Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena
Abstract
:1. Introduction
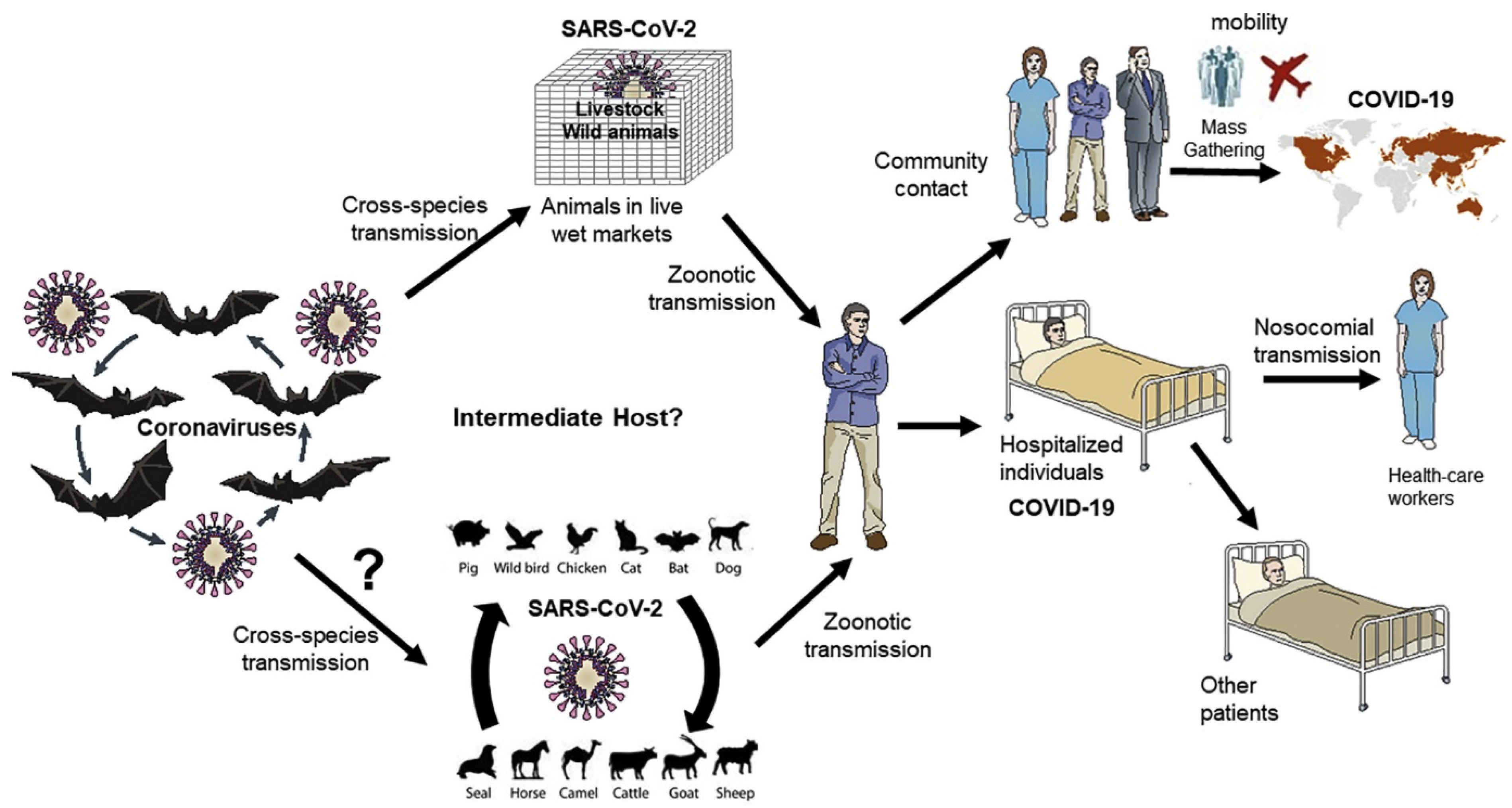
2. Zoonotic Spread of SARS-CoV-2
3. Viral Vector-Based Vaccines—Historical Perspective
4. Viral Vector-Based Vaccines for COVID-19
5. Replicating Viral Vector-Based COVID-19 Vaccines and Their Mechanism of Action
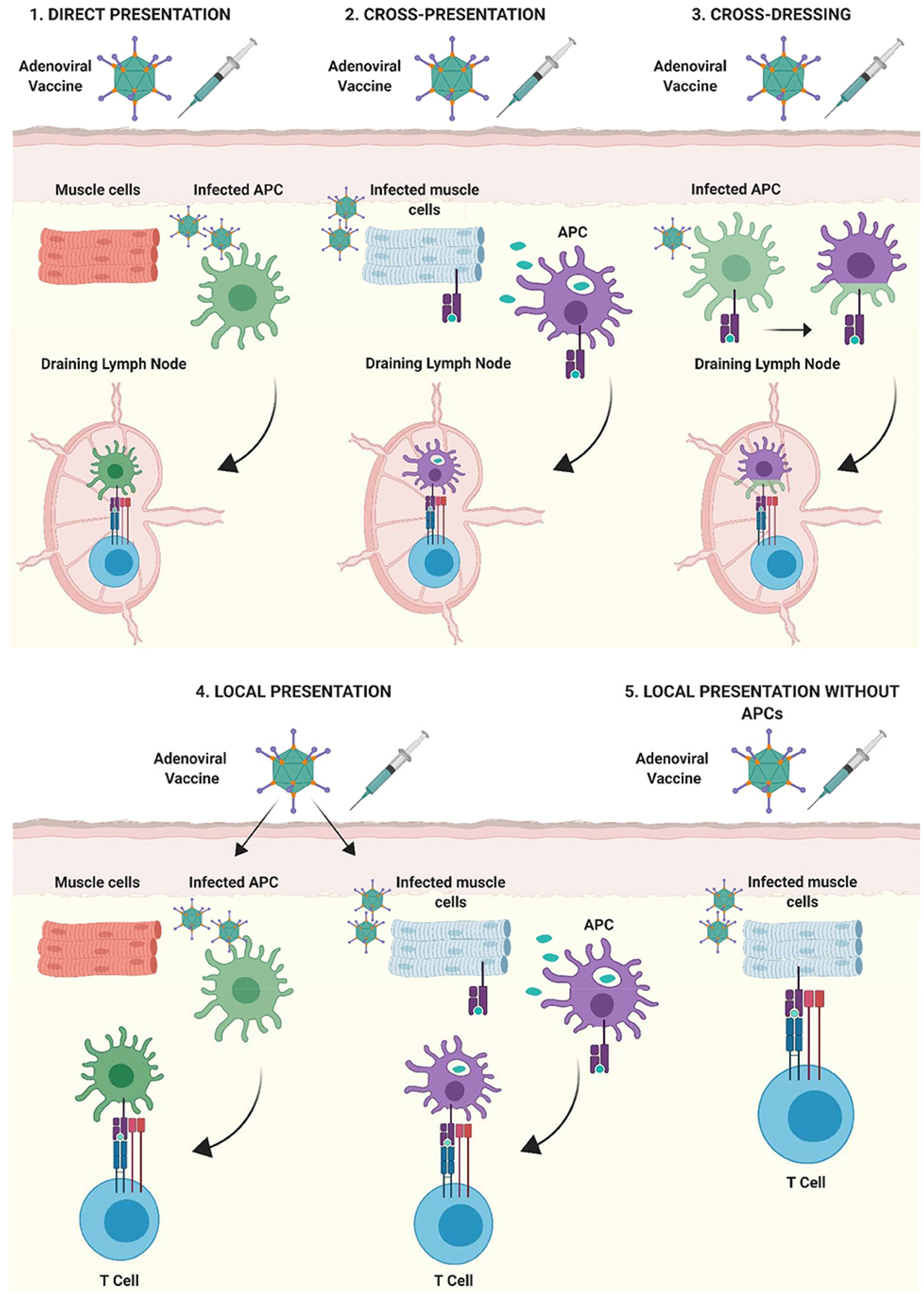
5.1. Mechanism of Action
5.2. Replicating Viral Vaccines under Various Clinical Trials
6. Drug Delivery Route and Delivery Systems
7. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alamer, F.; Alamir, A.; AlJohani, S.; AlSumih, N.; Hiji, F.; Alhammadi, M.; Almuneef, M. Childhood Vaccination Hesitancy in Saudi Arabia: A Time for Action. J. Infect. Public Health 2022, 15, 94–99. [Google Scholar] [CrossRef]
- Pollard, A.J.; Bijker, E.M. A Guide to Vaccinology: From Basic Principles to New Developments. Nat. Rev. Immunol. 2020, 21, 83–100. [Google Scholar] [CrossRef] [PubMed]
- Kaur, N.; Chaudhary, V. Biotherapeutics and Its Applications in Microbiology. Environ. Conserv. J. 2021, 22, 63–78. [Google Scholar] [CrossRef]
- Chavda, V.P.; Pandya, R.; Apostolopoulos, V. DNA Vaccines for SARS-CoV-2: Towards Third Generation Vaccination Era. Expert Rev. Vaccines 2021, 20, 1549–1560. [Google Scholar] [CrossRef]
- Hilleman, M.R. Vaccines in Historic Evolution and Perspective: A Narrative of Vaccine Discoveries. J. Hum. Virol. 2000, 3, 63–76. [Google Scholar] [CrossRef]
- Plotkin, S.A. Vaccines, Vaccination, and Vaccinology. J. Infect. Dis. 2003, 187, 1349–1359. [Google Scholar] [CrossRef] [PubMed]
- Kallerup, R.S.; Foged, C. Classification of Vaccines. In Subunit Vaccine Delivery; Springer: New York, NY, USA, 2015; pp. 15–29. ISBN 9781493914173. [Google Scholar]
