Comparison of Six Serological Immunoassays for the Detection of SARS-CoV-2 Neutralizing Antibody Levels in the Vaccinated Population
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Plaque Reduction Neutralization Test
2.3. SARS-CoV-2 Surrogate Virus Neutralization Test
2.4. SARS-CoV-2 Binding Antibody Tests
2.5. Statistical Analyses
3. Results
3.1. Neutralizing Antibody Titers by PRNT and sVNTs
3.2. Levels of SARS-CoV-2-Binding Antibodies
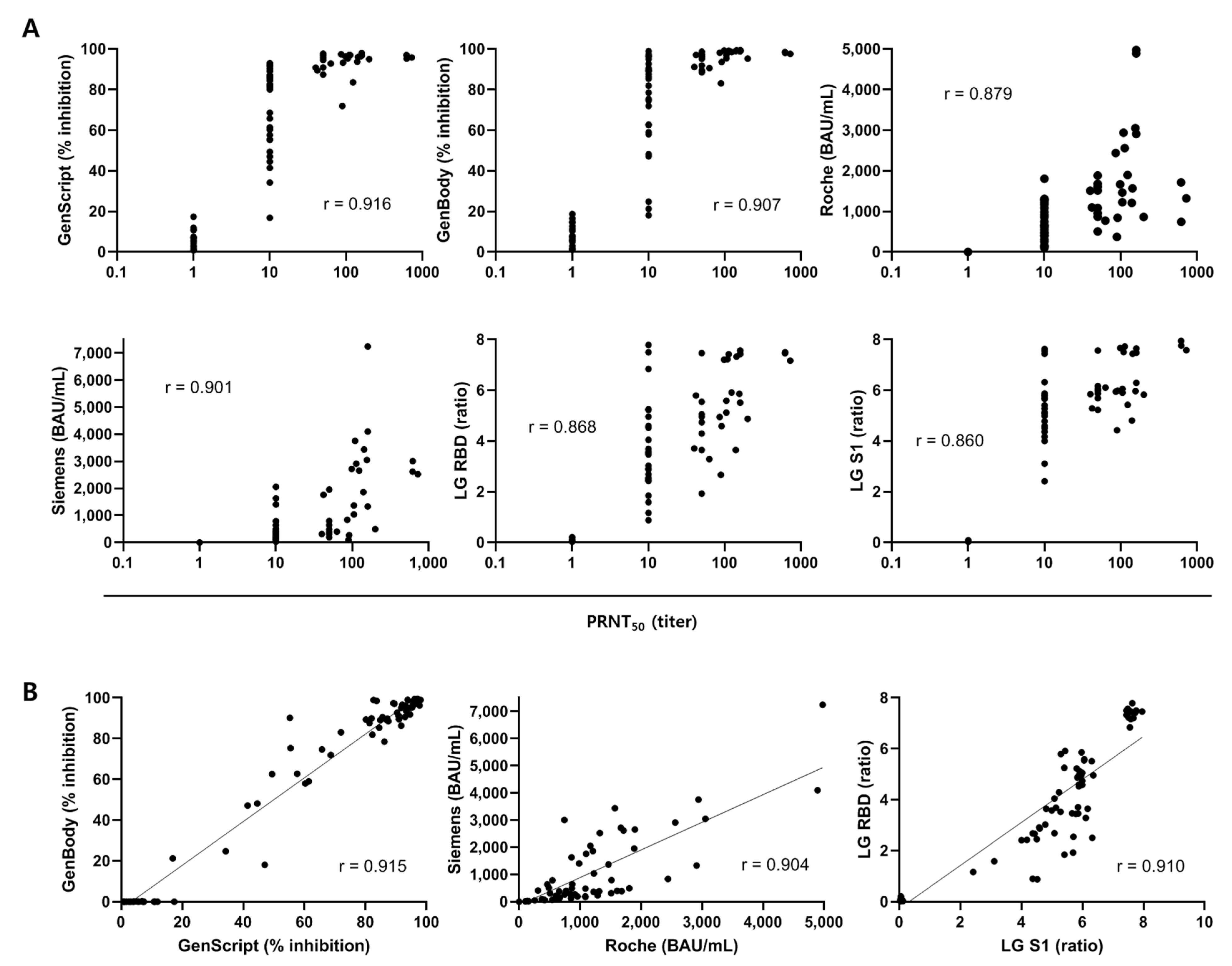
3.3. Correlation of SARS-CoV-2 Antibody Assays with PRNT
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| AZ/AZ (N = 15) | BNT/BNT (N = 15) | |
|---|---|---|
| Age (years), median (IQR) | 36 (28–51) | 39 (29–51) |
| Females, n (%) | 8 (53.3) | 7 (46.7) |
| Intervial between 1st and 2nd vaccination (days), median (IQR) | 77 (77–77) | 21 (21–22) |
| Days to sample collection after 2nd dose for 1 month samples, median (IQR) | 22 (21–24) | 29 (28–30) |
| Days to sample collection after 2nd dose for 3 month samples, median (IQR) | 81 (79–83) | 82 (78–83) |
| (A) | ||||||||||||||
| SARS-CoV-2 Surrogate Virus Neutralization Test Kit | GenBody FIA COVID-19 NAb | |||||||||||||
| Manufacturer | GenScript (USA) | GenBody (Korea) | ||||||||||||
| Target antigen | RBD | S | ||||||||||||
| Isotype | IgG, Neutralizing | IgG, Neutralizing | ||||||||||||
| Principle | Blocking enzyme-linked immunosorbent assay (ELISA) | Blocking fluorescent immunoassay (FIA) | ||||||||||||
| Analyzer | None | Confiscope F20 | ||||||||||||
| Specimen | Serum, Plasma | Serum | ||||||||||||
| Required sample volume | 10 μL | 20 µL | ||||||||||||
| Interpretation of results | Positive: % inhibition ≥ 30% | Positive: % inhibition ≥ 25% | ||||||||||||
| (B) | ||||||||||||||
| Elecsys Anti-SARS-CoV-2 S | SARS-CoV-2 IgG (sCOVG) | AdvanSure SARS CoV 2 IgG(RBD) ELISA | AdvanSure SARS CoV 2 IgG(S1) ELISA | |||||||||||
| Manufacturer | Roche Diagnostics (Switzerland) | Siemens (Germany) | LG Chem (Korea) | LG Chem (Korea) | ||||||||||
| Target antigen | S RBD | S1 RBD | RBD | S1 | ||||||||||
| Isotype | Total Ab | IgG | IgG | IgG | ||||||||||
| Principle | Electrochemiluminescence immunoassay (ECLIA) | Chemiluminescence immunoassay (CLIA) | One-step antigen capture format indirect ELISA | One-step antigen capture format indirect ELISA | ||||||||||
| Analyzer | Cobas e801 | Atellica IM | none | none | ||||||||||
| Calibration | 2-point calibration | 2-point calibration | ||||||||||||
