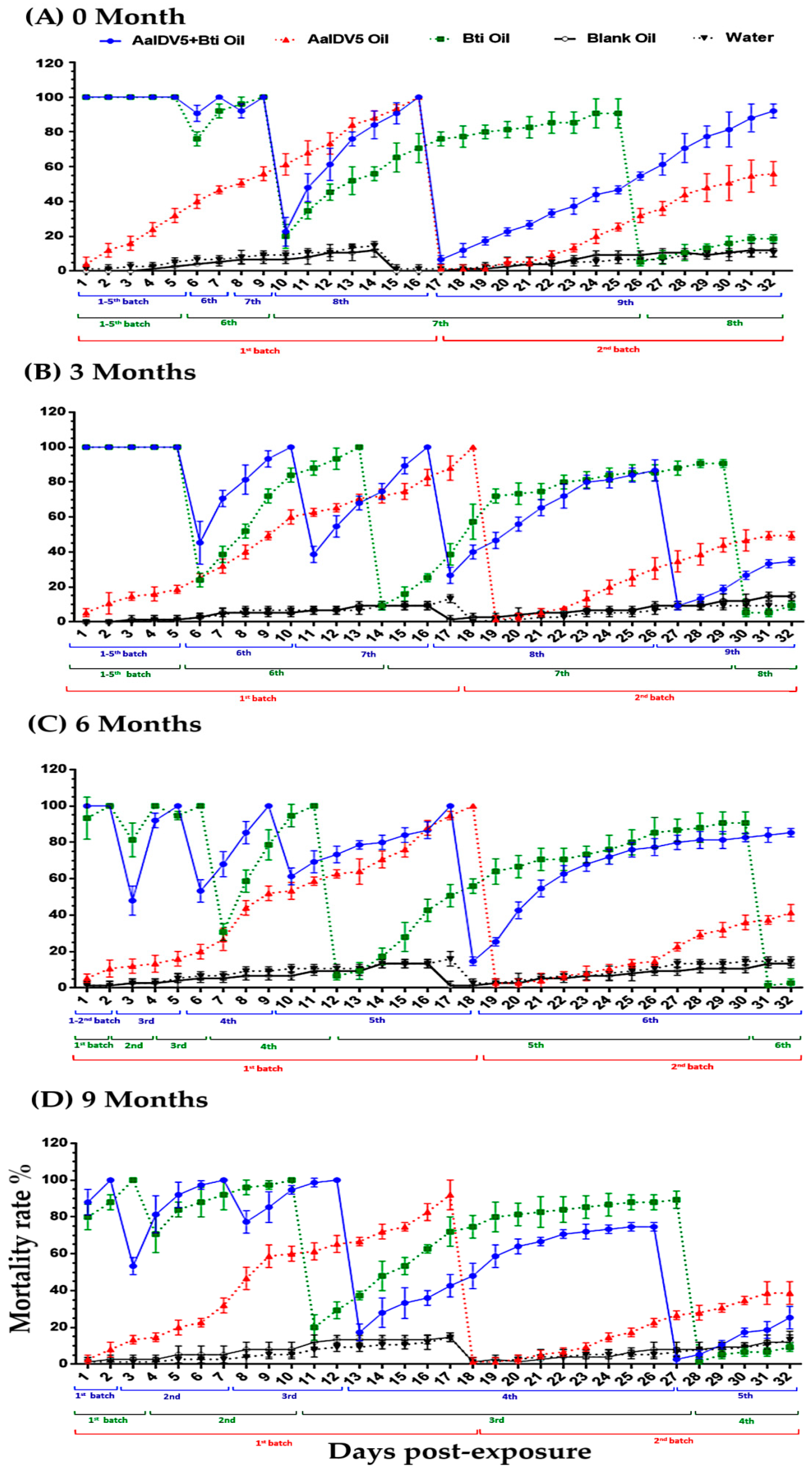
3.1. Bioactivity of Oil Suspensions under Semi-Field Conditions
3.2. Bioactivity of Oil Suspensions under Open-Field Conditions
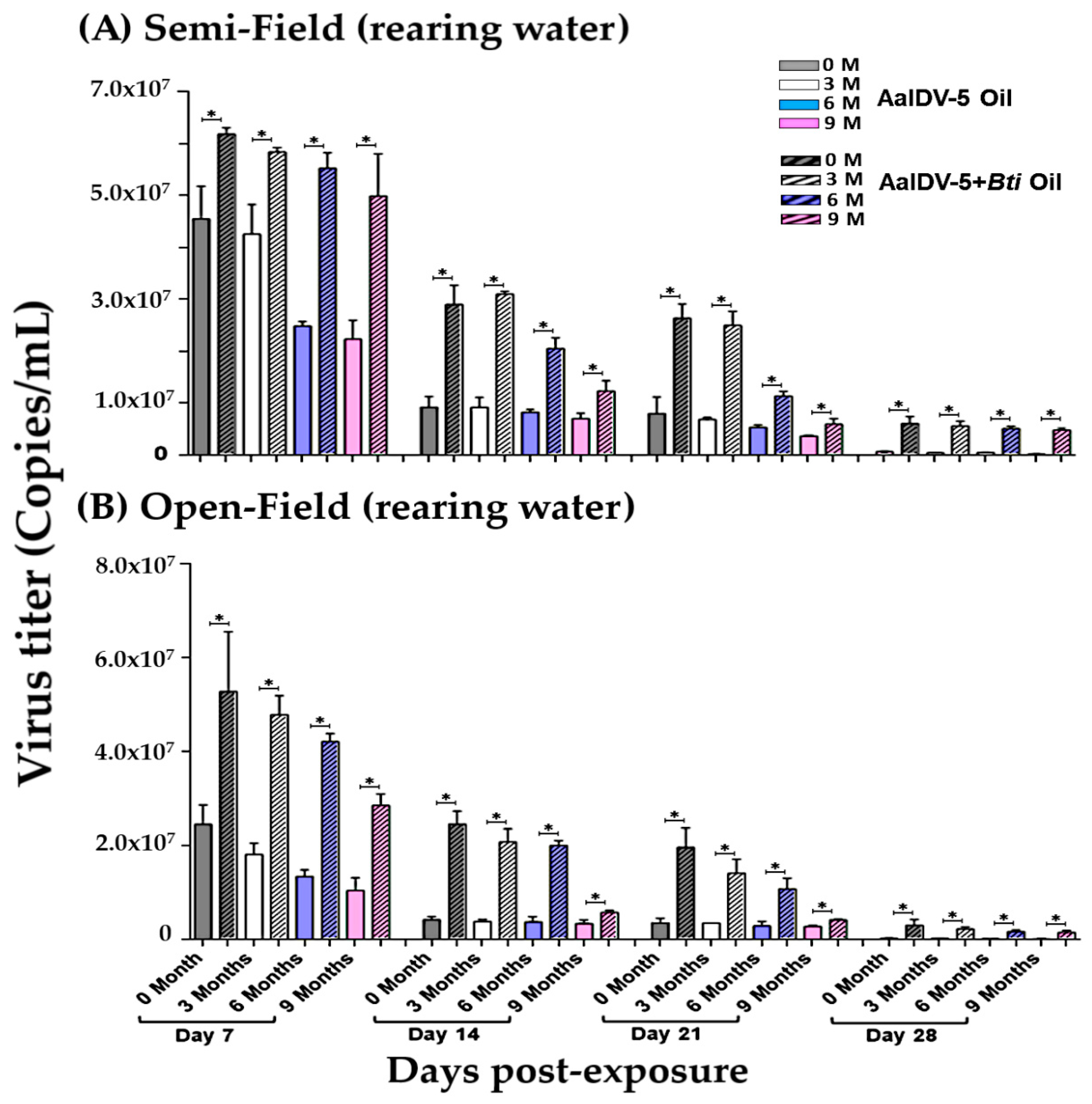
3.3. Accumulation of the AalDV-5 in Larval Rearing Water
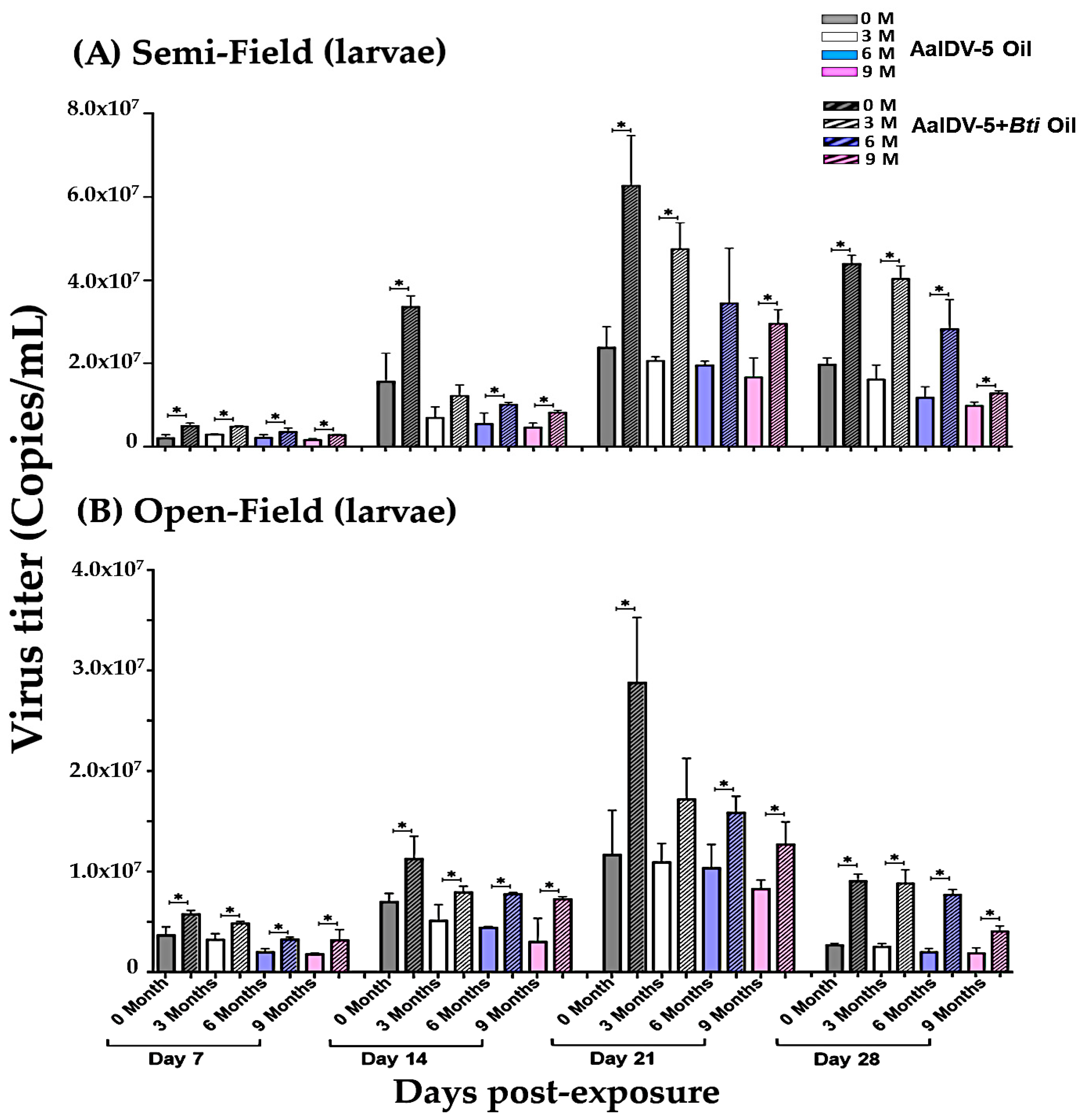
3.4. Accumulation of AalDV-5 in Dead Larvae
3.5. Bioactivity of Laboratory Strain AalDV-5 and Viral Quantification
3.6. Safety Assessment of AalDV-5 on Non-Target Species
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Lwande, O.W.; Obanda, V.; Lindström, A.; Ahlm, C.; Evander, M.; Näslund, J.; Bucht, G. Globe-trotting Aedes aegypti and Aedes albopictus: Risk factors for arbovirus pandemics. Vector Borne Zoonotic Dis. 2020, 20, 71–81. [Google Scholar] [CrossRef] [Green Version]
- Cuthbert, R.N.; Diagne, C.; Haubrock, P.J.; Turbelin, A.J.; Courchamp, F. Are the “100 of the world’s worst” invasive species also the costliest? Biol. Invasions 2021. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000; Volume 12. [Google Scholar]
- Liu, K.; Hou, X.; Ren, Z.; Lowe, R.; Wang, Y.; Li, R.; Liu, X.; Sun, J.; Lu, L.; Song, X. Climate factors and the East Asian summer monsoon may drive large outbreaks of dengue in China. Environ. Res. 2020, 183, 109190. [Google Scholar] [CrossRef]
- Metelmann, S.; Liu, X.; Lu, L.; Caminade, C.; Liu, K.; Cao, L.; Medlock, J.M.; Baylis, M.; Morse, A.P.; Liu, Q. Assessing the suitability for Aedes albopictus and dengue transmission risk in China with a delay differential equation model. PLoS Negl. Trop. Dis. 2021, 15, e0009153. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and Zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Weill, M.; Lutfalla, G.; Mogensen, K.; Chandre, F.; Berthomieu, A.; Berticat, C.; Pasteur, N.; Philips, A.; Fort, P.; Raymond, M. Insecticide resistance in mosquito vectors. Nature 2003, 423, 136–137. [Google Scholar] [CrossRef]
