An Insight into the Molecular Characteristics and Associated Pathology of Chicken Astroviruses
Abstract
:1. Introduction
2. Astrovirus
2.1. Virus Structure
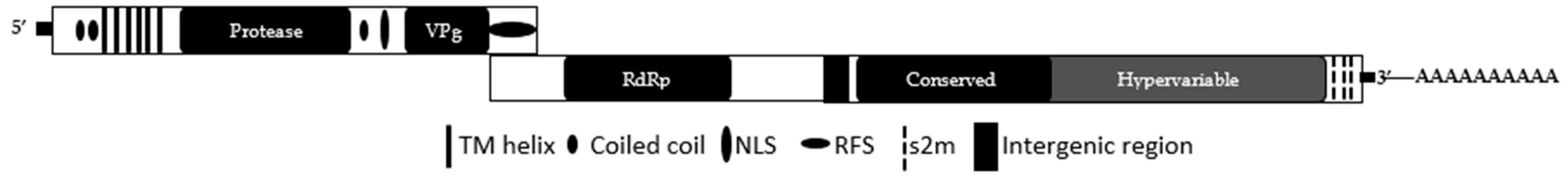
2.2. Genome Organization
2.3. Classification and Characterization
3. Chicken Astrovirus Infection
3.1. Transmission
3.2. CAstV-Associated Clinical Illnesses or Conditions
3.2.1. Uneven Flock Performance and the Runting-Stunting Syndrome (RSS)
3.2.2. Severe Kidney Disease, Urate Deposits, and Visceral Gout
3.2.3. White Chick Syndrome or White Chick Hatchery Disease
4. Control and Prevention
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Global Poultry Production to Reach 137M Tonnes in 2020, Mainly Driven by Growth in China, the EU, and the UK. Available online: https://www.globaltrademag.com/global-poultry-production-to-reach-137m-tonnes-in-2020-mainly-driven-by-growth-in-china-the-eu-and-the-uk/ (accessed on 30 November 2020).
- Saif, Y.M.; Swayne, D.E.; Pantin-Jackwood, M.J.; Spackman, E.; Johnson, T.J.; Day, J.M.; French, D.; Gingerich, E.; Bilgili, S.F.; Jones, K.; et al. Emerging Diseases and Diseases of Complex or Unknown Etiology. In Diseases of Poultry; Swayne, D.E., Boulianne, M., Logue, C.M., McDougald, L.R., Nair, V., Suarez, D.L., de Wit, S., Grimes, T., Johnson, D., Kromm, M., et al., Eds.; Wiley Online Books; Wiley-Blackwell: Ames, IA, USA, 2020; pp. 1383–1410. ISBN 9781119371199. [Google Scholar]
- Cortez, V.; Meliopoulos, V.A.; Karlsson, E.A.; Hargest, V.; Johnson, C.; Schultz-Cherry, S. Astrovirus biology and pathogenesis. Annu. Rev. Virol. 2017, 4, 327–348. [Google Scholar] [CrossRef]
- Smyth, V.J. A review of the strain diversity and pathogenesis of chicken astrovirus. Viruses 2017, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Iturriza-Gómara, M.; Cunliffe, N.A. 34—Viral Gastroenteritis. In Hunter’s Tropical Medicine and Emerging Infectious Diseases, 10th ed.; Ryan, E.T., Hill, D.R., Solomon, T., Aronson, N.E., Endy, T.P., Eds.; Elsevier: London, UK, 2020; pp. 289–307. ISBN 978-0-323-55512-8. [Google Scholar]
- Madeley, C.R.; Cosgrove, B.P. 28nm particles in faeces in infantile gastrornteritis. Lancet 1975, 6, 13–14. [Google Scholar]
- Snodgrass, D.R.; Angus, K.W.; Gray, E.W.; Menzies, J.D.; Paul, G. Pathogenesis of diarrhoea caused by astrovirus infections in lambs. Arch. Virol. 1979, 60, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Woode, G.N.; Gourley, N.E.; Pohlenz, J.F.; Liebler, E.M.; Mathews, S.L.; Hutchinson, M.P. Serotypes of bovine astrovirus. J. Clin. Microbiol. 1985, 22, 668–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gough, R.E.; Borland, E.D.; Keymer, I.F.; Stuart, J.C. An outbreak of duck hepatitis yype II in commercial ducks. Avian Pathol. 1985, 14, 227–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Benedictis, P.; Schultz-Cherry, S.; Burnham, A.; Cattoli, G. Astrovirus infections in humans and animals—Molecular biology, genetic diversity, and interspecies transmissions. Infect. Genet. Evol. 2011, 11, 1529–1544. [Google Scholar] [CrossRef]
- Asplin, F.D. Duck hepatitis: Vaccination against two serological types. Vet. Rec. 1965, 77, 1529–1530. [Google Scholar] [PubMed]