- Yadav, D.K.; Yadav, N.; Khurana, S.M.P. Vaccines: Present Status and Applications. In Animal Biotechnology; Academic Press: Cambridge, MA, USA, 2020; pp. 523–542. ISBN 9780128117101. [Google Scholar]
- Chavda, V.P.; Vora, L.K.; Pandya, A.K.; Patravale, V.B. Intranasal Vaccines for SARS-CoV-2: From Challenges to Potential in COVID-19 Management. Drug Discov. Today 2021, 26, 2619–2636. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Vora, L.K.; Vihol, D.R. COVAX-19 Vaccine: Completely Blocks Virus Transmission to Non-Immune Individuals. Clin. Complement. Med. Pharmacol. 2021, 1, 100004. [Google Scholar] [CrossRef]
- Uddin, M.; Mustafa, F.; Rizvi, T.A.; Loney, T.; Al Suwaidi, H.; Al-Marzouqi, A.H.H.; Eldin, A.K.; Alsabeeha, N.; Adrian, T.E.; Stefanini, C.; et al. SARS-CoV-2/COVID-19: Viral Genomics, Epidemiology, Vaccines, and Therapeutic Interventions. Viruses 2020, 12, 526. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.; Sultan, A.A.; Ding, H.; Triggle, C.R. A Review of the Progress and Challenges of Developing a Vaccine for COVID-19. Front. Immunol. 2020, 11, 2413. [Google Scholar] [CrossRef]
- Emrani, J.; Ahmed, M.; Jeffers-Francis, L.; Teleha, J.C.; Mowa, N.; Newman, R.H.; Thomas, M.D. SARS-COV-2, Infection, Transmission, Transcription, Translation, Proteins, and Treatment: A Review. Int. J. Biol. Macromol. 2021, 193, 1249–1273. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Hossain, M.K.; Beladiya, J.; Apostolopoulos, V. Nucleic Acid Vaccines for COVID-19: A Paradigm Shift in the Vaccine Development Arena. Biologics 2021, 1, 337–356. [Google Scholar] [CrossRef]
- WHO COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 9 December 2021).