- Li, Y.; Xu, J.; Zhong, D.; Zhang, H.; Yang, W.; Zhou, G.; Su, X.; Wu, Y.; Wu, K.; Cai, S. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasites Vectors 2018, 11, 4. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, G.; Zhong, D.; Wang, X.; Hemming-Schroeder, E.; David, R.E.; Lee, M.C.; Zhong, S.; Yi, G.; Liu, Z. Widespread multiple insecticide resistance in the major dengue vector Aedes albopictus in Hainan Province, China. Pest Manag. Sci. 2021, 77, 1945–1953. [Google Scholar] [CrossRef]
- Cisse, M.B.; Keita, C.; Dicko, A.; Dengela, D.; Coleman, J.; Lucas, B.; Mihigo, J.; Sadou, A.; Belemvire, A.; George, K. Characterizing the insecticide resistance of Anopheles gambiae in Mali. Malar. J. 2015, 14, 327. [Google Scholar] [CrossRef] [Green Version]
- Benelli, G.; Jeffries, C.L.; Walker, T. Biological control of mosquito vectors: Past, present, and future. Insects 2016, 7, 52. [Google Scholar] [CrossRef]
- Johnson, B.J.; Manby, R.; Devine, G.J. Performance of an aerially applied liquid Bacillus thuringiensis var. israelensis formulation (strain AM65-52) against mosquitoes in mixed saltmarsh–mangrove systems and fine-scale mapping of mangrove canopy cover using affordable drone-based imagery. Pest Manag. Sci. 2020, 76, 3822–3831. [Google Scholar] [CrossRef] [PubMed]
- Rumbos, C.I.; Athanassiou, C.G. Assessment of selected larvicides for the control of Culex pipiens biotype pipiens and Culex pipiens biotype molestus under laboratory and semi-field conditions. Pest Manag. Sci. 2020, 76, 3568–3576. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Xie, Y.G.; Lin, F.; Xie, L.H.; Yang, W.Q.; Su, X.H.; Ou, C.Q.; Luo, L.; Xiao, Q.; Gan, L. A long-lasting biological larvicide against the dengue vector mosquito Aedes albopictus. Pest Manag. Sci. 2021, 77, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Lin, M.; Jia, M.; Hu, J.; Zhu, L. Chemical composition and larvicidal activity against Aedes mosquitoes of essential oils from Arisaema fargesii. Pest Manag. Sci. 2020, 76, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Milugo, T.K.; Tchouassi, D.P.; Kavishe, R.A.; Dinglasan, R.R.; Torto, B. Derivatization increases mosquito larvicidal activity of the sesquiterpene lactone parthenin isolated from the invasive weed Parthenium hysterophorus. Pest Manag. Sci. 2021, 77, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Muturi, E.J.; Hay, W.T.; Behle, R.W.; Selling, G.W. Amylose inclusion complexes as emulsifiers for garlic and asafoetida essential oils for mosquito control. Insects 2019, 10, 337. [Google Scholar] [CrossRef] [Green Version]