- Imada, T.; Yamaguchi, S.; Mase, M.; Tsukamoto, K.; Kubo, M.; Morooka, A. Avian nephritis virus (ANV) as a new member of the family astroviridae and construction of infectious ANV cDNA. J. Virol. 2000, 74, 8487–8493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxendale, W.; Mebatsion, T. The isolation and characterisation of astroviruses from chickens. Avian Pathol. 2004, 33, 364–370. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, F.; Connor, T.J.; Calvert, V.M.; Smyth, J.A.; Curran, W.L.; Morley, A.J.; Thompson, D.; Singh, S.; Mcferran, J.B.; Adair, B.M.; et al. Studies on a new enterovirus-like virus isolated from chickens. Avian Pathol. 1994, 23, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.; El-gazzar, M.; Sellers, H.S.; Dorea, F.; Williams, S.M.; Kim, T.; Collett, S.; Mundt, E. Investigation into the aetiology of runting and stunting syndrome in chickens. Avian Pathol. 2012, 41, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulbule, N.R.; Mandakhalikar, K.D.; Kapgate, S.S.; Deshmukh, V.V.; Schat, K.A.; Chawak, M.M. Role of chicken astrovirus as a causative agent of gout in commercial broilers in India. Avian Pathol. 2013, 42, 464–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajewicz-Krukowska, J.; Pać, K.; Lisowska, A.; Pikuła, A.; Minta, Z.; Króliczewska, B.; Domańska-Blicharz, K. Astrovirus-induced “white chicks” condition—Field observation, virus detection and preliminary characterization. Avian Pathol. 2016, 45, 2–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sajewicz-Krukowska, J.; Domanska-Blicharz, K. Nearly full-length genome sequence of a novel astrovirus isolated from chickens with ‘white chicks’ condition. Arch. Virol. 2016, 161, 2581–2587. [Google Scholar] [CrossRef] [Green Version]
- Jacukowicz, A.; Domańska-Blicharz, K. Astrowirusy u drobiu. Med. Weter. 2017, 73, 329–333. [Google Scholar]
- Long, K.E.; Hastie, G.M.; Ojkić, D.; Brash, M.L. Economic impacts of white chick syndrome in Ontario, Canada. Avian Dis. 2017, 61, 402–408. [Google Scholar] [CrossRef]
- Caul, E.O.; Appleton, H. The electron microscopical and physical characteristics of small round human fecal viruses: An interim scheme for classification. J. Med. Virol. 1982, 9, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.F.; Dubois, R.M. The astrovirus capsid: A review. Viruses 2017, 9, 15. [Google Scholar] [CrossRef] [PubMed]
- del Rocío Banos-Lara, M.; Méndez, E. Role of individual caspases induced by astrovirus on the processing of its structural protein and its release from the cell through a non-lytic mechanism. Virology 2010, 401, 322–332. [Google Scholar] [CrossRef] [Green Version]
- Mendez, E.; Salas-Ocampo, E.; Arias, C.F. Caspases mediate processing of the capsid precursor and cell release of human astroviruses. J. Virol. 2004, 78, 8601–8608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.-I.; Linnemann, E.; Icard, A.H.; Durairaj, V.; Mundt, E.; Sellers, H.S. Chicken astrovirus as an aetiological agent of runting-stunting syndrome in broiler chickens. J. Gen. Virol. 2018, 99, 512–524. [Google Scholar] [CrossRef] [PubMed]
- Bosch, A.; Guix, S.; Krishna, N.K.; Méndez, E.; Monroe, S.S.; Pantin-Jackwood, M.; Schultz-Cherry, S. Family—Astroviridae. In Ninth Report of the International Committee on Taxonomy of Viruses; King, A.M.Q., Lefkowitz, E., Adams, M.J., Carstens, E.B., Eds.; Elsevier: San Diego, CA, USA, 2012; pp. 953–959. ISBN 9780123846846. [Google Scholar]
- Bosch, A.; Pintó, R.M.; Guix, S. Human astroviruses. Clin. Microbiol. Rev. 2014, 27, 1048–1074. [Google Scholar] [CrossRef] [Green Version]
- Mendez, E.; Arias, C.F. Astroviruses. In Fields Virology; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007; pp. 981–1000. [Google Scholar]
