A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses
Abstract
:1. Introduction
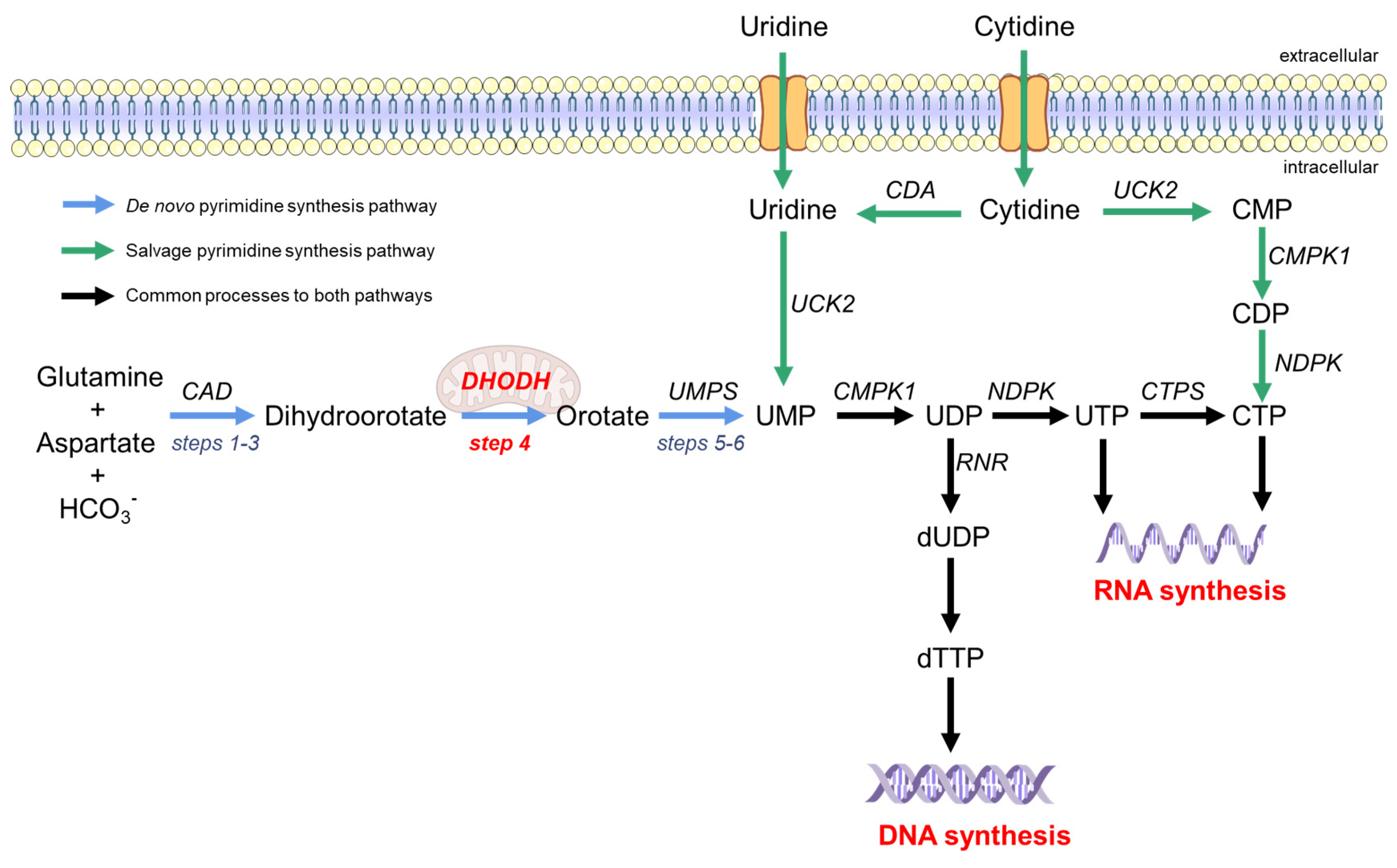
2. The Pyrimidine Synthesis Pathway Is a Reliable HTA Target
3. The Essential Role of DHODH in the De Novo Pyrimidine Synthesis Pathway
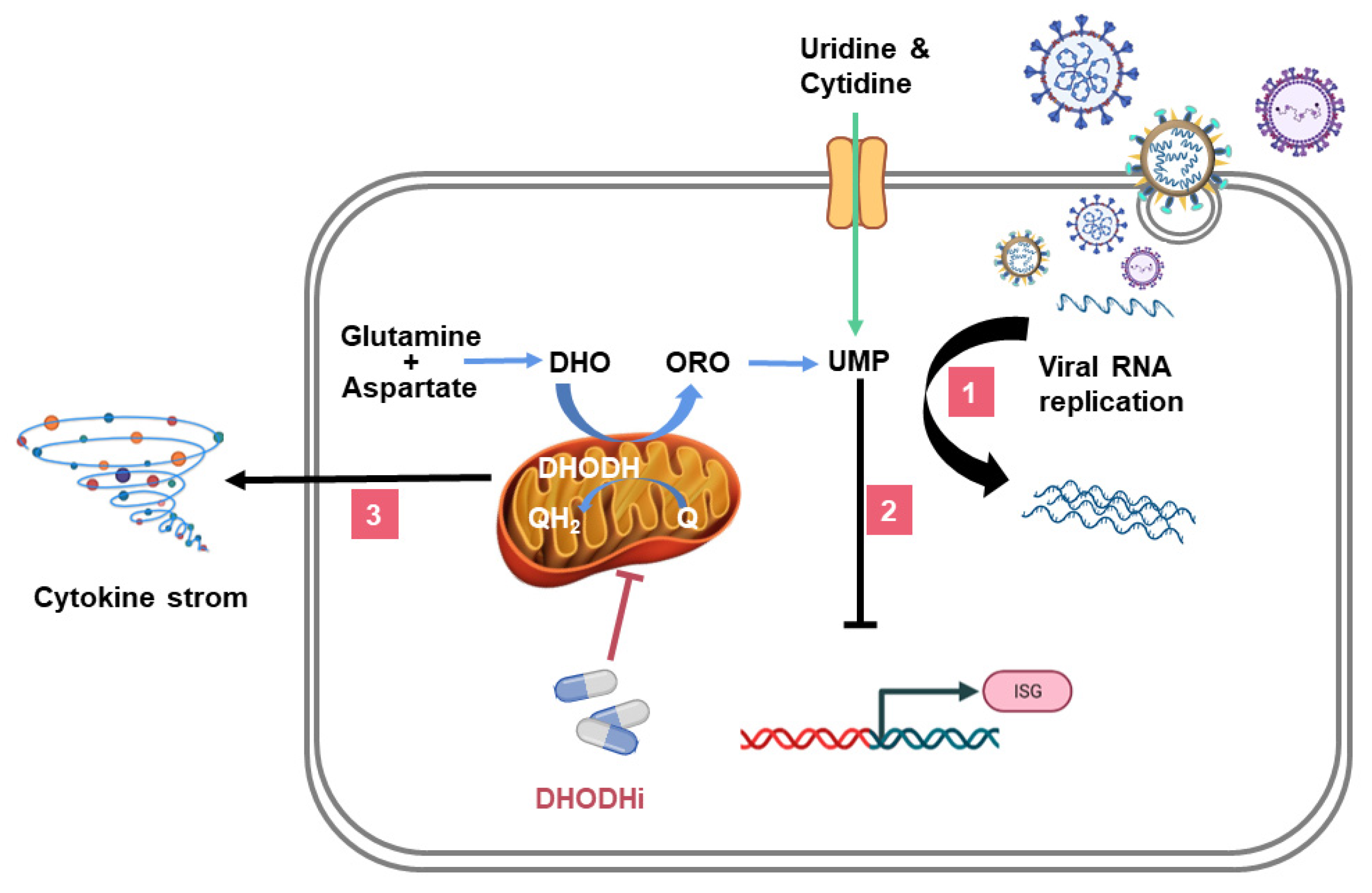
4. DHODHi Inhibit the Virus Replication Cycle
5. DHODHi Stimulate the Expression of ISGs
6. DHODHi Inhibit the Production of Inflammatory Cytokines
7. DHODHi Applications in Antiviral Treatment
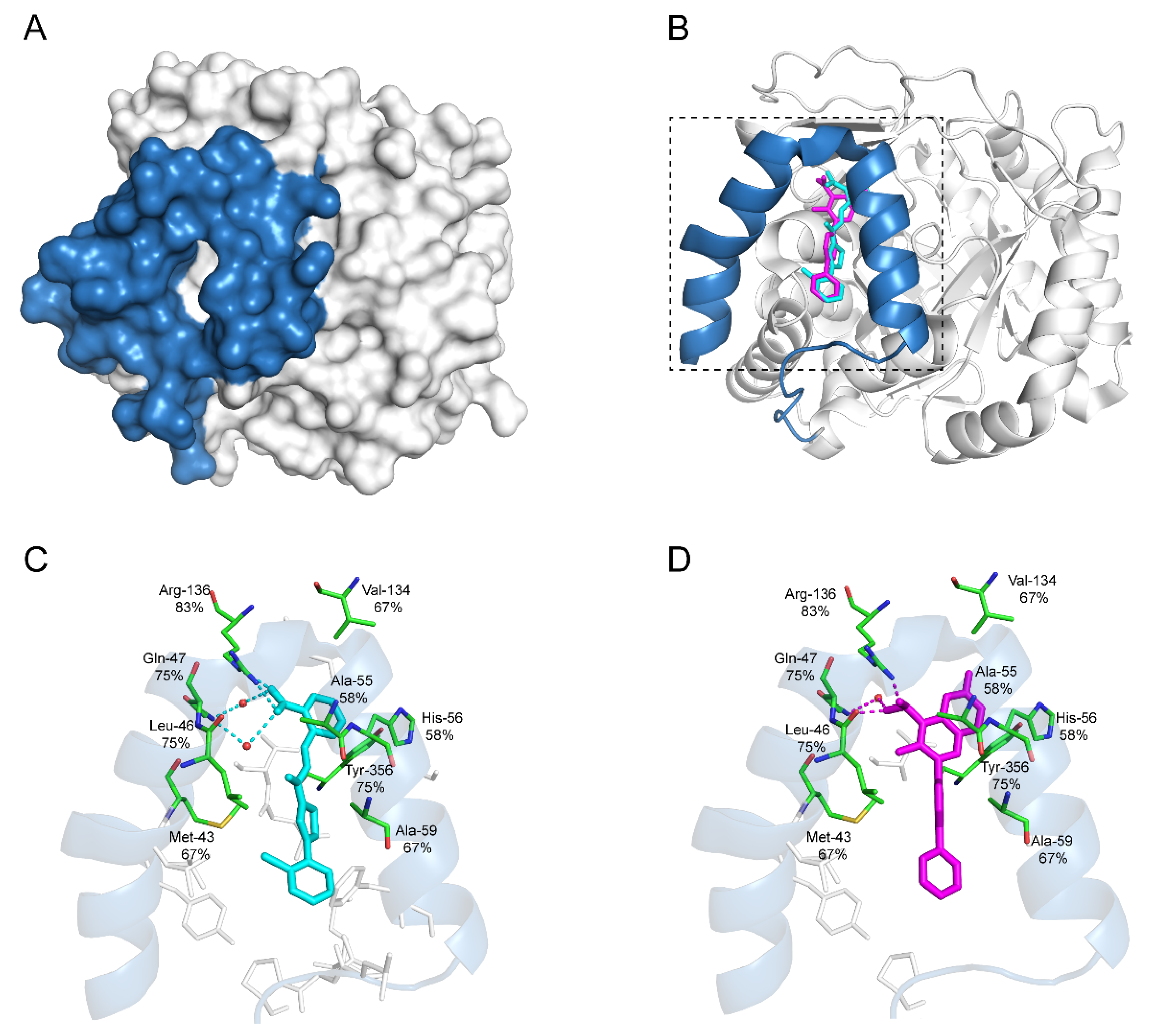
7.1. Leflunomide and Teriflunomide
7.2. Brequinar
7.3. S312 and S416
7.4. PTC299
7.5. IMU-838
7.6. Compound A3
7.7. FA-613
7.8. BAY2402234
7.9. MEDS433
7.10. RYL-634
8. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, G.F. From “A”IV to “Z”IKV: Attacks from Emerging and Re-emerging Pathogens. Cell 2018, 172, 1157–1159. [Google Scholar] [CrossRef] [Green Version]
- WHO. H1N1 IHR Emergency Committee. Available online: https://www.who.int/groups/h1n1-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. Poliovirus IHR Emergency Committee. Available online: https://www.who.int/groups/poliovirus-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. Ebola Virus Disease in West Africa (2014–2015) IHR Emergency Committee. Available online: https://www.who.int/groups/ebola-virus-disease-in-west-africa-(2014-2015)-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. Zika Virus IHR Emergency Committee. Available online: https://www.who.int/groups/zika-virus-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. Ebola Virus Disease in the Democratic Republic of the Congo (Equateur) IHR Emergency Committee. Available online: https://www.who.int/groups/ebola-virus-disease-in-the-democratic-republic-of-the-congo-equateur-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. Ebola Virus Disease in the Democratic Republic of the Congo (Kivu and Ituri) IHR Emergency Committee. Available online: https://www.who.int/groups/ebola-virus-disease-in-the-democratic-republic-of-the-congo-kivu-and-ituri-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. COVID-19 IHR Emergency Committee. Available online: https://www.who.int/groups/covid-19-ihr-emergency-committee (accessed on 15 March 2022).