- Marina, C.F.; Arredondo-Jiménez, J.I.; Castillo, A.; Williams, T. Sublethal effects of iridovirus disease in a mosquito. Oecologia 1999, 119, 383–388. [Google Scholar] [CrossRef]
- Becnel, J.J. Transmission of viruses to mosquito larvae mediated by divalent cations. J. Invertebr. Pathol. 2006, 92, 141–145. [Google Scholar] [CrossRef]
- Johnson, R.M.; Rasgon, J.L. Densonucleosis viruses (‘densoviruses’) for mosquito and pathogen control. Curr. Opin. Insect. Sci. 2018, 28, 90–97. [Google Scholar] [CrossRef]
- Vasilieva, V.; Lebedinets, N.; Gural, A.; Chigir, T.; Buchatsky, L.; Kuznetsova, M. Examination of the preparation viroden safety for vertebrates. Mikrobiol. Zh. 1990, 52, 73–79. [Google Scholar] [PubMed]
- El-Far, M.; Li, Y.; Fédière, G.; Abol-Ela, S.; Tijssen, P. Lack of infection of vertebrate cells by the densovirus from the maize worm Mythimna loreyi (MlDNV). Virus Res. 2004, 99, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Faisst, S.; Rommelaere, J. Parvoviruses: From Molecular Biology to Pathology and Therapeutic Uses; Karger Medical and Scientific Publishers: Basel, Switzerland, 2000; Volume 4. [Google Scholar]
- Carlson, J.; Suchman, E.; Buchatsky, L. Densoviruses for control and genetic manipulation of mosquitoes. Adv. Virus Res. 2006, 68, 361–392. [Google Scholar] [CrossRef]
- Li, J.; Dong, Y.; Sun, Y.; Lai, Z.; Zhao, Y.; Liu, P.; Gao, Y.; Chen, X.; Gu, J. A novel densovirus isolated from the asian tiger mosquito displays varied pathogenicity depending on its host species. Front. Microbiol. 2019, 10, 1549. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Li, X.; Gu, J.; Dong, Y.; Liu, Y.; Santhosh, P.; Chen, X. Development of non-defective recombinant densovirus vectors for microRNA delivery in the invasive vector mosquito, Aedes albopictus. Sci. Rep. 2016, 6, 20979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, H.M.; Ng’habi, K.R.; Walder, T.; Kadungula, D.; Moore, S.J.; Lyimo, I.; Russell, T.L.; Urassa, H.; Mshinda, H.; Killeen, G.F. Establishment of a large semi-field system for experimental study of African malaria vector ecology and control in Tanzania. Malar. J. 2008, 7, 158. [Google Scholar] [CrossRef] [Green Version]