- Kang, K.-I.; Icard, A.H.; Linnemann, E.; Sellers, H.S.; Mundt, E. Determination of the full length sequence of a chicken astrovirus suggests a different replication mechanism. Virus Genes 2012, 44, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.A.; Ideris, A.; Bejo, M.H.; Omar, A.R. Molecular characterisation and pathogenicity of novel Malaysian chicken astrovirus isolates. Avian Pathol. 2022, 51, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Koci, M.D.; Seal, B.S.; Schultz-Cherry, S. Molecular characterization of an avian astrovirus. J. Virol. 2000, 74, 6173–6177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koci, M.D.; Schultz-Cherry, S. Avian astroviruses. Avian Pathol. 2002, 31, 213–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pantin-Jackwood, M.J.; Strother, K.O.; Mundt, E.; Zsak, L.; Day, J.M.; Spackman, E. Molecular characterization of avian astroviruses. Arch. Virol. 2011, 156, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Krishna, N.K. Identification of structural domains involved in astrovirus capsid biology. Viral Immunol. 2005, 18, 17–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kofstad, T.; Jonassen, C.M. Screening of feral and wood pigeons for viruses harbouring a conserved mobile viral element: Characterization of novel astroviruses and picornaviruses. PLoS ONE 2011, 6, e25964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkbeiner, S.R.; Kirkwood, C.D.; Wang, D. Complete genome sequence of a highly divergent astrovirus isolated from a child with acute diarrhea. Virol. J. 2008, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pantin-Jackwood, M.J.; Spackman, E.; Woolcock, P.R. Molecular characterization and typing of chicken and turkey astroviruses circulating in the United States: Implications for diagnostics. Avian Dis. 2006, 50, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Monceyron, C.; Grinde, B.; Jonassen, T. Molecular characterisation of the 3’-end of the astrovirus genome. Arch. Virol. 1997, 142, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, E.A.; Small, C.T.; Freiden, P.; Feeroz, M.M.; Matsen, F.A.; San, S.; Hasan, M.K.; Wang, D.; Jones-Engel, L.; Schultz-Cherry, S. Non-Human primates harbor diverse mammalian and avian astroviruses including those associated with human infections. PLoS Pathog. 2015, 11, e1005225. [Google Scholar] [CrossRef]
- Woo, P.C.Y.; Lau, S.K.P.; Teng, J.L.L.; Tsang, A.K.L.; Joseph, S.; Xie, J.; Jose, S.; Fan, R.Y.Y.; Wernery, U.; Yuen, K.Y. A novel astrovirus from dromedaries in the Middle East. J. Gen. Virol. 2015, 96, 2697–2707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donato, C.; Vijaykrishna, D. The broad host range and genetic diversity of mammalian and avian astroviruses. Viruses 2017, 9, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Correa, I.; Truchado, D.A.; Gomez-Lucia, E.; Doménech, A.; Pérez-Tris, J.; Schmidt-Chanasit, J.; Cadar, D.; Benítez, L. A novel group of avian astroviruses from Neotropical passerine birds broaden the diversity and host range of Astroviridae. Sci. Rep. 2019, 9, 9513. [Google Scholar] [CrossRef] [PubMed]
- Fuller, T.; Bensch, S.; Müller, I.; Novembre, J.; Pérez-Tris, J.; Ricklefs, R.E.; Smith, T.B.; Waldenström, J. The ecology of emerging infectious diseases in migratory birds: An assessment of the role of climate change and priorities for future research. Ecohealth 2012, 9, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Smyth, V.J.; Todd, D.; Trudgett, J.; Lee, A.; Welsh, M.D. Capsid protein sequence diversity of chicken astrovirus. Avian Pathol. 2012, 41, 151–159. [Google Scholar] [CrossRef] [Green Version]