- WHO. Summary of probable SARS cases with onset of illness from 1 November 2002 to 31 July 2003. Available online: https://www.who.int/publications/m/item/summary-of-probable-sars-cases-with-onset-of-illness-from-1-november-2002-to-31-july-2003 (accessed on 15 March 2022).
- WHO. MERS-CoV IHR Emergency Committee. Available online: https://www.who.int/groups/mers-cov-ihr-emergency-committee (accessed on 15 March 2022).
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Burt, F.J.; Chen, W.; Miner, J.J.; Lenschow, D.J.; Merits, A.; Schnettler, E.; Kohl, A.; Rudd, P.A.; Taylor, A.; Herrero, L.J.; et al. Chikungunya virus: An update on the biology and pathogenesis of this emerging pathogen. Lancet Infect. Dis. 2017, 17, e107–e117. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, L.; Huang, Y.; Liu, F.; Zhao, Y.; Cao, L.; Wang, T.; Sun, Q.; Ming, Z.; Zhang, L.; et al. Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 2020, 368, 779–782. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Young, B.; Tan, T.T.; Leo, Y.S. The place for remdesivir in COVID-19 treatment. Lancet Infect. Dis. 2021, 21, 20–21. [Google Scholar] [CrossRef]
- Grein, J.; Ohmagari, N.; Shin, D.; Diaz, G.; Asperges, E.; Castagna, A.; Feldt, T.; Green, G.; Green, M.L.; Lescure, F.X.; et al. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020, 382, 2327–2336. [Google Scholar] [CrossRef]
- Amirian, E.S.; Levy, J.K. Current knowledge about the antivirals remdesivir (GS-5734) and GS-441524 as therapeutic options for coronaviruses. One Health 2020, 9, 100128. [Google Scholar] [CrossRef]
- Gordon, C.J.; Tchesnokov, E.P.; Feng, J.Y.; Porter, D.P.; Götte, M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020, 295, 4773–4779. [Google Scholar] [CrossRef] [Green Version]
- Warren, T.K.; Jordan, R.; Lo, M.K.; Ray, A.S.; Mackman, R.L.; Soloveva, V.; Siegel, D.; Perron, M.; Bannister, R.; Hui, H.C.; et al. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature 2016, 531, 381–385. [Google Scholar] [CrossRef] [PubMed]
- Ogando, N.S.; Zevenhoven-Dobbe, J.C.; van der Meer, Y.; Bredenbeek, P.J.; Posthuma, C.C.; Snijder, E.J. The Enzymatic Activity of the nsp14 Exoribonuclease Is Critical for Replication of MERS-CoV and SARS-CoV-2. J. Virol. 2020, 94, e01246-20. [Google Scholar] [CrossRef] [PubMed]
- Adalja, A.; Inglesby, T. Broad-Spectrum Antiviral Agents: A Crucial Pandemic Tool. Expert Rev. Anti. Infect. Ther. 2019, 17, 467–470. [Google Scholar] [CrossRef] [Green Version]
- Löffler, M.; Fairbanks, L.D.; Zameitat, E.; Marinaki, A.M.; Simmonds, H.A. Pyrimidine pathways in health and disease. Trends Mol. Med. 2005, 11, 430–437. [Google Scholar] [CrossRef]
- Zhou, Y.; Tao, L.; Zhou, X.; Zuo, Z.; Gong, J.; Liu, X.; Zhou, Y.; Liu, C.; Sang, N.; Liu, H.; et al. DHODH and cancer: Promising prospects to be explored. Cancer Metab. 2021, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Chitranshi, N.; Agarwal, A.K. Significance and biological importance of pyrimidine in the microbial world. Int. J. Med. Chem. 2014, 2014, 202784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evans, D.R.; Guy, H.I. Mammalian pyrimidine biosynthesis: Fresh insights into an ancient pathway. J. Biol. Chem. 2004, 279, 33035–33038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munger, J.; Bajad, S.U.; Coller, H.A.; Shenk, T.; Rabinowitz, J.D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006, 2, e132. [Google Scholar] [CrossRef] [Green Version]
- Consigli, R.A.; Ginsberg, H.S. Control of aspartate transcarbamylase activity in type 5 adenovirus-infected HeLa cells. J. Bacteriol. 1964, 87, 1027–1033. [Google Scholar] [CrossRef] [Green Version]
- Traut, T.W. Physiological concentrations of purines and pyrimidines. Mol. Cell Biochem. 1994, 140, 1–22. [Google Scholar] [CrossRef]
- Okesli, A.; Khosla, C.; Bassik, M.C. Human pyrimidine nucleotide biosynthesis as a target for antiviral chemotherapy. Curr. Opin. Biotechnol. 2017, 48, 127–134. [Google Scholar] [CrossRef]
- Reis, R.A.G.; Calil, F.A.; Feliciano, P.R.; Pinheiro, M.P.; Nonato, M.C. The dihydroorotate dehydrogenases: Past and present. Arch. Biochem. Biophys. 2017, 632, 175–191. [Google Scholar] [CrossRef]
- Munier-Lehmann, H.; Vidalain, P.-O.; Tangy, F.; Janin, Y.L. On Dihydroorotate Dehydrogenases and Their Inhibitors and Uses. J. Med. Chem. 2013, 56, 3148–3167. [Google Scholar] [CrossRef] [PubMed]
- Mei-Jiao, G.; Shi-Fang, L.; Yan-Yan, C.; Jun-Jun, S.; Yue-Feng, S.; Ting-Ting, R.; Yong-Guang, Z.; Hui-Yun, C. Antiviral effects of selected IMPDH and DHODH inhibitors against foot and mouth disease virus. Biomed. Pharmacother. 2019, 118, 109305. [Google Scholar] [CrossRef] [PubMed]
- Alamri, R.D.; Elmeligy, M.A.; Albalawi, G.A.; Alquayr, S.M.; Alsubhi, S.S.; El-Ghaiesh, S.H. Leflunomide an immunomodulator with antineoplastic and antiviral potentials but drug-induced liver injury: A comprehensive review. Int. Immunopharmacol. 2021, 93, 107398. [Google Scholar] [CrossRef]