- Liu, P.-W.; Xu, J.-B.; Dong, Y.-Q.; Chen, X.-G.; Gu, J.-B. Use of a recombinant mosquito densovirus as a gene delivery vector for the functional analysis of genes in mosquito larvae. JoVE 2017, 128, e56121. [Google Scholar] [CrossRef]
- Blazer, V.S.; Walsh, H.L.; Braham, R.P.; Smith, C. Necropsy-based wild fish health assessment. JoVE 2018, 139, e57946. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, L.D.; Bickford, A.A. Necropsy of chickens, turkeys, and other poultry. Vet. Clin. N. Am. Food Anim. 1986, 2, 43–60. [Google Scholar] [CrossRef]
- Parkinson, C.M.; O’Brien, A.; Albers, T.M.; Simon, M.A.; Clifford, C.B.; Pritchett-Corning, K.R. Diagnostic necropsy and selected tissue and sample collection in rats and mice. JoVE 2011, 54, e2966. [Google Scholar] [CrossRef] [Green Version]
- Perumalsamy, H.; Jang, M.J.; Kim, J.-R.; Kadarkarai, M.; Ahn, Y.-J. Larvicidal activity and possible mode of action of four flavonoids and two fatty acids identified in Millettia pinnata seed toward three mosquito species. Parasites Vectors 2015, 8, 237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledermann, J.P.; Suchman, E.L.; Black IV, W.C.; Carlson, J.O. Infection and pathogenicity of the mosquito densoviruses AeDNV, HeDNV, and APeDNV in Aedes aegypti mosquitoes (Diptera: Culicidae). J. Econ. Entomol. 2004, 97, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Hirunkanokpun, S.; Carlson, J.O.; Kittayapong, P. Evaluation of mosquito densoviruses for controlling Aedes aegypti (Diptera: Culicidae): Variation in efficiency due to virus strain and geographic origin of mosquitoes. Am. J. Trop. Med. 2008, 78, 784–790. [Google Scholar] [CrossRef] [Green Version]
- Zhai, Y.-G.; Lv, X.-J.; Sun, X.-H.; Fu, S.-H.; Fen, Y.; Tong, S.-X.; Wang, Z.-X.; Tang, Q.; Attoui, H.; Liang, G.-D. Isolation and characterization of the full coding sequence of a novel densovirus from the mosquito Culex pipiens pallens. J. Gen. Virol. 2008, 89, 195–199. [Google Scholar] [CrossRef]