- WHO. COVID19 Vaccine Tracker; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Parums, D. V Editorial: First Full Regulatory Approval of a COVID-19 Vaccine, the BNT162b2 Pfizer-BioNTech Vaccine, and the Real-World Implications for Public Health Policy. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2021, 27, e934625. [Google Scholar] [CrossRef] [PubMed]
- Tregoning, J.S.; Flight, K.E.; Higham, S.L.; Wang, Z.; Pierce, B.F. Progress of the COVID-19 Vaccine Effort: Viruses, Vaccines and Variants versus Efficacy, Effectiveness and Escape. Nat. Rev. Immunol. 2021, 21, 626–636. [Google Scholar] [CrossRef]
- Li, C.-X.; Noreen, S.; Zhang, L.; Saeed, M.; Wu, P.; Ijaz, M.; Dai, D.; Maqbool, I.; Madni, A.; Akram, F.; et al. A Critical Analysis of SARS-CoV-2 (COVID-19) Complexities, Emerging Variants, and Therapeutic Interventions and Vaccination Strategies. Biomed. Pharmacother. 2022, 146, 112550. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.; Wu, J.T. Managing Waning Vaccine Protection against SARS-CoV-2 Variants. Lancet 2021, 399, 2–3. [Google Scholar] [CrossRef]
- Khan, W.H.; Hashmi, Z.; Goel, A.; Ahmad, R.; Gupta, K.; Khan, N.; Alam, I.; Ahmed, F.; Ansari, M.A. COVID-19 Pandemic and Vaccines Update on Challenges and Resolutions. Front. Cell. Infect. Microbiol. 2021, 11, 690621. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A. What Is the Vaccine Effect on Reducing Transmission in the Context of the SARS-CoV-2 Delta Variant? Lancet Infect. Dis. 2021, 380, 4–5. [Google Scholar] [CrossRef]
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.-L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.-B. In the Shadow of Biological Warfare: Conspiracy Theories on the Origins of COVID-19 and Enhancing Global Governance of Biosafety as a Matter of Urgency. J. Bioeth. Inq. 2020, 17, 567–574. [Google Scholar] [CrossRef]
- van der Linden, S.; Roozenbeek, J.; Compton, J. Inoculating Against Fake News About COVID-19. Front. Psychol. 2020, 11, 2928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A Pneumonia Outbreak Associated with a New Coronavirus of Probable Bat Origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burki, T. The Origin of SARS-CoV-2. Lancet. Infect. Dis. 2020, 20, 1018. [Google Scholar] [CrossRef]
- Rasmussen, A.L. On the Origins of SARS-CoV-2. Nat. Med. 2021, 27, 9. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Feehan, J.; Apostolopoulos, V. A Veterinary Vaccine for SARS-CoV-2: The First COVID-19 Vaccine for Animals. Vaccines 2021, 9, 631. [Google Scholar] [CrossRef]
- Almazán, F.; Sola, I.; Zuñiga, S.; Marquez-Jurado, S.; Morales, L.; Becares, M.; Enjuanes, L. Coronavirus Reverse Genetic Systems: Infectious Clones and Replicons. Virus Res. 2014, 189, 262. [Google Scholar] [CrossRef]
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor Recognition by the Novel Coronavirus from Wuhan: An Analysis Based on Decade-Long Structural Studies of SARS Coronavirus. J. Virol. 2020, 94, e00127-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheahan, T.; Rockx, B.; Donaldson, E.; Sims, A.; Pickles, R.; Corti, D.; Baric, R. Mechanisms of Zoonotic Severe Acute Respiratory Syndrome Coronavirus Host Range Expansion in Human Airway Epithelium. J. Virol. 2008, 82, 2274–2285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haider, N.; Rothman-Ostrow, P.; Osman, A.Y.; Arruda, L.B.; Macfarlane-Berry, L.; Elton, L.; Thomason, M.J.; Yeboah-Manu, D.; Ansumana, R.; Kapata, N.; et al. COVID-19—Zoonosis or Emerging Infectious Disease? Front. Public Health 2020, 8, 763. [Google Scholar] [CrossRef]