- McNulty, M.S.; Connor, T.J.; McNeilly, F.; McFerran, J.B. Biological characterisation of avian enteroviruses and enterovirus-like viruses. Avian Pathol. 1990, 19, 75–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Todd, D.; Wilkinson, D.S.; Jewhurst, H.L.; Wylie, M.; Gordon, A.W.; Adair, B.M. A seroprevalence investigation of chicken astrovirus infections. Avian Pathol. 2009, 38, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Kwoka, K.T.T.; de Rooij, M.M.T.; Messink, A.B.; Wouters, I.M.; Smit, L.A.M.; Heederik, D.J.J.; Koopmans, M.P.G.; Phan, M.V.T. Comparative viral metagenomics from chicken feces and farm dust in the Netherlands. bioRxiv 2021. [Google Scholar] [CrossRef]
- Worobey, M.; Holmes, E.C. Evolutionary aspects of recombination in RNA viruses. J. Gen. Virol. 1999, 80, 2535–2543. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.D.; Desai, P.T.; Zhang, Y.; Scharber, S.K.; Baller, J.; Xing, Z.S.; Cardona, C.J. Development of the intestinal RNA virus community of healthy broiler chickens. PLoS ONE 2016, 11, e0150094. [Google Scholar] [CrossRef] [Green Version]
- Pantin-Jackwood, M.J.; Day, J.M.; Jackwood, M.W.; Spackman, E. Enteric viruses detected by molecular methods in commercial chicken and turkey flocks in the United States between 2005 and 2006. Avian Dis. 2008, 52, 235–244. [Google Scholar] [CrossRef] [Green Version]
- Pantin-Jackwood, M.J.; Spackman, E.; Day, J.M.; Rives, D. Periodic monitoring of commercial turkeys for enteric viruses indicates continuous presence of astrovirus and rotavirus on the farms. Avian Dis. Dig. 2007, 2, e4. [Google Scholar] [CrossRef] [Green Version]
- Yin, L.; Zhou, Q.; Mai, K.; Huang, J.; Yan, Z.; Wei, X.; Shen, H.; Li, Q.; Chen, L.; Zhou, Q. Isolation and characterization of a novel chicken astrovirus in China. Poult. Sci. 2021, 100, 101363. [Google Scholar] [CrossRef]
- Palomino-Tapia, V.; Mitevski, D.; Inglis, T.; van der Meer, F.; Martin, E.; Brash, M.; Provost, C.; Gagnon, C.A.; Abdul-Careem, M.F. Chicken Astrovirus (CAstV) molecular studies reveal evidence of multiple past recombination events in sequences originated from clinical samples of White Chick Syndrome (WCS) in Western Canada. Viruses 2020, 12, 1096. [Google Scholar] [CrossRef] [PubMed]
- Adebiyi, A.I.; Mcilwaine, K.; Oluwayelu, D.O.; Smyth, V.J. Detection and characterization of chicken astrovirus associated with hatchery disease in commercial day-old turkeys in southwestern Nigeria. Arch. Virol. 2021, 166, 1607–1614. [Google Scholar] [CrossRef] [PubMed]
- de Wit, J.J.; ten Dam, G.B.; vande Laar, J.M.A.M.; Biermann, Y.; Verstegen, I.; Edens, F.; Schrier, C.C. Detection and characterization of a new astrovirus in chicken and turkeys with enteric and locomotion disorders. Avian Pathol. 2011, 40, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.A.; Ideris, A.; Bejo, M.H.; Omar, A.R. Molecular detection, characterisation and serological survey of chicken astrovirus from broiler flocks in Malaysia. Pertanika J. Sci. Technol. 2022, 1–15. [Google Scholar] [CrossRef]
- Oluwayelu, D.O.; Todd, D. Chicken astrovirus infection: Minireview and preliminary serologic evidence of antigenically and genetically distinct chicken astroviruses in Nigerian indigenous chickens. African J. Biomed. Res. 2012, 15, 71–76. [Google Scholar]
- Sharma, R.N.; Dufayet, R.; Maufras, T.; O’Connell, K.; Tiwari, K. Seroprevalence of antibodies to astrovirus in chickens in Grenada, West Indies. Vet. World 2017, 10, 636–639. [Google Scholar] [CrossRef]