- Berini, F.; Katz, C.; Gruzdev, N.; Casartelli, M.; Tettamanti, G.; Marinelli, F. Microbial and viral chitinases: Attractive biopesticides for integrated pest management. Biotechnol. Adv. 2018, 36, 818–838. [Google Scholar] [CrossRef]
- Chen, C.; Lee, H.; Stella-Wong, S.; Lau, K.; Sofian-Azirun, M. Container survey of mosquito breeding sites in a university campus in Kuala Lumpur, Malaysia. Dengue Bull. 2009, 33, 187–193. [Google Scholar]
- Alkenani, N.A. Influence of the mixtures composed of slow–release insecticide formulations against Aedes aegypti mosquito larvae reared in pond water. Saudi J. Biol. Sci. 2017, 24, 1181–1185. [Google Scholar] [CrossRef]
- Kovendan, K.; Murugan, K.; Kumar, A.N.; Vincent, S.; Hwang, J.-S. Bioefficacy of larvicdial and pupicidal properties of Carica papaya (Caricaceae) leaf extract and bacterial insecticide, spinosad, against chikungunya vector, Aedes aegypti (Diptera: Culicidae). Parasitol. Res. 2012, 110, 669–678. [Google Scholar] [CrossRef]
- Guidi, V.; Lehner, A.; Lüthy, P.; Tonolla, M. Dynamics of Bacillus thuringiensis var. israelensis and Lysinibacillus sphaericus spores in urban catch basins after simultaneous application against mosquito larvae. PLoS ONE 2013, 8, e55658. [Google Scholar] [CrossRef]
- Bernal, L.; Dussán, J. Synergistic effect of Lysinibacillus sphaericus and glyphosate on temephos-resistant larvae of Aedes aegypti. Parasites Vectors 2020, 13, 68. [Google Scholar] [CrossRef] [Green Version]
- Bravo, A.; Gill, S.S.; Soberon, M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon 2007, 49, 423–435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, J.-B.; Dong, Y.-Q.; Peng, H.-J.; Chen, X.-G. A recombinant AeDNA containing the insect-specific toxin, BmK IT1, displayed an increasing pathogenicity on Aedes albopictus. Am. J. Trop. Med. 2010, 83, 614. [Google Scholar] [CrossRef] [Green Version]
- Tetreau, G.; Stalinski, R.; Kersusan, D.; Veyrenc, S.; David, J.-P.; Reynaud, S.; Després, L. Decreased toxicity of Bacillus thuringiensis subsp. israelensis to mosquito larvae after contact with leaf litter. Appl. Environ. 2012, 78, 5189–5195. [Google Scholar] [CrossRef] [Green Version]
