Efficacy of Liming Forest Soil in the Context of African Swine Fever Virus
Abstract
:1. Introduction
2. Materials and Methods
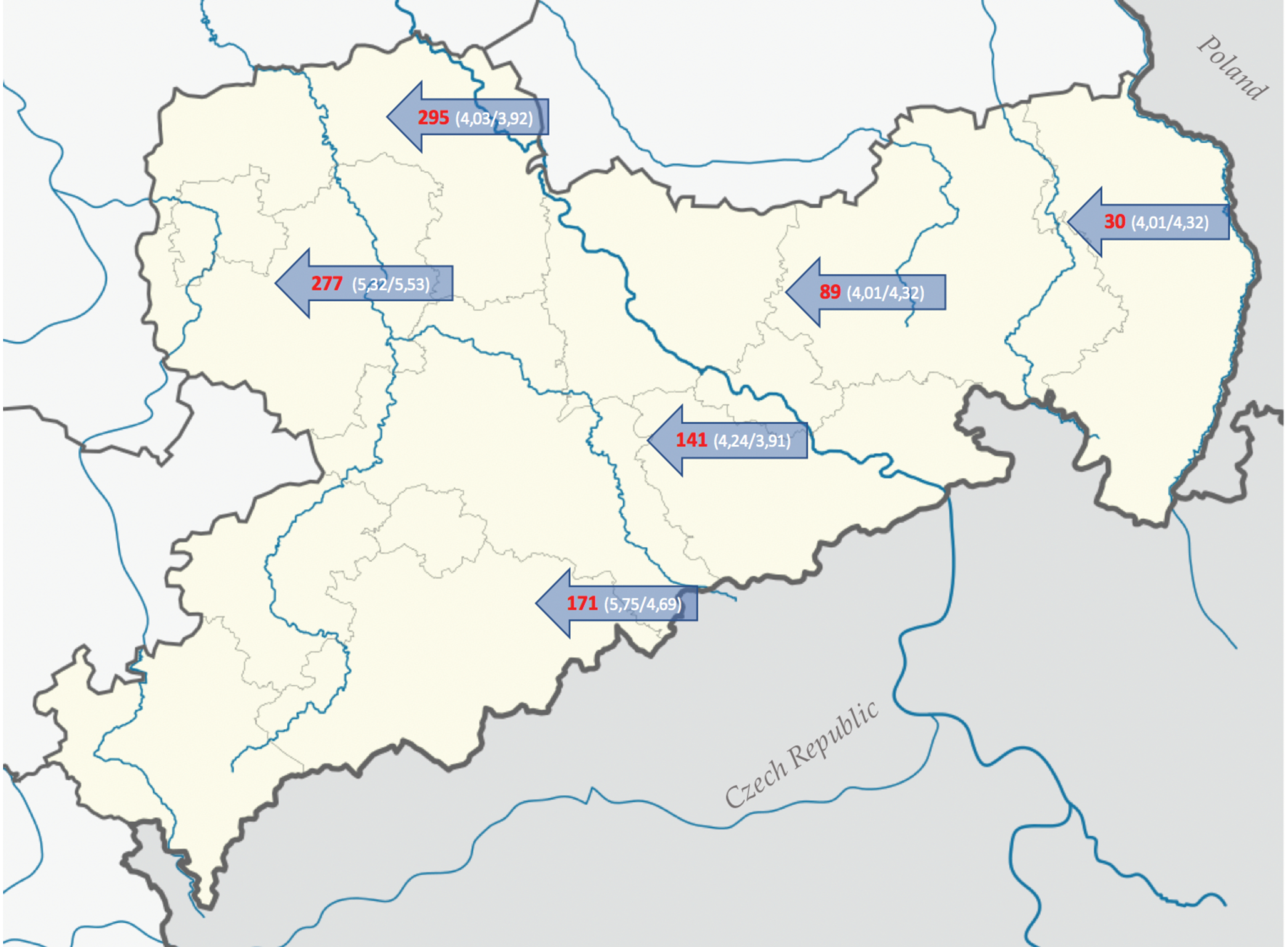
2.1. Study Sites and Soil Types
2.2. Disinfection Experiment with Slaked Lime, Lime Milk and Quicklime (Lime Experiments)
2.3. Lime/Water Ratio
2.4. Avoidance of Cell Toxicity
3. Results
3.1. Disinfection of MVAV and ASFV with Lime (Lime Experiments)
3.2. Lime/Water Ratio
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sauter-Louis, C.; Forth, J.H.; Probst, C.; Staubach, C.; Hlinak, A.; Rudovsky, A.; Holland, D.; Schlieben, P.; Goldner, M.; Schatz, J.; et al. Joining the club: First detection of African swine fever in wild boar in Germany. Transbound. Emerg. Dis. 2020, 68, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institute (FLI). Karten: ASP in Deutschland, 08.01.2021 bis 22.12.2021. Available online: https://www.openagrar.de/receive/openagrar_mods_00076885 (accessed on 7 January 2022).