- Singh, A.; Maqbool, M.; Mobashir, M.; Hoda, N. Dihydroorotate dehydrogenase: A drug target for the development of antimalarials. Eur. J. Med. Chem. 2017, 125, 640–651. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, H.H.; Kunz, A.; Simon, V.A.; Palese, P.; Shaw, M.L. Broad-spectrum antiviral that interferes with de novo pyrimidine biosynthesis. Proc. Natl. Acad. Sci. USA 2011, 108, 5777–5782. [Google Scholar] [CrossRef] [Green Version]
- Xiong, R.; Zhang, L.; Li, S.; Sun, Y.; Ding, M.; Wang, Y.; Zhao, Y.; Wu, Y.; Shang, W.; Jiang, X.; et al. Novel and potent inhibitors targeting DHODH are broad-spectrum antivirals against RNA viruses including newly-emerged coronavirus SARS-CoV-2. Protein Cell 2020, 11, 723–739. [Google Scholar] [CrossRef]
- Sepúlveda, C.S.; García, C.C.; Damonte, E.B. Antiviral activity of A771726, the active metabolite of leflunomide, against Junín virus. J. Med. Virol. 2018, 90, 819–827. [Google Scholar] [CrossRef]
- Qing, M.; Zou, G.; Wang, Q.-Y.; Xu, H.Y.; Dong, H.; Yuan, Z.; Shi, P.-Y. Characterization of dengue virus resistance to brequinar in cell culture. Antimicrob. Agents Chemother. 2010, 54, 3686–3695. [Google Scholar] [CrossRef] [Green Version]
- Evers, D.L.; Wang, X.; Huong, S.M.; Andreoni, K.A.; Huang, E.S. Inhibition of human cytomegalovirus signaling and replication by the immunosuppressant FK778. Antivir. Res. 2005, 65, 1–12. [Google Scholar] [CrossRef]
- Lucas-Hourani, M.; Dauzonne, D.; Jorda, P.; Cousin, G.; Lupan, A.; Helynck, O.; Caignard, G.; Janvier, G.; André-Leroux, G.; Khiar, S.; et al. Inhibition of pyrimidine biosynthesis pathway suppresses viral growth through innate immunity. PLoS Pathog. 2013, 9, e1003678. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Li, Y.; Pu, F.; Wang, H.; Zhang, D.; Bai, J.; Shang, Y.; Ma, Z.; Ma, X.X. Inhibiting pyrimidine biosynthesis impairs Peste des Petits Ruminants Virus replication through depletion of nucleoside pools and activation of cellular immunity. Vet. Microbiol. 2021, 260, 109186. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sun, J.; Hu, J.; Wang, C.; Prinz, R.A.; Peng, D.; Liu, X.; Xu, X. A77 1726, the active metabolite of the anti-rheumatoid arthritis drug leflunomide, inhibits influenza A virus replication in vitro and in vivo by inhibiting the activity of Janus kinases. Faseb. J. 2020, 34, 10132–10145. [Google Scholar] [CrossRef] [PubMed]
- Cheung, N.N.; Lai, K.K.; Dai, J.; Kok, K.H.; Chen, H.; Chan, K.H.; Yuen, K.Y.; Kao, R.Y.T. Broad-spectrum inhibition of common respiratory RNA viruses by a pyrimidine synthesis inhibitor with involvement of the host antiviral response. J. Gen. Virol. 2017, 98, 946–954. [Google Scholar] [CrossRef]
- Smits, S.L.; de Lang, A.; van den Brand, J.M.; Leijten, L.M.; van IJcken, W.F.; Eijkemans, M.J.; van Amerongen, G.; Kuiken, T.; Andeweg, A.C.; Osterhaus, A.D.; et al. Exacerbated innate host response to SARS-CoV in aged non-human primates. PLoS Pathog. 2010, 6, e1000756. [Google Scholar] [CrossRef] [Green Version]
- Shaw, A.C.; Goldstein, D.R.; Montgomery, R.R. Age-dependent dysregulation of innate immunity. Nat. Rev. Immunol. 2013, 13, 875–887. [Google Scholar] [CrossRef] [Green Version]
- Channappanavar, R.; Fehr, A.R.; Vijay, R.; Mack, M.; Zhao, J.; Meyerholz, D.K.; Perlman, S. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe 2016, 19, 181–193. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.; Maini, M.K.; Wack, A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J. Interferon. Cytokine. Res. 2015, 35, 252–264. [Google Scholar] [CrossRef]
- Rockx, B.; Baas, T.; Zornetzer, G.A.; Haagmans, B.; Sheahan, T.; Frieman, M.; Dyer, M.D.; Teal, T.H.; Proll, S.; van den Brand, J.; et al. Early upregulation of acute respiratory distress syndrome-associated cytokines promotes lethal disease in an aged-mouse model of severe acute respiratory syndrome coronavirus infection. J. Virol. 2009, 83, 7062–7074. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Xu, J.; Zhou, C.; Wu, Z.; Zhong, S.; Liu, J.; Luo, W.; Chen, T.; Qin, Q.; Deng, P. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005, 171, 850–857. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, M.L.; Schleyerbach, R.; Kirschbaum, B.J. Leflunomide: An immunomodulatory drug for the treatment of rheumatoid arthritis and other autoimmune diseases. Immunopharmacology 2000, 47, 273–289. [Google Scholar] [CrossRef]
- Breedveld, F.C.; Dayer, J.M. Leflunomide: Mode of action in the treatment of rheumatoid arthritis. Ann. Rheum. Dis. 2000, 59, 841–849. [Google Scholar] [CrossRef]