- Lai, C.C.; Shih, T.P.; Ko, W.C.; Tang, H.J.; Hsueh, P.R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Gajjar, N.; Shah, N.; Dave, D.J. Darunavir ethanolate: Repurposing an anti-HIV drug in COVID-19 treatment. Eur. J. Med. Chem. Rep. 2021, 3, 100013. [Google Scholar] [CrossRef]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 2309. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Shi, Z.L. Origin and Evolution of Pathogenic Coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Sallard, E.; Halloy, J.; Casane, D.; Decroly, E.; van Helden, J. Tracing the Origins of SARS-COV-2 in Coronavirus Phylogenies: A Review. Environ. Chem. Lett. 2021, 19, 769–785. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Zhao, X.; Li, J.; Niu, P.; Yang, B.; Wu, H.; Wang, W.; Song, H.; Huang, B.; Zhu, N.; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Luk, H.K.H.; Li, X.; Fung, J.; Lau, S.K.P.; Woo, P.C.Y. Molecular Epidemiology, Evolution and Phylogeny of SARS Coronavirus. Infect. Genet. Evol. 2019, 71, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Baddal, B.; Cakir, N. Co-Infection of MERS-CoV and SARS-CoV-2 in the Same Host: A Silent Threat. J. Infect. Public Health 2020, 13, 1251–1252. [Google Scholar] [CrossRef] [PubMed]
- Menachery, V.D.; Yount, B.L.; Debbink, K.; Agnihothram, S.; Gralinski, L.E.; Plante, J.A.; Graham, R.L.; Scobey, T.; Ge, X.Y.; Donaldson, E.F.; et al. A SARS-like Cluster of Circulating Bat Coronaviruses Shows Potential for Human Emergence. Nat. Med. 2015, 21, 1508–1513. [Google Scholar] [CrossRef]
- Lau, S.K.P.; Luk, H.K.H.; Wong, A.C.P.; Li, K.S.M.; Zhu, L.; He, Z.; Fung, J.; Chan, T.T.Y.; Fung, K.S.C.; Woo, P.C.Y. Possible Bat Origin of Severe Acute Respiratory Syndrome Coronavirus 2. Emerg Infect Dis. 2020, 26, 1542–1547. [Google Scholar] [CrossRef] [PubMed]
- Paraskevis, D.; Kostaki, E.G.; Magiorkinis, G.; Panayiotakopoulos, G.; Sourvinos, G.; Tsiodras, S. Full-Genome Evolutionary Analysis of the Novel Corona Virus (2019-NCoV) Rejects the Hypothesis of Emergence as a Result of a Recent Recombination Event. Infect. Genet. Evol. 2020, 79, 104212. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zeng, L.P.; Yang, X.L.; Ge, X.Y.; Zhang, W.; Li, B.; Xie, J.Z.; Shen, X.R.; Zhang, Y.Z.; Wang, N.; et al. Discovery of a Rich Gene Pool of Bat SARS-Related Coronaviruses Provides New Insights into the Origin of SARS Coronavirus. PLoS Pathog. 2017, 13, e1006698. [Google Scholar] [CrossRef] [PubMed]
- Frutos, R.; Serra-Cobo, J.; Chen, T.; Devaux, C.A. COVID-19: Time to Exonerate the Pangolin from the Transmission of SARS-CoV-2 to Humans. Infect. Genet. Evol. 2020, 84, 104493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Q.; Zhang, Z. Probable Pangolin Origin of SARS-CoV-2 Associated with the COVID-19 Outbreak. Curr. Biol. 2020, 30, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The Proximal Origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Wu, Q.; Zhang, Z. Pangolin Homology Associated with 2019-NCoV. Curr. Biol. 2020; Preprint. [Google Scholar] [CrossRef] [Green Version]
- Wei, C.; Shan, K.-J.; Wang, W.; Zhang, S.; Huan, Q.; Qian, W. Evidence for a Mouse Origin of the SARS-CoV-2 Omicron Variant. J. Genet. Genomics 2021. [Google Scholar] [CrossRef]
- Ye, Z.-W.; Yuan, S.; Yuen, K.-S.; Fung, S.-Y.; Chan, C.-P.; Jin, D.-Y. Zoonotic Origins of Human Coronaviruses. Int. J. Biol. Sci. 2020, 16, 1686–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seidah, N.G.; Pasquato, A.; Andréo, U. How Do Enveloped Viruses Exploit the Secretory Proprotein Convertases to Regulate Infectivity and Spread? Viruses 2021, 13, 1229. [Google Scholar] [CrossRef] [PubMed]
- Basu, D.; Chavda, V.P.; Mehta, A.A. Therapeutics for COVID-19 and Post COVID-19 Complications: An Update. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100086. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Apostolopoulos, V. Omicron Variant (B.1.1.529) of SARS-CoV-2: Threat for the Elderly? Maturitas 2022, 158, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Kapadia, C.; Soni, S.; Prajapati, R.; Chauhan, S.C.; Yallapu, M.M.; Apostolopoulos, V. A Global Picture: Therapeutic Perspectives for COVID-19. Immunotherapy 2022, 14, 351–371. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Apostolopoulos, V. Global Impact of Delta plus Variant and Vaccination. Expert Rev. Vaccines, 2022; Preprint. [Google Scholar] [CrossRef] [PubMed]