- Xue, J.; Han, T.; Xu, M.; Zhao, J.; Zhang, G. The first serological investigation of chicken astrovirus infection in China. Biologicals 2017, 47, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Smyth, J.A.; Connor, T.J.; McNeilly, F.; Moffet, D.A.; Calvert, V.M.; McNulty, M.S. Studies on the pathogenicity of enterovirus-like viruses in chickens. Avian Pathol. 2007, 36, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wu, Z.; Yao, Y.; Qin, A.; Qian, K. The isolation and molecular characterization of an astrovirus from “yellow” chickens, China. Front. Vet. Sci. 2020, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Pandit, R.J.; Thakkar, J.R.; Hinsu, A.T.; Pandey, V.C.; Pal, J.K.; Prajapati, K.S.; Jakhesara, S.J.; Joshi, C.G. Complete genome sequence analysis of chicken astrovirus isolate from India. Vet. Res. Commun. 2017, 41, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.; Smyth, V.; Ball, N.W.; Donnelly, B.M.; Wylie, M.; Knowles, N.J.; Adair, B.M. Identification of chicken enterovirus-like viruses, duck hepatitis virus type 2 and duck hepatitis virus type 3 as astroviruses. Avian Pathol. 2009, 38, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brugère-Picoux, J.; Vaillancourt, J.P.; Bouzouaia, M.; Shivaprasad, H.L.; Venne, D. Manual of Poultry Diseases; Ed. AFAS: Paris, France, 2015; pp. 300–316. [Google Scholar]
- Rebel, J.M.J.; Balk, F.R.M.; Post, J.; Van Hemert, S.; Zekarias, B.; Stockhofe, N. Malabsorption syndrome in broilers. Worlds. Poult. Sci. J. 2006, 62, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Rosenberger, J.; Direction, C.S. Update on the Runting-Stunting Syndrome. Ceva Eggs Program Online. Available online: http://fs-1.5mpublishing.com/images/ceva/EPO_No3-May2012.pdf (accessed on 19 March 2022).
- Nuñez, L.F.N.; Santander-Parra, S.H.; Kyriakidis, N.C.; Astolfi-Ferreira, C.S.; Buim, M.R.; De la Torre, D.; Ferreira, A.J.P. Molecular characterization and determination of relative cytokine expression in naturally infected day-old chicks with chicken astrovirus associated to white chick syndrome. Animals 2020, 10, 1195. [Google Scholar] [CrossRef] [PubMed]
- Smyth, V.; Trudgett, J.; Wylie, M.; Jewhurst, H.; Conway, B.; Welsh, M.; Kaukonen, E.; Perko-Mäkelä, P. Chicken astrovirus detected in hatchability problems associated with ‘white chicks’. Vet. Rec. 2013, 173, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Olsen, D.E. Isolation of a Reovirus-like Agent from Broiler Chicks with Diarrhea and Stunting. In Proceedings of the 26th Western Poultry Diseases Conference, Davis, CA, USA, 21–24 March 1977; pp. 131–139. [Google Scholar]
- Kouwenhoven, B.; Vertommen, M.; Goren, E. Investigations into the role of reovirus in the malabsorption syndrome. Avian Pathol. 1988, 17, 879–892. [Google Scholar] [CrossRef] [PubMed]
- Zsak, L.; Cha, R.M.; Day, J.M. Chicken parvovirus-induced runting-stunting syndrome in young broilers. Avian Dis. 2013, 57, 123–127. [Google Scholar] [CrossRef]
- Smyth, V.J.; Jewhurst, H.L.; Wilkinson, D.S.; Adair, B.M.; Gordon, A.W.; Todd, D. Development and evaluation of real-time TaqMan ® RT-PCR assays for the detection of avian nephritis virus and chicken astrovirus in chickens. Avian Pathol. 2010, 39, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Mettifogo, E.; Nuñez, L.F.N.; Chacón, J.L.; Santander Parra, S.H.; Astolfi-Ferreira, C.S.; Jerez, J.A.; Jones, R.C.; Piantino Ferreira, A.J. Emergence of enteric viruses in production chickens is a concern for avian health. Sci. World J. 2014, 2014, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; Day, J.M.; Pantin-Jackwood, M.J. Astrovirus, reovirus, and rotavirus concomitant infection causes decreased weight gain in broad-breasted white poults. Avian Dis. Dig. 2010, 5, e3–e4. [Google Scholar] [CrossRef]