- Friedrich-Loeffler-Institute (FLI). Karten zur Afrikanischen Schweinepest. Available online: https://www.fli.de/de/aktuelles/tierseuchengeschehen/afrikanische-schweinepest/karten-zur-afrikanischen-schweinepest/ (accessed on 17 September 2021).
- Forth, J.H.; (Institute of Diagnostic Virology, Friedrich-Loeffler-Institut, Greifswald, Germany). African Swine Fever Virus—Variants on the Rise. Personal communication, 2022. in press.
- European Food Safety Authority (EFSA). Epidemiological Analyses of African Swine Fever in the European Union (November 2018 to October 2019). Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2020.5996 (accessed on 30 January 2020).
- European Food Safety Authority (EFSA). Epidemiological Analyses of African Swine fever in the European Union (November 2017 until November 2018). Available online: https://efsa.onlinelibrary.wiley.com/doi/full/10.2903/j.efsa.2018.5494 (accessed on 29 November 2018).
- Bundesministerium für Gesundheit und Konsumentenschutz GZ 39.505/6-III/A/4b/96. Mittel und Verfahren für die Durchführung der Desinfektion bei Anzeigepflichtigen Tierseuchen; Durchführungsbestimmungen des Bundesministeriums für Gesundheit und Konsumentenschutz GZ 39.505/6-III/A/4b/96. Available online: https://www.verbrauchergesundheit.gv.at/tiere/tierseuchenuebungen/tierseuchen_desinfektionserlass.pdf?7vj8ky (accessed on 5 March 2021).
- Kalmar, I.D.; Cay, A.B.; Tignon, M. Sensitivity of African swine fever virus (ASFV) to heat, alkalinity and peroxide treatment in presence or absence of porcine plasma. Vet. Microbiol. 2018, 219, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Mazur-Panasiuk, N.; Zmudzki, J.; Wozniakowski, G. African Swine Fever Virus—Persistence in Different Environmental Conditions and the Possibility of its Indirect Transmission. J. Vet. Res. 2019, 63, 303–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanneberger, F.; Abd El Wahed, A.; Fischer, M.; Blome, S.; Truyen, U. The efficacy of disinfection on modified vaccinia Ankara and African Swine fever Virus in various forest soil types. Viruses 2021, 14, 2173. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institute (FLI). Empfehlungen zur Desinfektion bei Tierseuchen—Peressigsäure. Greifswald—Insel Riems, Friedrich-Loeffler-Inst. Available online: https://www.openagrar.de/servlets/MCRFileNodeServlet/openagrar_derivate_00025821/FLI-V3-3-6-Chemische-Desinfektionsmittel-Peressigsaeure-RL-Desinfektion-V02-2020-07-30.pdf (accessed on 30 July 2020).