- Cutolo, M.; Sulli, A.; Ghiorzo, P.; Pizzorni, C.; Craviotto, C.; Villaggio, B. Anti-inflammatory effects of leflunomide on cultured synovial macrophages from patients with rheumatoid arthritis. Ann. Rheum. Dis. 2003, 62, 297–302. [Google Scholar] [CrossRef] [Green Version]
- Vergne-Salle, P.; Léger, D.Y.; Bertin, P.; Trèves, R.; Beneytout, J.L.; Liagre, B. Effects of the active metabolite of leflunomide, A77 1726, on cytokine release and the MAPK signalling pathway in human rheumatoid arthritis synoviocytes. Cytokine 2005, 31, 335–348. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.; Begué-Pastor, N.; Benavent, S.; Gruaz, L.; Kaufmann, M.T.; Chicheportiche, R.; Dayer, J.M. The active metabolite of leflunomide, A77 1726, inhibits the production of prostaglandin E(2), matrix metalloproteinase 1 and interleukin 6 in human fibroblast-like synoviocytes. Rheumatology 2003, 42, 89–96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Influenza infection, SARS, MERS and COVID-19: Cytokine storm—The common denominator and the lessons to be learned. Clin. Immunol. 2021, 223, 108652. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Jiang, M.; Chen, X.; Montaner, L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: Review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020, 108, 17–41. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, J.Y.; Yang, J.W.; Lee, K.H.; Effenberger, M.; Szpirt, W.; Kronbichler, A.; Shin, J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics 2021, 11, 316–329. [Google Scholar] [CrossRef]
- Gustine, J.N.; Jones, D. Immunopathology of Hyperinflammation in COVID-19. Am. J. Pathol. 2021, 191, 4–17. [Google Scholar] [CrossRef] [PubMed]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 hyperinflammation and post-Covid-19 illness may be rooted in mast cell activation syndrome. Int. J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef]
- Kluge, S.; Janssens, U.; Welte, T.; Weber-Carstens, S.; Schälte, G.; Salzberger, B.; Gastmeier, P.; Langer, F.; Welper, M.; Westhoff, M.; et al. Recommendations for treatment of critically ill patients with COVID-19: Version 3 S1 guideline. Anaesthesist 2021, 70, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Wang, M.; Zhao, Y.; Zhang, Y.; Wang, T.; Zheng, Z.; Li, X.; Zeng, S.; Zhao, D.; Li, H.; et al. A Small-Scale Medication of Leflunomide as a Treatment of COVID-19 in an Open-Label Blank-Controlled Clinical Trial. Virol. Sin. 2020, 35, 725–733. [Google Scholar] [CrossRef]
- Merad, M.; Blish, C.A.; Sallusto, F.; Iwasaki, A. The immunology and immunopathology of COVID-19. Science 2022, 375, 1122–1127. [Google Scholar] [CrossRef] [PubMed]
- Merad, M.; Martin, J.C. Pathological inflammation in patients with COVID-19: A key role for monocytes and macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Xu, Y.; Jiang, H. Potential treatment of COVID-19 by inhibitors of human dihydroorotate dehydrogenase. Protein Cell 2020, 11, 699–702. [Google Scholar] [CrossRef] [PubMed]
- Luban, J.; Sattler, R.A.; Mühlberger, E.; Graci, J.D.; Cao, L.; Weetall, M.; Trotta, C.; Colacino, J.M.; Bavari, S.; Strambio-De-Castillia, C.; et al. The DHODH inhibitor PTC299 arrests SARS-CoV-2 replication and suppresses induction of inflammatory cytokines. Virus Res. 2021, 292, 198246. [Google Scholar] [CrossRef]
- McLean, J.E.; Neidhardt, E.A.; Grossman, T.H.; Hedstrom, L. Multiple inhibitor analysis of the brequinar and leflunomide binding sites on human dihydroorotate dehydrogenase. Biochemistry 2001, 40, 2194–2200. [Google Scholar] [CrossRef]
- Fragoso, Y.D.; Brooks, J.B. Leflunomide and teriflunomide: Altering the metabolism of pyrimidines for the treatment of autoimmune diseases. Expert Rev. Clin. Pharmacol. 2015, 8, 315–320. [Google Scholar] [CrossRef]
- Davis, I.C.; Lazarowski, E.R.; Chen, F.P.; Hickman-Davis, J.M.; Sullender, W.M.; Matalon, S. Post-infection A77-1726 blocks pathophysiologic sequelae of respiratory syncytial virus infection. Am. J. Respir. Cell Mol. Biol. 2007, 37, 379–386. [Google Scholar] [CrossRef]
- Bilger, A.; Plowshay, J.; Ma, S.; Nawandar, D.; Barlow, E.A.; Romero-Masters, J.C.; Bristol, J.A.; Li, Z.; Tsai, M.H.; Delecluse, H.J.; et al. Leflunomide/teriflunomide inhibit Epstein-Barr Virus (EBV)-induced lymphoproliferative disease and lytic viral replication. Oncotarget 2017, 8, 44266–44280. [Google Scholar] [CrossRef] [Green Version]
- Davis, I.C.; Lazarowski, E.R.; Hickman-Davis, J.M.; Fortenberry, J.A.; Chen, F.P.; Zhao, X.; Sorscher, E.; Graves, L.M.; Sullender, W.M.; Matalon, S. Leflunomide prevents alveolar fluid clearance inhibition by respiratory syncytial virus. Am. J. Respir. Crit. Care Med. 2006, 173, 673–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernhoff, E.; Tylden, G.D.; Kjerpeseth, L.J.; Gutteberg, T.J.; Hirsch, H.H.; Rinaldo, C.H. Leflunomide inhibition of BK virus replication in renal tubular epithelial cells. J. Virol. 2010, 84, 2150–2156. [Google Scholar] [CrossRef] [Green Version]