- Chavda, V.P.; Patel, A.B.; Vihol, D.; Vaghasiya, D.D.; Ahmed, K.M.S.B.; Trivedi, K.U.; Dave, D.J. Herbal Remedies, Nutraceuticals, and Dietary Supplements for COVID-19 Management: An Update. Clin. Complement. Med. Pharmacol. 2022; in press. [Google Scholar] [CrossRef]
- Chavda, V.P.; Apostolopoulos, V. Is Booster Dose Strategy Sufficient for Omicron Variant of SARS-CoV-2? Vaccines 2022, 10, 367. [Google Scholar] [CrossRef] [PubMed]
- El Zowalaty, M.E.; Järhult, J.D. From SARS to COVID-19: A Previously Unknown SARS- Related Coronavirus (SARS-CoV-2) of Pandemic Potential Infecting Humans—Call for a One Health Approach. One Heal. 2020, 9, 100124. [Google Scholar] [CrossRef]
- Gerberding, J.L.; Haynes, B.F. Vaccine Innovations—Past and Future. N. Engl. J. Med. 2021, 384, 393–396. [Google Scholar] [CrossRef]
- Ura, T.; Okuda, K.; Shimada, M. Developments in Viral Vector-Based Vaccines. Vaccines 2014, 2, 624–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, D.A.; Symons, R.H.; Berg, P. Biochemical Method for Inserting New Genetic Information into DNA of Simian Virus 40: Circular SV40 DNA Molecules Containing Lambda Phage Genes and the Galactose Operon of Escherichia Coli. Proc. Natl. Acad. Sci. USA 1972, 69, 2904–2909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Tai, P.W.L.; Gao, G. Adeno-Associated Virus Vector as a Platform for Gene Therapy Delivery. Nat. Rev. Drug Discov. 2019, 18, 358–378. [Google Scholar] [CrossRef] [PubMed]
- Kandimalla, R.; Chakraborty, P.; Vallamkondu, J.; Chaudhary, A.; Samanta, S.; Reddy, P.H.; De Feo, V.; Dewanjee, S. Counting on COVID-19 Vaccine: Insights into the Current Strategies, Progress and Future Challenges. Biomed. 2021, 9, 1740. [Google Scholar] [CrossRef]
- Chanukya, G.V.; Srikantam, A. Comparative Quantitative Analysis of SARS-CoV-2 Spike Neutralizing Antibody Titers Following Two Anti COVID-19 Vaccines in India. medRxiv, 2021; in press. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, C.; Liu, M.; Liu, J. Effectiveness and Safety of SARS-CoV-2 Vaccine in Real-World Studies: A Systematic Review and Meta-Analysis. Infect. Dis. Poverty 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Rogliani, P.; Mazzeo, F.; Matera, M.G. Controversy Surrounding the Sputnik V Vaccine. Respir. Med. 2021, 187, 106569. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaieli, M.; Ekrami, E.; Akbari, A.; Noorbakhsh, N.; Moghadam, N.B.; Mamoudifard, M. A Comprehensive Review on Efficient Approaches for Combating Coronaviruses. Biomed. Pharmacother. 2021, 144, 112353. [Google Scholar] [CrossRef] [PubMed]
- Malik, J.A.; Ahmed, S.; Mir, A.; Shinde, M.; Bender, O.; Alshammari, F.; Ansari, M.; Anwar, S. The SARS-CoV-2 Mutations versus Vaccine Effectiveness: New Opportunities to New Challenges. J. Infect. Public Health 2022, 15, 228–240. [Google Scholar] [CrossRef] [PubMed]
- Fiolet, T.; Kherabi, Y.; MacDonald, C.-J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 Vaccines for Their Characteristics, Efficacy and Effectiveness against SARS-CoV-2 and Variants of Concern: A Narrative Review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Andrews, N.; Tessier, E.; Stowe, J.; Gower, C.; Kirsebom, F.; Simmons, R.; Gallagher, E.; Thelwall, S.; Groves, N.; Dabrera, G.; et al. Duration of Protection against Mild and Severe Disease by Covid-19 Vaccines. N. Engl. J. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Cascella, M.; Rajnik, M.; Aleem, A. Features, Evaluation, and Treatment of Coronavirus (COVID-19); StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books (accessed on 24 January 2022).