- Dupont, C.; Boisvert, J. Persistence of Bacillus thuringiensis serovar. israelensis toxic activity in the environment and interaction with natural substrates. Water Air Soil Pollut. 1986, 29, 425–438. [Google Scholar] [CrossRef]
- Sun, Y.; Dong, Y.; Li, J.; Lai, Z.; Hao, Y.; Liu, P.; Chen, X.; Gu, J. Development of large-scale mosquito densovirus production by in vivo methods. Parasites Vectors 2019, 12, 255. [Google Scholar] [CrossRef]
- Agboli, E.; Leggewie, M.; Altinli, M.; Schnettler, E. Mosquito-Specific Viruses—Transmission and Interaction. Viruses 2019, 11, 873. [Google Scholar] [CrossRef] [Green Version]
- Marcombe, S.; Darriet, F.; Agnew, P.; Etienne, M.; Yp-Tcha, M.-M.; Yébakima, A.; Corbel, V. Field efficacy of new larvicide products for control of multi-resistant Aedes aegypti populations in Martinique (French West Indies). Am. J. Trop. Med. 2011, 84, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thavara, U.; Tawatsin, A.; Chansang, C.; Asavadachanukorn, P.; Zaim, M.; Mulla, M.S. Simulated field evaluation of the efficacy of two formulations of diflubenzuron, a chitin synthesis inhibitor against larvae of Aedes aegypti (L.) (Diptera: Culicidae) in water-storage containers. Southeast Asian J. Trop. Med. Public Health 2007, 38, 269. [Google Scholar] [PubMed]
- Becker, N.; Petric, D.; Zgomba, M.; Boase, C.; Dahl, C.; Lane, J.; Kaiser, A. Mosquitoes and Their Control; Plenum Publishers: New Heidelberg, Germany, 2003; 498p. [Google Scholar]
- Margalit, J.; Bobroglo, H. The effect of organic materials and solids in water on the persistence of Bacillus thuringiensis var. israelensis Serotype H-14 1. Z. Angew. Entomol. 1984, 97, 516–520. [Google Scholar] [CrossRef]
- Karch, S.; Manzambi, Z.; Salaun, J. Field trials with Vectolex (Bacillus sphaericus) and Vectobac (Bacillus thuringiensis (H-14)) against Anopheles gambiae and Culex quinquefasciatus breeding in Zaire. J. Am. Mosq. Control Assoc. 1991, 7, 176–179. [Google Scholar] [PubMed]