- Chacko, B.; John, G.T. Leflunomide for cytomegalovirus: Bench to bedside. Transpl. Infect. Dis. 2012, 14, 111–120. [Google Scholar] [CrossRef]
- Knecht, W.; Löffler, M. Species-related inhibition of human and rat dihydroorotate dehydrogenase by immunosuppressive isoxazol and cinchoninic acid derivatives. Biochem. Pharmacol. 1998, 56, 1259–1264. [Google Scholar] [CrossRef]
- Andersen, P.I.; Krpina, K.; Ianevski, A.; Shtaida, N.; Jo, E.; Yang, J.; Koit, S.; Tenson, T.; Hukkanen, V.; Anthonsen, M.W.; et al. Novel Antiviral Activities of Obatoclax, Emetine, Niclosamide, Brequinar, and Homoharringtonine. Viruses 2019, 11, 964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luthra, P.; Naidoo, J.; Pietzsch, C.A.; De, S.; Khadka, S.; Anantpadma, M.; Williams, C.G.; Edwards, M.R.; Davey, R.A.; Bukreyev, A.; et al. Inhibiting pyrimidine biosynthesis impairs Ebola virus replication through depletion of nucleoside pools and activation of innate immune responses. Antiviral. Res. 2018, 158, 288–302. [Google Scholar] [CrossRef]
- Morales Vasquez, D.; Park, J.-G.; Ávila-Pérez, G.; Nogales, A.; de la Torre, J.C.; Almazan, F.; Martinez-Sobrido, L. Identification of Inhibitors of ZIKV Replication. Viruses 2020, 12, 1041. [Google Scholar] [CrossRef]
- Li, S.-f.; Gong, M.-j.; Sun, Y.-f.; Shao, J.-j.; Zhang, Y.-g.; Chang, H.-y. Antiviral activity of brequinar against foot-and-mouth disease virus infection in vitro and in vivo. Biomed. Pharmacother. 2019, 116, 108982. [Google Scholar] [CrossRef]
- Schultz, D.C.; Johnson, R.M.; Ayyanathan, K.; Miller, J.; Whig, K.; Kamalia, B.; Dittmar, M.; Weston, S.; Hammond, H.L.; Dillen, C.; et al. Pyrimidine inhibitors synergize with nucleoside analogues to block SARS-CoV-2. Nature 2022, 604, 134–140. [Google Scholar] [CrossRef]
- Zhu, J.; Han, L.; Diao, Y.; Ren, X.; Xu, M.; Xu, L.; Li, S.; Li, Q.; Dong, D.; Huang, J.; et al. Design, synthesis, X-ray crystallographic analysis, and biological evaluation of thiazole derivatives as potent and selective inhibitors of human dihydroorotate dehydrogenase. J. Med. Chem. 2015, 58, 1123–1139. [Google Scholar] [CrossRef]
- Li, S.; Luan, G.; Ren, X.; Song, W.; Xu, L.; Xu, M.; Zhu, J.; Dong, D.; Diao, Y.; Liu, X.; et al. Rational Design of Benzylidenehydrazinyl-Substituted Thiazole Derivatives as Potent Inhibitors of Human Dihydroorotate Dehydrogenase with in Vivo Anti-arthritic Activity. Sci. Rep. 2015, 5, 14836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Weetall, M.; Trotta, C.; Cintron, K.; Ma, J.; Kim, M.J.; Furia, B.; Romfo, C.; Graci, J.D.; Li, W.; et al. Targeting of Hematologic Malignancies with PTC299, A Novel Potent Inhibitor of Dihydroorotate Dehydrogenase with Favorable Pharmaceutical Properties. Mol. Cancer Ther. 2019, 18, 3–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Şimşek-Yavuz, S.; Komsuoğlu Çelikyurt, F.I. An update of anti-viral treatment of COVID-19. Turk. J. Med. Sci. 2021, 51, 3372–3390. [Google Scholar] [CrossRef]
- Muehler, A.; Peelen, E.; Kohlhof, H.; Gröppel, M.; Vitt, D. Vidofludimus calcium, a next generation DHODH inhibitor for the Treatment of relapsing-remitting multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 43, 102129. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Wangen, C.; Häge, S.; Peter, A.S.; Dobler, G.; Hurst, B.; Julander, J.; Fuchs, J.; Ruzsics, Z.; Überla, K.; et al. IMU-838, a Developmental DHODH Inhibitor in Phase II for Autoimmune Disease, Shows Anti-SARS-CoV-2 and Broad-Spectrum Antiviral Efficacy In Vitro. Viruses 2020, 12, 1394. [Google Scholar] [CrossRef]
- Ortiz-Riaño, E.; Ngo, N.; Devito, S.; Eggink, D.; Munger, J.; Shaw, M.L.; de la Torre, J.C.; Martínez-Sobrido, L. Inhibition of arenavirus by A3, a pyrimidine biosynthesis inhibitor. J. Virol. 2014, 88, 878–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christian, S.; Merz, C.; Evans, L.; Gradl, S.; Seidel, H.; Friberg, A.; Eheim, A.; Lejeune, P.; Brzezinka, K.; Zimmermann, K.; et al. The novel dihydroorotate dehydrogenase (DHODH) inhibitor BAY 2402234 triggers differentiation and is effective in the treatment of myeloid malignancies. Leukemia 2019, 33, 2403–2415. [Google Scholar] [CrossRef] [Green Version]
- Mathieu, C.; Touret, F.; Jacquemin, C.; Janin, Y.L.; Nougairède, A.; Brailly, M.; Mazelier, M.; Décimo, D.; Vasseur, V.; Hans, A.; et al. A Bioluminescent 3CL(Pro) Activity Assay to Monitor SARS-CoV-2 Replication and Identify Inhibitors. Viruses 2021, 13, 1814. [Google Scholar] [CrossRef]
- Stegmann, K.M.; Dickmanns, A.; Heinen, N.; Groß, U.; Görlich, D.; Pfaender, S.; Dobbelstein, M. N4-hydroxycytidine and inhibitors of dihydroorotate dehydrogenase synergistically suppress SARS-CoV-2 replication. bioRxiv 2021. preprint. [Google Scholar] [CrossRef]