| Specimen | Serum, Li-heparin, EDTA and sodium citrate plasma | Serum and plasma (lithium heparin) | Serum, heparin, EDTA and citrate plasma | Serum, heparin, EDTA and citrate plasma | ||||||||||
| Required sample volume | 12 μL | 40 µL | 10 µL | 10 µL | ||||||||||
| Interpretation of results | Positive: ≥0.80 U/mL | Reactive: ≥1.00 index (U/mL) | Positive: ≥1.1 (S/Co ratio) | Positive: ≥1.1 (S/Co ratio) | ||||||||||
| Analytical measuring interval | 0.40–250 U/mL | 0.50–150.00 index (U/mL) | none | none | ||||||||||
| Reportable range | 0.40–4,840 U/mL | 0.50–332.3 U/mL | 0.01–7.80 S/Co ratio | 0.01–8.00 S/Co ratio | ||||||||||
| PRNT | sVNT | CLIA | EIA | |||||
|---|---|---|---|---|---|---|---|---|
| Sample No. | Collection time, vaccination | (PRNT50 ≥1:10) * | GenScript (≥30%) | GenBody (≥25%) | Roche (≥0.8 U/mL) | Siemens (≥1.0 U/mL) | LG RBD (≥1.1 ratio) | LG S1 (≥1.1 ratio) |
| 1 | 3 mo, AZ/AZ | P (1:10) ** | N (17.0) | N (21.3) | P (102.0) | N (0.72) | P (1.2) | P (2.4) |
| 2 | 3 mo, AZ/AZ | P (1:10) | P (47.0) | N (18.2) | P (150.0) | P (1.02) | P (1.6) | P (3.1) |
| 3 | 1 mo, AZ/AZ | P (1:10) | P (34.2) | P (25.0) | P (134.0) | P (1.68) | N (0.9) | P (4.5) |
| 4 | 1 mo, AZ/AZ | P (1:10) | P (41.4) | P (47.2) | P (250.0) | P (2.33) | N (0.9) | P (4.4) |
| PRNT | sVNT | CLIA | EIA | |||||
|---|---|---|---|---|---|---|---|---|
| GenScript | GenBody | Roche | Siemens | LG RBD | LG S1 | |||
| PRNT | 98.9% * | 97.8% | 100.0% | 98.9% | 97.8% | 100.0% | ||
| 0.975 ** | 0.951 | 1.000 | 0.975 | 0.951 | 1.000 | |||
| sVNT | GenScript | 98.9% | 98.9% | 100.0% | 96.7% | 98.9% | ||
| 0.975 | 0.975 | 1.000 | 0.927 | 0.975 | ||||
| GenBody | 97.8% | 98.9% | 95.6% | 97.8% | ||||
| 0.951 | 0.975 | 0.903 | 0.951 | |||||
| CLIA | Roche | 98.9% | 97.8% | 100.0% | ||||
| 0.975 | 0.951 | 1.000 | ||||||
| Siemens | 96.7% | 98.9% | ||||||
| 0.927 | 0.975 | |||||||
| EIA | LG RBD | 97.8% | ||||||
| 0.951 | ||||||||
| LG S1 | ||||||||
References
- Sharma, A.; Tiwari, S.; Deb, M.K.; Marty, J.L. Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2): A global pandemic and treatment strategies. Int. J. Antimicrob. Agents 2020, 56, 106054. [Google Scholar] [CrossRef] [PubMed]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. Anti-Coronavirus Vaccines: Past Investigations on SARS-CoV-1 and MERS-CoV, the Approved Vaccines from BioNTech/Pfizer, Moderna, Oxford/AstraZeneca and others under Development Against SARSCoV-2 Infection. Curr. Med. Chem. 2022, 29, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Alhatlani, B. An overview of current COVID-19 vaccine platforms. Comput. Struct. Biotechnol. J. 2021, 19, 2508–2517. [Google Scholar] [CrossRef] [PubMed]