- Yang, P.; Carey, J.R.; Dowell, R.V. Temperature influences on the development and demography of Bactrocera dorsalis (Diptera: Tephritidae) in China. Environ. Entomol. 1994, 23, 971–974. [Google Scholar] [CrossRef]
- Dreyer, H.; Baumgärtner, J. Temperature influence on cohort parameters and demographic characteristics of the two cowpea coreids Clavigralla tomentosicollis and C. shadabi. Entomol. Exp. Appl. 1996, 78, 201–213. [Google Scholar] [CrossRef]
- Infante, F. Development and population growth rates of Prorops nasuta (Hym., Bethylidae) at constant temperatures. J. Appl. Entomol 2000, 124, 343–348. [Google Scholar] [CrossRef]
- Muturi, E.J.; Alto, B.W. Larval environmental temperature and insecticide exposure alter Aedes aegypti competence for arboviruses. Vector Borne Zoonotic Dis. 2011, 11, 1157–1163. [Google Scholar] [CrossRef]
- Buckner, E.A.; Alto, B.W.; Lounibos, L.P. Larval temperature–food effects on adult mosquito infection and vertical transmission of dengue-1 virus. J. Med. Entomol. 2016, 53, 91–98. [Google Scholar] [CrossRef] [Green Version]
- Perrin, A.; Gosselin-Grenet, A.-S.; Rossignol, M.; Ginibre, C.; Scheid, B.; Lagneau, C.; Chandre, F.; Baldet, T.; Ogliastro, M.; Bouyer, J. Variation in the susceptibility of urban Aedes mosquitoes infected with a densovirus. Sci. Rep. 2020, 10, 18654. [Google Scholar] [CrossRef]
- Bravo, A.; Likitvivatanavong, S.; Gill, S.S.; Soberón, M. Bacillus thuringiensis: A story of a successful bioinsecticide. Insect Biochem. Mol. Biol. 2011, 41, 423–431. [Google Scholar] [CrossRef] [Green Version]
- Buchatsky, L. Densonucleosis of bloodsucking mosquitoes. Dis. Aquat. Org. 1989, 6, 145–150. [Google Scholar] [CrossRef]
- Fediere, G. Epidemiology and pathology of densovirinae. In Parvoviruses: From Molecular Biology to Pathology and Therapeutic Uses; Faisst, G., Rommerlaere, S., Eds.; S. Karger: Basal, Switzerland, 2000; Volume 4. [Google Scholar]
- Jousset, F.-X.; Barreau, C.; Boublik, Y.; Cornet, M. A parvo-like virus persistently infecting a C6/36 clone of Aedes albopictus mosquito cell line and pathogenic for Aedes aegypti larvae. Virus Res. 1993, 29, 99–114. [Google Scholar] [CrossRef]