- Forni, G.; Mantovani, A.; Forni, G.; Mantovani, A.; Moretta, L.; Rappuoli, R.; Rezza, G.; Bagnasco, A.; Barsacchi, G.; Bussolati, G.; et al. COVID-19 Vaccines: Where We Stand and Challenges Ahead. Cell Death Differ. 2021, 28, 626–639. [Google Scholar] [CrossRef]
- Bezbaruah, R.; Borah, P.; Kakoti, B.B.; Al-Shar’I, N.A.; Chandrasekaran, B.; Jaradat, D.M.M.; Al-Zeer, M.A.; Abu-Romman, S. Developmental Landscape of Potential Vaccine Candidates Based on Viral Vector for Prophylaxis of COVID-19. Front. Mol. Biosci. 2021, 8, 635337. [Google Scholar] [CrossRef]
- Robert-Guroff, M. Replicating and Non-Replicating Viral Vectors for Vaccine Development. Curr. Opin. Biotechnol. 2007, 18, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Callaway, E. The Race for Coronavirus Vaccines: A Graphical Guide. Nature 2020, 580, 576–577. [Google Scholar] [CrossRef]
- Sadoff, J.; Gray, G.; Vandebosch, A.; Cárdenas, V.; Shukarev, G.; Grinsztejn, B.; Goepfert, P.A.; Truyers, C.; Fennema, H.; Spiessens, B.; et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021, 384, 2187–2201. [Google Scholar] [CrossRef] [PubMed]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S Vaccine against SARS-CoV-2 Variants in Humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef] [PubMed]
- WHO. The Janssen Ad26.COV2.S COVID-19 Vaccine: What You Need to Know; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Watanabe, Y.; Mendonça, L.; Allen, E.R.; Howe, A.; Lee, M.; Allen, J.D.; Chawla, H.; Pulido, D.; Donnellan, F.; Davies, H.; et al. Native-like SARS-CoV-2 Spike Glycoprotein Expressed by ChAdOx1 NCoV-19/AZD1222 Vaccine. ACS Cent. Sci. 2021, 7, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Dicks, M.D.J.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.S.; Cottingham, M.G. A Novel Chimpanzee Adenovirus Vector with Low Human Seroprevalence: Improved Systems for Vector Derivation and Comparative Immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). The Oxford/AstraZeneca (ChAdOx1-S [Recombinant] Vaccine) COVID-19 Vaccine: What You Need to Know; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Vanaparthy, R.; Mohan, G.; Vasireddy, D.; Medicina, P.A.-I. Review of COVID-19 Viral Vector-Based Vaccines and COVID-19 Variants. Infez. Med. 2021, 29, 328–338. [Google Scholar] [CrossRef]
- Klassen, S.A.; Senefeld, J.W.; Senese, K.A.; Johnson, P.W.; Wiggins, C.C.; Baker, S.E.; van Helmond, N.; Bruno, K.A.; Pirofski, L.A.; Shoham, S.; et al. Convalescent Plasma Therapy for COVID-19: A Graphical Mosaic of the Worldwide Evidence. Front. Med. 2021, 8, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Hindustan Times. All You Need to Know about SII Vaccine ‘Covishield’|10 Points|Latest News India Hindustan Times. Available online: https://www.hindustantimes.com/india-news/ingredients-side-effects-response-time-all-you-need-to-know-about-covishield-101610469717522.html (accessed on 12 January 2022).
- Business Standard. Rejection of Sputnik V by US, EU a Mistake: Argentine Epidemiologist. Business Standard News. Available online: https://www.business-standard.com/article/international/rejection-of-sputnik-v-by-us-eu-a-mistake-argentine-epidemiologist-121122800647_1.html (accessed on 12 January 2022).
- Chavda, V.P.; Patel, A.B.; Vaghasiya, D.D. SARS-CoV-2 variants and vulnerability at the global level. J. Med. Virol. 2022; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- PHO. COVID-19 Vaccines: Viral Vector-Based Vaccines The Basics: Viral Vector-Based Vaccines; Public Health Ontario: Toronto, ON, Canada, 2021; pp. 1–9. [Google Scholar]
- Kyriakidis, N.C.; López-Cortés, A.; González, E.V.; Grimaldos, A.B.; Prado, E.O. SARS-CoV-2 Vaccines Strategies: A Comprehensive Review of Phase 3 Candidates. NPJ Vaccines 2021, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of Action of Adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pulendran, B.; Arunachalam, P.S.; O’Hagan, D.T. Emerging Concepts in the Science of Vaccine Adjuvants. Nat. Rev. Drug Discov. 2021, 20, 454–475. [Google Scholar] [CrossRef] [PubMed]
- van Riel, D.; de Wit, E. Next-Generation Vaccine Platforms for COVID-19. Nat. Mater. 2020, 19, 810–812. [Google Scholar] [CrossRef] [PubMed]
- Mayo Clinic. Different Types of COVID-19 Vaccines: How They Work; Mayo Clinic: Rochester, MN, USA, 2020. [Google Scholar]
- Coughlan, L. Factors Which Contribute to the Immunogenicity of Non-Replicating Adenoviral Vectored Vaccines. Front. Immunol. 2020, 11, 909. [Google Scholar] [CrossRef]
- Mendonça, S.A.; Lorincz, R.; Boucher, P.; Curiel, D.T. Adenoviral Vector Vaccine Platforms in the SARS-CoV-2 Pandemic. NPJ Vaccines 2021, 6, 97. [Google Scholar] [CrossRef]
- Falsey, A.R.; Sobieszczyk, M.E.; Hirsch, I.; Sproule, S.; Robb, M.L.; Corey, L.; Neuzil, K.M.; Hahn, W.; Hunt, J.; Mulligan, M.J.; et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 NCoV-19) Covid-19 Vaccine. N. Engl. J. Med. 2021, 385, 2348–2360. [Google Scholar] [CrossRef]
- Zheng, C.; Shao, W.; Chen, X.; Zhang, B.; Wang, G.; Zhang, W. Real-world effectiveness of COVID-19 vaccines: A literature review and meta-analysis. Int. J. Infect. Dis. 2022, 114, 252–260, PMCID: PMC8595975. [Google Scholar] [CrossRef] [PubMed]
- Funk, C.D.; Laferrière, C.; Ardakani, A. A Snapshot of the Global Race for Vaccines Targeting SARS-CoV-2 and the COVID-19 Pandemic. Front. Pharmacol. 2020, 11, 937. [Google Scholar] [CrossRef]
- World Health Organization (WHO). List of Candidate Vaccines Developed against SARS-CoV; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- McGill COVID19 Vaccine Tracker Team. COVID-19 VACCINE TRACKER. 2022. Available online: https://covid19.trackvaccines.org/vaccines/ (accessed on 11 February 2022).