- Sette, A.; Crotty, S. Adaptive immunity to SARS-CoV-2 and COVID-19. Cell 2021, 184, 861–880. [Google Scholar] [CrossRef]
- Tada, T.; Zhou, H.; Samanovic, M.I.; Dcosta, B.M.; Cornelius, A.; Mulligan, M.J.; Landau, N.R. Comparison of Neutralizing Antibody Titers Elicited by mRNA and Adenoviral Vector Vaccine against SARS-CoV-2 Variants. BioRxiv 2021. [Google Scholar] [CrossRef]
- Bohn, M.K.; Loh, T.P.; Wang, C.B.; Mueller, R.; Koch, D.; Sethi, S.; Rawlinson, W.D.; Clementi, M.; Erasmus, R.; Leportier, M.; et al. IFCC Interim Guidelines on Serological Testing of Antibodies against SARS-CoV-2. Clin. Chem. Lab. Med. 2020, 58, 2001–2008. [Google Scholar] [CrossRef]
- Joyner, M.J.; Carter, R.E.; Senefeld, J.W.; Klassen, S.A.; Mills, J.R.; Johnson, P.W.; Theel, E.S.; Wiggins, C.C.; Bruno, K.A.; Klompas, A.M.; et al. Convalescent Plasma Antibody Levels and the Risk of Death from Covid-19. N. Engl. J. Med. 2021, 384, 1015–1027. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Zang, R.; Gomez Castro, M.F.; McCune, B.T.; Zeng, Q.; Rothlauf, P.W.; Sonnek, N.M.; Liu, Z.; Brulois, K.F.; Wang, X.; Greenberg, H.B.; et al. TMPRSS2 and TMPRSS4 promote SARS-CoV-2 infection of human small intestinal enterocytes. Sci. Immunol. 2020, 5. [Google Scholar] [CrossRef]
- Amanat, F.; White, K.M.; Miorin, L.; Strohmeier, S.; McMahon, M.; Meade, P.; Liu, W.C.; Albrecht, R.A.; Simon, V.; Martinez-Sobrido, L.; et al. An In Vitro Microneutralization Assay for SARS-CoV-2 Serology and Drug Screening. Curr. Protoc. Microbiol. 2020, 58, e108. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2-spike protein-protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef] [PubMed]
- Ebinger, J.E.; Fert-Bober, J.; Printsev, I.; Wu, M.; Sun, N.; Figueiredo, J.C.; Eyk, J.E.V.; Braun, J.G.; Cheng, S.; Sobhani, K. Prior COVID-19 Infection and Antibody Response to Single Versus Double Dose mRNA SARS-CoV-2 Vaccination. MedRxiv 2021. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Yu, S.; Chen, K.; Fang, L.; Mao, H.; Lou, X.; Li, C.; Zhang, Y. Comparison and Analysis of Neutralizing Antibody Levels in Serum after Inoculating with SARS-CoV-2, MERS-CoV, or SARS-CoV Vaccines in Humans. Vaccines 2021, 9, 588. [Google Scholar] [CrossRef] [PubMed]