- European Food Safety Authority (EFSA). Available data on notified biocides efficacy under field conditions. EFSA J. 2009, 2009, 34. [Google Scholar]
- Grabow, W.O.; Middendorff, I.G.; Basson, N.C. Role of lime treatment in the removal of bacteria, enteric viruses, and coliphages in a wastewater reclamation plant. Appl. Environ. Microbiol. 1978, 35, 663–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, G. Removal of viruses from sewage, effluents, and waters. I. A review. Bull. World Health Organ. 1973, 49, 451–460. [Google Scholar] [PubMed]
- Matsuzaki, S.; Azuma, K.; Lin, X.; Kuragano, M.; Uwai, K.; Yamanaka, S.; Tokuraku, K. Farm use of calcium hydroxide as an effective barrier against pathogens. Sci. Rep. 2021, 11, 7941. [Google Scholar] [CrossRef] [PubMed]
- Deutsche Veterinärmedizinische Gesellschaft (DVG). DVG-Prüfrichtlinien, V. Tierhaltung Viruzidie—Methoden der PrüEfung von Chemischen Desinfektionsmitteln für die Tierhaltung. Available online: https://www.desinfektion-d.dvg.de/fileadmin/FG_Desinfektion/Dokumente/Fuer_Gutachter/Pruefrichtlinien/V.4_Viruzidie_7Nov2017.pdf (accessed on 7 November 2017).
- Location Map Saxony, Germany (Modified for Experimental Background). 2009. Available online: https://de.wikipedia.org/wiki/Datei:Saxony_location_map.svg (accessed on 29 August 2012).
- Hübner, A.; Keßler, C.; Pannhorst, K.; Forth, J.H.; Kabuuka, T.; Karger, A.; Mettenleiter, T.C.; Fuchs, W. Identification and characterization of the 285L and K145R proteins of African swine fever virus. J. Gen. Virol. 2019, 100, 1303–1314. [Google Scholar] [CrossRef] [PubMed]
- Leitlinie der Deutschen Vereinigung zur Bekämpfung der Viruskrankheiten. Leitlinie der Deutschen Vereinigung zur Bekämpfung der Viruskrankheiten (DVV) e.V.2 und des Robert Koch-Instituts (RKI) zur Prüfung von Chemischen Desinfektionsmitteln auf Wirksamkeit Gegen Viren in der Humanmedizin Fassung vom 1. August 2008. Available online: https://www.springermedizin.de/leitlinie1-der-deutschen-vereinigung-zur-bekaempfung-der-viruskr/8010706 (accessed on 1 August 2008).
- De Lorenzi, G.; Borella, L.; Alborali, G.L.; Prodanov-Radulovic, J.; Stukelj, M.; Bellini, S. African swine fever: A review of cleaning and disinfection procedures in commercial pig holdings. Res. Vet. Sci. 2020, 132, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Gabbert, L.R.; Neilan, J.G.; Rasmussen, M. Recovery and chemical disinfection of foot-and-mouth disease and African swine fever viruses from porous concrete surfaces. J. Appl. Microbiol. 2020, 129, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Davis, T.; O’Brien, C.; LaRocco, M.; Rodriguez, L.L. Disinfection of transboundary animal disease viruses on surfaces used in pork packing plants. Vet. Microbiol. 2018, 219, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Krug, P.W.; Lee, L.J.; Eslami, A.C.; Larson, C.R.; Rodriguez, L. Chemical disinfection of high-consequence transboundary animal disease viruses on nonporous surfaces. Biologicals 2011, 39, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Franz, J.; Conraths, K.D.; Probst, C.; Sauter-Louis, C.; Beer, M.; Mettenleiter, T.; Blome, S. Maßnahmen im Falle Eines Ausbruchs von ASP bei Wildschweinen. Available online: https://www.bauernverband-mv.de/sites/default/files/2018-03/Vortrag2_Prof._Conr.raths.pdf (accessed on 8 March 2021).
- Guberti, V.; Khomenko, S.; Masiulis, M.; Kerba, S. African Swine Fever in Wild Boar—Ecology and Biosecurity. In FAO Animal Production and Health Manual No. 22; FAO: Rome, Italy, 2019; Available online: https://www.oie.int/app/uploads/2021/03/en-manual-asfinwildboar-2019-web.pdf (accessed on 19 March 2021).