- Sainas, S.; Pippione, A.C.; Lupino, E.; Giorgis, M.; Circosta, P.; Gaidano, V.; Goyal, P.; Bonanni, D.; Rolando, B.; Cignetti, A.; et al. Targeting Myeloid Differentiation Using Potent 2-Hydroxypyrazolo [1,5-a]pyridine Scaffold-Based Human Dihydroorotate Dehydrogenase Inhibitors. J. Med. Chem. 2018, 61, 6034–6055. [Google Scholar] [CrossRef]
- Calistri, A.; Luganini, A.; Mognetti, B.; Elder, E.; Sibille, G.; Conciatori, V.; Del Vecchio, C.; Sainas, S.; Boschi, D.; Montserrat, N.; et al. The New Generation hDHODH Inhibitor MEDS433 Hinders the In Vitro Replication of SARS-CoV-2 and Other Human Coronaviruses. Microorganisms 2021, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Luganini, A.; Sibille, G.; Mognetti, B.; Sainas, S.; Pippione, A.C.; Giorgis, M.; Boschi, D.; Lolli, M.L.; Gribaudo, G. Effective deploying of a novel DHODH inhibitor against herpes simplex type 1 and type 2 replication. Antiviral. Res. 2021, 189, 105057. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Cao, L.; Gao, H.; Wu, Y.; Wang, Y.; Fang, F.; Lan, T.; Lou, Z.; Rao, Y. Discovery, Optimization, and Target Identification of Novel Potent Broad-Spectrum Antiviral Inhibitors. J. Med. Chem. 2019, 62, 4056–4073. [Google Scholar] [CrossRef] [PubMed]
- Gong, M.; Yang, Y.; Huang, Y.; Gan, T.; Wu, Y.; Gao, H.; Li, Q.; Nie, J.; Huang, W.; Wang, Y.; et al. Novel quinolone derivatives targeting human dihydroorotate dehydrogenase suppress Ebola virus infection in vitro. Antiviral. Res. 2021, 194, 105161. [Google Scholar] [CrossRef]
- Song, P.; Li, W.; Xie, J.; Hou, Y.; You, C. Cytokine storm induced by SARS-CoV-2. Clin. Chim. Acta 2020, 509, 280–287. [Google Scholar] [CrossRef]
- Rane, J.S.; Pandey, P.; Chatterjee, A.; Khan, R.; Kumar, A.; Prakash, A.; Ray, S. Targeting virus-host interaction by novel pyrimidine derivative: An in silico approach towards discovery of potential drug against COVID-19. J. Biomol. Struct. Dyn. 2021, 39, 5768–5778. [Google Scholar] [CrossRef]
- Kaltwasser, J.P.; Nash, P.; Gladman, D.; Rosen, C.F.; Behrens, F.; Jones, P.; Wollenhaupt, J.; Falk, F.G.; Mease, P. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: A multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum. 2004, 50, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Koller, G.; Cusnir, I.; Hall, J.; Ye, C. Reversible alopecia areata: A little known side effect of leflunomide. Clin. Rheumatol. 2019, 38, 2015–2016. [Google Scholar] [CrossRef]
- Alfaro-Lara, R.; Espinosa-Ortega, H.F.; Arce-Salinas, C.A. Systematic review and meta-analysis of the efficacy and safety of leflunomide and methotrexate in the treatment of rheumatoid arthritis. Reumatol. Clin. 2019, 15, 133–139. [Google Scholar] [CrossRef]
- Pally, C.; Smith, D.; Jaffee, B.; Magolda, R.; Zehender, H.; Dorobek, B.; Donatsch, P.; Papageorgiou, C.; Schuurman, H.J. Side effects of brequinar and brequinar analogues, in combination with cyclosporine, in the rat. Toxicology 1998, 127, 207–222. [Google Scholar] [CrossRef]
- Makowka, L.; Sher, L.S.; Cramer, D.V. The development of Brequinar as an immunosuppressive drug for transplantation. Immunol. Rev. 1993, 136, 51–70. [Google Scholar] [CrossRef] [PubMed]
- Schiff, M.H.; Strand, V.; Oed, C.; Loew-Friedrich, I. Leflunomide: Efficacy and safety in clinical trials for the treatment of rheumatoid arthritis. Drugs Today 2000, 36, 383–394. [Google Scholar] [CrossRef] [PubMed]
| Host Targets | Description of Host Targets | HTAs | Known Antiviral Effects |
|---|---|---|---|
| DHODH | The rate-limiting enzyme in the de novo pyrimidine synthesis pathway | Leflunomide, teriflunomide, and brequinar | Influenza virus, HBV, HCV, EBOV, DENV, SARS-CoV-2, HIV, and ZIKV |
| Chemokine receptors type 5 | A G-protein coupled receptor, which is an HIV-1 co-receptor associated with CXCR4 | Maraviroc, PF-232798, TAK-220, and INCB9471 | HIV |
| Inosine monophosphate dehydrogenase | The rate-limiting enzyme in the de novo biosynthesis of guanine nucleotides | Ribavirin, mycophenolic acid, mycophenolate mofetil, and mizoribine | RSV, HCV, HBV, HCMV, EMCV, ZIKV, and EBOV |
| Cyclophilins | A peptidyl-prolyl isomerase, catalyzing the isomerization of peptide bonds from trans to cis form at proline residues to facilitate protein folding | Cyclosporin A, NIM811, and alisporivir | HCV |
| Eukaryotic initiation factor 2α | A eukaryotic initiation factor required for most eukaryotic translation initiation | Nitazoxanide, tizoxanide, and RM5061 | Influenza virus, HBV, HCV, EBOV, DENV, JEV, HIV, and ZIKV |
| Dihydrofolate reductase | An enzyme converting dihydrofolate into tetrahydrofolate for the de novo synthesis of purines, thymidylic acid, and certain amino acids | Methotrexate, trimetrexate, and 1-aryl-4,6-diamino-1,2-dihydrotriazines | ZIKV, influenza virus, and RSV |