- Bewley, K.R.; Coombes, N.S.; Gagnon, L.; McInroy, L.; Baker, N.; Shaik, I.; St-Jean, J.R.; St-Amant, N.; Buttigieg, K.R.; Humphries, H.E.; et al. Quantification of SARS-CoV-2 neutralizing antibody by wild-type plaque reduction neutralization, microneutralization and pseudotyped virus neutralization assays. Nat. Protoc. 2021, 16, 3114–3140. [Google Scholar] [CrossRef] [PubMed]
- Dogan, M.; Kozhaya, L.; Placek, L.; Gunter, C.; Yigit, M.; Hardy, R.; Plassmeyer, M.; Coatney, P.; Lillard, K.; Bukhari, Z.; et al. SARS-CoV-2 specific antibody and neutralization assays reveal the wide range of the humoral immune response to virus. Commun. Biol. 2021, 4, 129. [Google Scholar] [CrossRef] [PubMed]
- Padoan, A.; Bonfante, F.; Pagliari, M.; Bortolami, A.; Negrini, D.; Zuin, S.; Bozzato, D.; Cosma, C.; Sciacovelli, L.; Plebani, M. Analytical and clinical performances of five immunoassays for the detection of SARS-CoV-2 antibodies in comparison with neutralization activity. EBioMedicine 2020, 62, 103101. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, L.; Park, Y.J.; Tortorici, M.A.; Czudnochowski, N.; Walls, A.C.; Beltramello, M.; Silacci-Fregni, C.; Pinto, D.; Rosen, L.E.; Bowen, J.E.; et al. Mapping Neutralizing and Immunodominant Sites on the SARS-CoV-2 Spike Receptor-Binding Domain by Structure-Guided High-Resolution Serology. Cell 2020, 183, 1024–1042.e1021. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Charlton, C.L.; Bailey, A.; Kanji, J.N.; McCarthy, M.K.; Morrison, T.E.; Huey, L.; Annen, K.; DomBourian, M.G.; et al. A New SARS-CoV-2 Dual-Purpose Serology Test: Highly Accurate Infection Tracing and Neutralizing Antibody Response Detection. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Mathur, G.; Mathur, S. Antibody Testing for COVID-19. Am. J. Clin. Pathol. 2020, 154, 1–3. [Google Scholar] [CrossRef]
- Lisboa Bastos, M.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.P.; Johnston, J.C.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for covid-19: Systematic review and meta-analysis. BMJ 2020, 370, m2516. [Google Scholar] [CrossRef] [PubMed]
- Kruttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.; Imohl, M.; Kleines, M. Comparison of four new commercial serologic assays for determination of SARS-CoV-2 IgG. J. Clin. Virol. 2020, 128, 104394. [Google Scholar] [CrossRef] [PubMed]