- Bayry, J.; Goudar, M.S.; Nighot, P.K.; Kshirsagar, S.G.; Ladman, B.S.; Gelb, J.; Ghalsasi, G.R.; Kolte, G.N. Erratum: Emergence of a nephropathogenic avian infectious bronchitis virus with a novel genotype in India (Journal of Clinical Microbiology (2005) 43:2 (916–918)). J. Clin. Microbiol. 2005, 43, 2039. [Google Scholar] [CrossRef] [Green Version]
- Sellers, H.; Linneman, E.; Icard, A.H.; Mundt, E. A purified recombinant baculovirus expressed capsid protein of a new astrovirus provides partial protection to runting–stunting syndrome in chickens. Vaccine 2010, 28, 1253–1263. [Google Scholar] [CrossRef] [PubMed]
- Connor, T.J.; McNeilly, F.; McFerran, J.B.; McNulty, M.S. A survey of avian sera from northern ireland for antibody to avian nephritis virus. Avian Pathol. 1987, 16, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Moser, L.A.; Schultz-Cherry, S. Pathogenesis of astrovirus infection. Viral Immunol. 2005, 18, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Rajendra Bulbule, N.; Sanjay Kapgate, S.; Madhukar Chawak, M. Infectious causes of gout. Adv. Anim. Vet. Sci. 2014, 2, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Nuñez, L.F.N.; Parra, S.H.S.; Carranza, C.; Astolfi-Ferreira, C.S.; Buim, M.R.; Ferreira, A.J.P. Detection and molecular characterization of chicken astrovirus associated with chicks that have an unusual condition known as “white chicks” in Brazil. Poult. Sci. 2016, 95, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Koci, M.D.; Kelley, L.A.; Larsen, D.; Schultz-Cherry, S. Astrovirus-induced synthesis of nitric oxide contributes to virus control during infection. J. Virol. 2004, 78, 1564–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nighot, P.K.; Moeser, A.; Ali, R.A.; Blikslager, A.T.; Koci, M.D. Astrovirus infection induces sodium malabsorption and redistributes sodium hydrogen exchanger expression. Virology 2010, 401, 146–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
| No. | Accession Number | Isolate | Group/Subgroup | Isolate Origin | Isolation of Virus | Changes in SPF-Embryonated Chicken Egg and Cell Line | Clinical and Pathological Findings in Chicken | Reference |
|---|---|---|---|---|---|---|---|---|
| 1 | JN582328 | FP3 | Bi | Meconium of dead-in-shell embryo | Yolk sac of SPF embryonated chicken eggs | Stunted embryo growth | Shrunken degenerate cells and nuclear debris in the epithelium of many villi; Multifocal cellular infiltration of the renal interstitium and scattered necrotic tubules | [60,63] |
| 2 | MK746105 | CHN/2017/NJ1701 | Bi | Small intestine of “Yellow” chickens with mild growth problems | LMH and yolk sac of SPF embryonated chicken eggs | CPE detection of virus antigens; stunted embryo; significant decrease in hatching rate | [61] | |
| 3 | MW846319 | GD202013 | Bii | Kidneys and intestine | Yolk sac of embryonated chicken eggs | Stunted embryo | Enteritis, swollen kidneys; abscission in small intestines; necrotic and dilated renal tubules filled and infiltrated with red blood cells and heterophils | [52] |