- Friedrich-Loeffler-Institute (FLI). Empfehlungen zur Desinfektion bei Tierseuchen—Grundsätze zu Anforderungen und Anwendungen von Wirkstoffklassen; Friedrich-Loeffler-Institute: Riems, Germany, 2020. [Google Scholar]
- Anon. Infection Route of the 2007 Outbreak of Highly Pathogenic Avian Influenza in Japan; Food Safety and Consumer Affairs Bureau Ministry of Agriculture, Forestry & Fisheries: Tokyo, Japan, 2007. [Google Scholar]
- Ruenphet, S.; Punyadarsaniya, D.; Jantafong, T.; Takehara, K. Stability and virucidal efficacies using powder and liquid forms of fresh charcoal ash and slaked lime against Newcastle disease virus and Avian influenza virus. Vet. World 2019, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Euler, B. Lime as Disinfactant für Pig Slurry Contaminated with Aujeszky’s Disease (Pseudorabies) Virus (ADV). Agric. Wastes 1984, 9, 289–297. [Google Scholar] [CrossRef]
- Schmitz, A.P.; Le Bouquin, M.S.; Rousset, N.; Ogor, K.; LeBras, M.O.; Martenot, C.; Daniel, P.; Belen Cepeda Hontecillas, A.; Scoizec, A.; Morin, H. Natural and Experimental Persistence of Highly Pathogenic H5 Influenza Viruses in Slurry of Domestic Ducks, with or without Lime Treatment. Appl. Environ. Microbiol. 2020, 86, e02288-20. [Google Scholar] [CrossRef] [PubMed]
- Friedrich-Loeffler-Institute (FLI). Empfehlungen zur Desinfektion bei Tierseuchen—Kalk; Friedrich-Loeffler-Institute: Riems, Germany, 2020. [Google Scholar]
- Schneider, T.; Strauch, B.R.D. Untersuchungen über den Einsatz von Branntkalk zur Desinfektion von Stallböden. Tierarztl. Umsch. 1992, 47, 534–538. [Google Scholar]
- Tanneberger, F.; Institute of Animal Hygiene and Veterinary Public Health. Survey Experiment on the Change in Soil pH after Disinfection with Lime Products; data not shown; Leipzig University: Leipzig, Germany, 2021. [Google Scholar]


| Virus | BSL | Disinfection |
|---|---|---|
| MVAV | BSL-2 | 1%, 5%, 10% slaked lime 1%, 5%, 10% quicklime 1%, 5%, 10% lime milk |
| ASFV Armenia ∆258L GFP huCD4 | BSL-4 | 10% slaked lime 10% quicklime 10% lime milk |
| Ratio | Soil (mL) | MVAV (mL) | BSA (g) | Slaked Lime or Quicklime (g) | WSH (µL) | PBS (Reaction Stop) (mL) | Total Virus Dilution | Virus Titer (Log10 TCID50/mL) |
|---|---|---|---|---|---|---|---|---|
| 0 | 3 | 1 | 0.24 | 0.42 | 0 | 7 | 1:8 | ND |
| 1:2 | 420 | 6.58 | ND | |||||
| 1:3 | 840 | 6.16 | ND | |||||
| 1:5 | 1680 | 5.32 | ND | |||||
| 1:9 | 3360 | 3.64 | ND | |||||
| Control | 0 | 0 | 7 | 6.04 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanneberger, F.; Abd El Wahed, A.; Fischer, M.; Deutschmann, P.; Roszyk, H.; Carrau, T.; Blome, S.; Truyen, U. Efficacy of Liming Forest Soil in the Context of African Swine Fever Virus. Viruses 2022, 14, 734. https://doi.org/10.3390/v14040734
Tanneberger F, Abd El Wahed A, Fischer M, Deutschmann P, Roszyk H, Carrau T, Blome S, Truyen U. Efficacy of Liming Forest Soil in the Context of African Swine Fever Virus. Viruses. 2022; 14(4):734. https://doi.org/10.3390/v14040734
Chicago/Turabian StyleTanneberger, Franziska, Ahmed Abd El Wahed, Melina Fischer, Paul Deutschmann, Hanna Roszyk, Tessa Carrau, Sandra Blome, and Uwe Truyen. 2022. "Efficacy of Liming Forest Soil in the Context of African Swine Fever Virus" Viruses 14, no. 4: 734. https://doi.org/10.3390/v14040734
APA StyleTanneberger, F., Abd El Wahed, A., Fischer, M., Deutschmann, P., Roszyk, H., Carrau, T., Blome, S., & Truyen, U. (2022). Efficacy of Liming Forest Soil in the Context of African Swine Fever Virus. Viruses, 14(4), 734. https://doi.org/10.3390/v14040734