- KBR. Cellid’s Covid-19 Vaccine Candidate Shows No Difference between Middle, High Doses; Korea Biomedical Review: Seoul, Korea, 2021. [Google Scholar]
- U.S. National Library of Medicine. Evaluate the Safety, Immunogenicity and Potential Efficacy of an RVSV-SARS-CoV-2-S Vaccine; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Phase 2b/3 Trial of VSV-ΔG SARS-CoV-2 Vaccine (BRILIFE) Against Approved Comparator Vaccine; (BRILIFE002); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- Chinese Clinical Trail Registry. A Phase I Clinical Trial of Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray (DelNS1-2019-NCoV-RBD-OPT1); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. A Phase II Clinical Trial of Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray (DelNS1-2019-NCoV-RBD-OPT1); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. A Phase III Clinical Trial of Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray (DelNS1-2019-NCoV-RBD-OPT1); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Safety and Immunogenicity Study of AdCLD-CoV19: A COVID-19 Preventive Vaccine in Healthy Volunteers; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Preventive Dendritic Cell Vaccine, AV-COVID-19, in Subjects Not Actively Infected With COVID-19; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Phase I–II Trial of Dendritic Cell Vaccine to Prevent COVID-19 in Adults; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Dendritic Cell Vaccine to Prevent COVID-19; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Dendritic Cell Vaccine, AV-COVID-19, to Prevent COVID-19 Infection; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Safety and Immunogenicity of Two Different Strengths of the Inactivated COVID-19 Vaccine ERUCOV-VAC (ERUCOV-VAC); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. Efficacy, Immunogenicity and Safety of Inactivated ERUCOV-VAC Compared With Placebo in COVID-19; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- Pavel, S.T.I.; Yetiskin, H.; Uygut, M.A.; Aslan, A.F.; Aydın, G.; İnan, Ö.; Kaplan, B.; Ozdarendeli, A. Development of an Inactivated Vaccine against SARS CoV-2. Vaccines 2021, 9, 1266. [Google Scholar] [CrossRef]
- U.S. National Library of Medicine. A Synthetic MVA-Based SARS-CoV-2 Vaccine, COH04S1, for the Prevention of COVID-19 Infection; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. SARS-CoV-2 Vaccine (COH04S1) Versus Emergency Use Authorization SARS-COV-2 Vaccine for the Treatment of COVID-19 in Patients With Blood Cancer; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- Rauch, S.; Jasny, E.; Schmidt, K.E.; Petsch, B. New Vaccine Technologies to Combat Outbreak Situations. Front. Immunol. 2018, 9, 1963. [Google Scholar] [CrossRef] [Green Version]
- Eroglu, B.; Nuwarda, R.F.; Ramzan, I.; Kayser, V. A Narrative Review of COVID-19 Vaccines. Vaccines 2022, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Kumar, A. Mucosal and Transdermal Vaccine Delivery Strategies against COVID-19. Drug Deliv. Transl. Res. 2021, 12, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.; Chavda, V.; Tandel, H.; Domadiya, K. Nasal Medication Conveyance Framework: An Approach for Brain Delivery from Essential to Cutting Edge. Res. Rev. J. Med. 2016, 6, 14–27. [Google Scholar]
- U.S. National Library of Medicine. A Phase I Clinical Trial of Influenza Virus Vector COVID-19 Vaccine for Intranasal Spray (DelNS1-2019-NCoV-RBD-OPT1); U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- U.S. National Library of Medicine. A Ph 2 Trial With an Oral Tableted COVID-19 Vaccine; U.S. National Library of Medicine: Bethesda, MD, USA, 2021. [Google Scholar]
- Health Research Authority. A First in Human Study of OraPro-COVID-19 in Healthy Volunteers; Health Research Authority: London, UK, 2021. [Google Scholar]
- Abd Elkodous, M.; Olojede, S.O.; Morsi, M.; El-Sayyad, G.S. Nanomaterial-Based Drug Delivery Systems as Promising Carriers for Patients with COVID-19. RSC Adv. 2021, 11, 26463–26480. [Google Scholar] [CrossRef]
- Theobald, N. Emerging Vaccine Delivery Systems for COVID-19 Functionalised Silica Nanoparticles Offer a Potentially Safe and Effective Alternative Delivery System for DNA/RNA Vaccines. Drug Discov. Today 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed]

| Vaccine Name | Viral-Vector Used | Manufacturer | Route/Dose | Efficacy | References |
|---|---|---|---|---|---|
| Vaxzevria Or Covishield | Chimpanzee adenovirus ChAdOx1 (Non-replicating) | Oxford University in collaboration with AstraZeneca. | Intramuscular injection (IM)/0.5 mL two doses of vaccine. Currently, the requirement for a booster dose. | 76.0% effective at preventing symptomatic COVID-19 commencing 22 days from the first dose and 81.3% effective after the second dose. 81% and 61% effective against the B.1.1.7 and B.1.617.2 variants, respectively, after the second dose. Also effective for B.1.351. | [64,65] |