| Type | Test Species | AalDV-5 Concentration Used (Copies/mL) | Application | PCR Detection | Pathological Changes | Target Species | Non-Target Species |
|---|---|---|---|---|---|---|---|
| Mammal | Mice | 1 × 108 | Respiratory toxicity | Not detected | Not observed | _ | + |
| Mice | 1 × 108 | Injection | _ | _ | _ | + | |
| 1 × 1011 | Oral | _ | _ | _ | + | ||
| Bird | Chicken | 1 × 1011 | Oral | _ | _ | _ | + |
| Aquatic | Carp | 1 × 1011 | Oral | _ | _ | _ | + |
| Cell lines | HBMEC | 1 × 1011 | Inoculation | _ | _ | _ | + |
| U251 | 1 × 1011 | Inoculation | _ | _ | _ | + | |
| Vero | 1 × 1011 | Inoculation | _ | _ | _ | + | |
| Cos-7 | 1 × 1011 | Inoculation | _ | _ | _ | + | |
| BHK-21 | 1 × 1011 | Inoculation | _ | _ | _ | + | |
| S2 | 1 × 1011 | Inoculation | _ | _ | _ | + | |
| Sf9 | 1 × 1011 | Inoculation | _ | _ | _ | + | |
| C6/36 | 1 × 1011 | Inoculation | + | + | + | _ | |
| Aag2 | 1 × 1011 | Inoculation | + | + | + | _ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batool, K.; Xiao, J.; Xu, Y.; Yang, T.; Tao, P.; Zhao, S.; Chen, J.; Alam, I.; Xie, Y.; Gu, J.; et al. Densovirus Oil Suspension Significantly Improves the Efficacy and Duration of Larvicidal Activity against Aedes albopictus. Viruses 2022, 14, 475. https://doi.org/10.3390/v14030475
Batool K, Xiao J, Xu Y, Yang T, Tao P, Zhao S, Chen J, Alam I, Xie Y, Gu J, et al. Densovirus Oil Suspension Significantly Improves the Efficacy and Duration of Larvicidal Activity against Aedes albopictus. Viruses. 2022; 14(3):475. https://doi.org/10.3390/v14030475
Chicago/Turabian StyleBatool, Khadija, Jie Xiao, Ye Xu, Ting Yang, Peiwen Tao, Siyu Zhao, Jiao Chen, Intikhab Alam, Yugu Xie, Jinbao Gu, and et al. 2022. "Densovirus Oil Suspension Significantly Improves the Efficacy and Duration of Larvicidal Activity against Aedes albopictus" Viruses 14, no. 3: 475. https://doi.org/10.3390/v14030475
APA StyleBatool, K., Xiao, J., Xu, Y., Yang, T., Tao, P., Zhao, S., Chen, J., Alam, I., Xie, Y., Gu, J., & Chen, X. (2022). Densovirus Oil Suspension Significantly Improves the Efficacy and Duration of Larvicidal Activity against Aedes albopictus. Viruses, 14(3), 475. https://doi.org/10.3390/v14030475