| α-Glucosidase | An enzyme catalyzing the hydrolysis of glycosidic bonds in complex sugars | NB-DNJ and Celgosivir | HIV, HCV, human coronavirus, influenza A virus, and DENV |
| Kinases | An enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates | Sunitinib and erlotinib | DENV and EBOV |
| Sodium taurocholate cotransporting polypeptide | A multiple transmembrane transporter involved in the circulation of bile acids, and served as a common receptor of HBV and HDV | Myrcludex B, CsA, ezetimibe, and ritonavir | HBV and HDV |
| Farnesoid X receptor | A nuclear bile acid receptor that regulates the expression of bile acid transporters | GW4064, WAY362450, fexaramine, and chenodeoxycholic acid | HBV |
| Diacylglycerol acyltransferases | An enzyme catalyzing the terminal step in triacylglycerol synthesis | pradigastat | HCV |
| DHODHi | Key Binding Site Residues | Molecular Structure | Antiviral Activities | Clinical Applications |
|---|---|---|---|---|
| Leflunomide | Tyr356, Met 43, His56, Ala55, Ala59, Pro364, Val134, Gln47, Arg136, Phe98 | 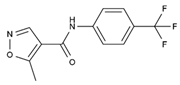 | Influenza A virus (H1N1), ZIKV, EBOV, SARS-CoV-2, BK virus, DENV, porcine epidemic diarrhea virus, CMV, RSV, herpes simplex virus type 1, and HCMV | Phase I/II/III (SARS-CoV-2) Phase I (HIV) Phase II (BK virus) |
| Teriflunomide | Tyr356, Met 43, His56, Ala55, Ala59, Pro364, Val134, Arg136, Gln47, Phe98 | 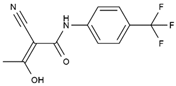 | SARS-CoV-2, Human T-lymphotropic virus type-1, JUNV, influenza virus (H5N1), EBV, EV71, and HIV | Phase I/II (HTLV-1) |
| Brequinar | Arg136, Met 43, Gln47, Leu46, Leu42, His56, Tyr38, Pro326, Tyr356, Pro69, Val143, Val134 | 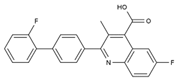 | SARS-CoV-2 flaviviruses, alphavirus, rhabdovirus, influenza viruses, EV71, EV70, and Coxsackievirus B3 | Phase I/II (SARS-CoV-2) |
| IMU838 | Arg136, Met 43, Gln47, Leu46, Leu42, His56, Tyr38, Pro326, Tyr356, Pro69, Val143, Val134 | 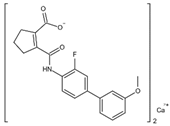 | SARS-CoV-2, HCMV, HIV-1, and HCV | Phase II/III (SARS-CoV-2) |
| S416 | Tyr38, Leu42, Met43, Leu46, Gln47, Pro52, Ala55, His56, Ala59, Phe62, Thr63, Leu67, Leu68, Pro69, Phe98, Met111, Val134, Arg136, Val143, Tyr356, Leu359, Thr360 | 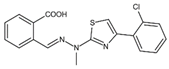 | Influenza A virus (H1N1, H3N2, H9N2), ZIKV, EBOV, and SARS-CoV-2 | —— |
| S312 | Tyr38, Leu42, Met43, Leu46, Gln47, Pro52, Ala55, His56, Ala59, Phe62, Thr63, Leu67, Leu68, Pro69, Phe98, Met111, Val134, Arg136, Val143, Tyr356, Leu359, Thr360 | 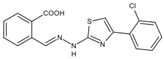 | Influenza virus (H1N1, H3N2, H9N2), ZIKV, EBOV, and SARS-CoV-2 | —— |
| FA-613 | Tyr356, Arg136, Ala55, Ala59, Leu 46, Thr360 | 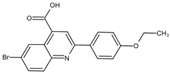 | Influenza A virus (H5N1 and H7N9), EV-A71, RSV, human rhinovirus A, SARS-CoV, and MERS-CoV | —— |
| PTC299 | Tyr356, Phe98, Met111, Leu68, Pro364, Phe62, Met43, Leu58, Leu46, Leu50, Ala55, Arg136, His56, Ala59, Gln47, Val134, VAL143, Thr63 | 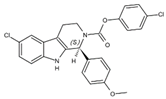 | SARS-CoV-2, HCV, Poliovirus, EBOV, and Rift Valley Fever | —— |
| Compound A3 | Tyr356, Arg136, Ala55, Ala59, Leu46, Pro364, Phe336 |  | Influenza A virus (A/WSN/33), influenza B virus (B/Yamagata/88), Newcastle disease virus (La Sota), Sendai virus (SV52), Vesicular stomatitis virus, Sindbis virus, HCV, West Nile virus, DENV-1, NYVAC, hAd5, and HIV-1 | —— |
| BAY2402234 | Thr63, Tyr38, Leu42, Met43, Leu46, Leu50, Leu58, Ala59, Phe62, Leu67, Leu68, Pro69, Met111, Leu359, Pro364, Thr360 | 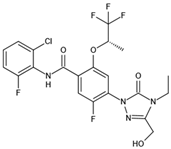 | SARS-CoV-2 | —— |
| MEDS433 | Gln47, Phe62, Arg136, Thr360 |  | HCoV-OC43, HCoV-229E, SARS-CoV-2, and HSV | —— |
| RYL-634 | Tyr38, Leu42, Leu46, Gln47, Phe62, Leu67, Arg136 |  | HCV, DENV, ZIKV, chikungunya virus, EV71, HIV, RSV, severe fever with thrombocytopenia syndrome virus, and influenza virus | —— |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, Y.; Li, S.; Song, K.; Ye, J.; Li, W.; Zhong, Y.; Feng, Z.; Liang, S.; Cai, Z.; Xu, K. A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Viruses 2022, 14, 928. https://doi.org/10.3390/v14050928
Zheng Y, Li S, Song K, Ye J, Li W, Zhong Y, Feng Z, Liang S, Cai Z, Xu K. A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Viruses. 2022; 14(5):928. https://doi.org/10.3390/v14050928
Chicago/Turabian StyleZheng, Yucheng, Shiliang Li, Kun Song, Jiajie Ye, Wenkang Li, Yifan Zhong, Ziyan Feng, Simeng Liang, Zeng Cai, and Ke Xu. 2022. "A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses" Viruses 14, no. 5: 928. https://doi.org/10.3390/v14050928
APA StyleZheng, Y., Li, S., Song, K., Ye, J., Li, W., Zhong, Y., Feng, Z., Liang, S., Cai, Z., & Xu, K. (2022). A Broad Antiviral Strategy: Inhibitors of Human DHODH Pave the Way for Host-Targeting Antivirals against Emerging and Re-Emerging Viruses. Viruses, 14(5), 928. https://doi.org/10.3390/v14050928