| JNJ-78436735 | Human adenovirus (Ad26) (Non-replicating) | Janssen (Johnson & Johnson) | IM/0.5 mL single dose. Currently, the requirement for a booster dose. | 66% effective in preventing symptomatic COVID-19 in a one-dose regimen 28 days after completion, with an 85% efficacy in preventing severe COVID-19 and a 100% efficacy in preventing hospitalization or death caused by the disease. Also effective for B.1.1.7 variant, B.1.351variant and P.2 variant. | [66] |
| Sputnik V (Gam-COVID-Vac) | Adeno (Ad26) viral vector (Non-replicating) | Gamaleya Research Institute of Epidemiology and Microbiology | IM/0.5 mL two doses. Currently, the requirement for a booster dose. | After the second dose efficacy is 91.6% for all age groups; about 90% effective against the B.1.617.2 variant. However, there was a noticeable decrease in neutralizing antibodies against B.1.351, P.1, and B.1.1.28 variants. | [67,68] |
| Sputnik light | Adeno (Ad26) viral vector (non-replicating) | Gamaleya Research Institute of Epidemiology and Microbiology | IM/0.5 mL single dose. Currently, the requirement for a booster dose. | The single-injection vaccine is 79% effective; 88% effective in preventing hospitalization, and 85% in preventing death (as per an Argentinian study with 60–79-year-old subjects). According to the Gamaleya Center, it is effective against all new variants. | [69,70] |
| CONVIDECIA (Ad5-nCoV) | Adeno (Ad5) viral vector (Non-replicating) | CanSino Biologics and the Beijing Institute of Biotechnology of the Academy of Military Medical Sciences. | IM/0.5ml single dose. Currently, the requirement for a booster dose. | 65.7% efficacy in preventing moderate symptoms of COVID-19, and 91% efficacy in preventing severe disease. There is currently no clear information on variant efficacy. | [71,72] |
| Viral Vector | Pros | Cons |
|---|---|---|
| Non-Replicating Viral Vector | ||
| Adenovirus |
|
|
| Adeno-associated virus |
|
|
| Alphavirus |
|
|
| Herpesvirus |
|
|
| Poxviruses: NYVAC; MVA |
|
|
| Poxviruses: ALCAC; FPV |
|
|
| Replicating Viral Vector | ||
| Adenovirus |
|
|
| Measles virus |
|
|
| Poxviruses: Vaccinia |
|
|
| Vesicular stomatitis virus |
|
|
| Vaccine | Developer | Country | Clinical Trial Registry No. | Clinical Trial Status | Viral Vector |
|---|---|---|---|---|---|
| Brilife (IIBR-100) | The Israel Institute for Biological Research (IIBR) | Israel | NCT04608305 | Phase I/II | Vesicular stomatitis virus |
| NeuroRx, Inc. in collaboration with Cromos, Brilife Georgia, Israel Institute for Biological Research | Georgia | NCT04990466 | Phase IIb/III | Vesicular stomatitis virus | |
| DelNS1-2019-nCoV-RBD-OPT1 | Wantai Biopharm | China | ChiCTR2000037782 | Phase I | H1N1 Influenza virus |
| China | ChiCTR2000039715 | Phase-II | H1N1 Influenza virus | ||
| Philippines | ChiCTR2100051391 | Phase III | H1N1 Influenza virus | ||
| AdCLD-CoV19 | Cellid Co., Ltd. | Republic of Korea | NCT04666012 | Phase I/IIa | Adenovirus |
| AV-COVID-19 | Aivita Biomedical, Inc. in collaboration with PT AIVITA Biomedika Indonesia, Kariadi Hospital, Central Army Hospital RSPAD Gatot Soebroto | Indonesia | NCT05007496 | Phase I/II | Autologous dendritic cells and lymphocytes (DCL) |
| Aivita Biomedical, Inc. | United States of America | NCT04386252 | Phase I/II | Autologous dendritic cells and lymphocytes (DCL) | |
| Indonesia-MoH in collaboration with Aivita Biomedical, Inc. | Indonesia | NCT04685603 NCT04690387 | Phase I | Autologous dendritic cells and lymphocytes (DCL) | |
| ERUCOV-VAC | The Health Institutes of Turkey in collaboration with TC Erciyes University | Turkey | NCT04691947 | Phase I | Whole-virion inactivated |
| NCT04824391 | Phase II | Whole-virion inactivated | |||
| COH04S1 | City of Hope Medical Center | United States of America | NCT04639466 | Phase I | Synthetically modified vaccinia Ankara (MVA) |
| NCT04977024 | Phase II | MVA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chavda, V.P.; Bezbaruah, R.; Athalye, M.; Parikh, P.K.; Chhipa, A.S.; Patel, S.; Apostolopoulos, V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses 2022, 14, 759. https://doi.org/10.3390/v14040759
Chavda VP, Bezbaruah R, Athalye M, Parikh PK, Chhipa AS, Patel S, Apostolopoulos V. Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses. 2022; 14(4):759. https://doi.org/10.3390/v14040759
Chicago/Turabian StyleChavda, Vivek P., Rajashri Bezbaruah, Mansi Athalye, Palak K. Parikh, Abu Sufiyan Chhipa, Snehal Patel, and Vasso Apostolopoulos. 2022. "Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena" Viruses 14, no. 4: 759. https://doi.org/10.3390/v14040759
APA StyleChavda, V. P., Bezbaruah, R., Athalye, M., Parikh, P. K., Chhipa, A. S., Patel, S., & Apostolopoulos, V. (2022). Replicating Viral Vector-Based Vaccines for COVID-19: Potential Avenue in Vaccination Arena. Viruses, 14(4), 759. https://doi.org/10.3390/v14040759