| 4 | KY038163 | ANAND/2016 | Biii | Spleen, kidneys, and lungs of broiler chickens with visceral gout | Allantoic fluid of SPF embryonated chicken eggs | Embryo death within 5 to 6 days post inoculation; Hemorrhages on embryos | [62] | |
| 5 | JX945861 | PDRC (Indian isolates) | Biii | Kidneys from broiler flocks with severe mortality within first week of hatch | CEKC: and allantoic fluid of SPF embryonated chicken eggs | CPE: Stunting and yellowish discoloration of embryo | Dullness, drowsiness; 67.5 to 100% mortality in SPF and broiler chickens, respectively; necrosis of the liver; pale swollen kidneys with urate deposits, visceral and articular gout; tubular congestion, parenchymatous interstitial nephritis, and lymphocyte infiltration in the kidneys | [16] |
| 6 | KX397575 | CC_CkAstV | Biv | Intestines of chickens with RSS | LMH and yolk sac of SPF embryonated chicken eggs | Retarded growth | Cystic lesions in the intestinal (duodenum) crypts | [25] |
| 7 | MT491731 | UPM1019/2018 | Bv | Kidneys of broiler chickens with gout and urate deposit | Stunted and hemorrhagic embryo, with mortality | Diarrhea, drowsiness, ruffled feathers, retarded growth, and depression. Air-filled empty crop with distended intestines; swollen kidneys, enlarged ureters with extensive urate deposits and visceral gout; heart covered with white chalky urate deposit | [30] | |
| 8 | JN582317 | 612 | Ai | From broilers with respiratory distress | CAM of SPF embryonated chicken eggs | Pock-like lesions and generalized edematous thickening of CAM: Stunted and hemorrhagic embryos. | Slight mottling of liver and mild swelling of kidneys; very mild lesions in the intestines; shrunken degenerate cells in the crypts and cell debris in the lumen; multifocal randomly distributed mononuclear cell infiltrates with degenerate hepatocytes; mild to moderate mononuclear cell and occasionally heterophilic infiltration of the interstitium and necrotic tubules in the kidneys | [14,60] |
| 9 | JN582318 | P22.18.8.00 | Ai | From broiler chicks with RSS | CEL and LMH | CPE and plaque production | Mild diarrhea, partly digested feed in the feces; distention of the small intestine; small areas of limited damage at the base of the villi of the small intestine | [13] |
| 10 | KT886453 | G059/2014 | Aiii | Liver and kidneys of dead in shell embryos, and weak chicks with white plumage | CAM and yolk- sac of SPF embryonated chicken eggs | Delay and prolong hatching; weak chicks; growth inhibition and white chicks | Visible subcutaneous edema; greenish exudate in the body cavity; enlarged and congested liver with brittle consistency and numerous pinpoint hemorrhages on the surface; kidneys, spleens, and pancreas were enlarged and very congested | [18] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raji, A.A.; Omar, A.R. An Insight into the Molecular Characteristics and Associated Pathology of Chicken Astroviruses. Viruses 2022, 14, 722. https://doi.org/10.3390/v14040722
Raji AA, Omar AR. An Insight into the Molecular Characteristics and Associated Pathology of Chicken Astroviruses. Viruses. 2022; 14(4):722. https://doi.org/10.3390/v14040722
Chicago/Turabian StyleRaji, Abdullahi Abdullahi, and Abdul Rahman Omar. 2022. "An Insight into the Molecular Characteristics and Associated Pathology of Chicken Astroviruses" Viruses 14, no. 4: 722. https://doi.org/10.3390/v14040722
APA StyleRaji, A. A., & Omar, A. R. (2022). An Insight into the Molecular Characteristics and Associated Pathology of Chicken Astroviruses. Viruses, 14(4), 722. https://doi.org/10.3390/v14040722