- Guven, E.; Duus, K.; Lydolph, M.C.; Jorgensen, C.S.; Laursen, I.; Houen, G. Non-specific binding in solid phase immunoassays for autoantibodies correlates with inflammation markers. J. Immunol. Methods 2014, 403, 26–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Thier, P.; Bacharouche, J.; Duval, J.F.; Skali-Lami, S.; Francius, G. Atomic force microscopy analysis of IgG films at hydrophobic surfaces: A promising method to probe IgG orientations and optimize ELISA tests performance. Biochim. Biophys. Acta 2015, 1854, 138–145. [Google Scholar] [CrossRef]
- Whitman, J.D.; Hiatt, J.; Mowery, C.T.; Shy, B.R.; Yu, R.; Yamamoto, T.N.; Rathore, U.; Goldgof, G.M.; Whitty, C.; Woo, J.M.; et al. Evaluation of SARS-CoV-2 serology assays reveals a range of test performance. Nat. Biotechnol. 2020, 38, 1174–1183. [Google Scholar] [CrossRef]
- Kristiansen, P.A.; Page, M.; Bernasconi, V.; Mattiuzzo, G.; Dull, P.; Makar, K.; Plotkin, S.; Knezevic, I. WHO International Standard for anti-SARS-CoV-2 immunoglobulin. Lancet 2021, 397, 1347–1348. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- Wec, A.Z.; Wrapp, D.; Herbert, A.S.; Maurer, D.P.; Haslwanter, D.; Sakharkar, M.; Jangra, R.K.; Dieterle, M.E.; Lilov, A.; Huang, D.; et al. Broad neutralization of SARS-related viruses by human monoclonal antibodies. Science 2020, 369, 731–736. [Google Scholar] [CrossRef]
- Schafer, A.; Muecksch, F.; Lorenzi, J.C.C.; Leist, S.R.; Cipolla, M.; Bournazos, S.; Schmidt, F.; Maison, R.M.; Gazumyan, A.; Martinez, D.R.; et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 2021, 218. [Google Scholar] [CrossRef]
- Sholukh, A.M.; Fiore-Gartland, A.; Ford, E.S.; Miner, M.D.; Hou, Y.J.; Tse, L.V.; Kaiser, H.; Zhu, H.; Lu, J.; Madarampalli, B.; et al. Evaluation of Cell-Based and Surrogate SARS-CoV-2 Neutralization Assays. J. Clin. Microbiol. 2021, 59, e0052721. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Hillyer, C.; Du, L. Neutralizing Antibodies against SARS-CoV-2 and Other Human Coronaviruses. Trends Immunol. 2020, 41, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Bosnjak, B.; Stein, S.C.; Willenzon, S.; Cordes, A.K.; Puppe, W.; Bernhardt, G.; Ravens, I.; Ritter, C.; Schultze-Florey, C.R.; Godecke, N.; et al. Low serum neutralizing anti-SARS-CoV-2 S antibody levels in mildly affected COVID-19 convalescent patients revealed by two different detection methods. Cell Mol. Immunol. 2021, 18, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Ju, B.; Zhang, Q.; Ge, J.; Wang, R.; Sun, J.; Ge, X.; Yu, J.; Shan, S.; Zhou, B.; Song, S.; et al. Human neutralizing antibodies elicited by SARS-CoV-2 infection. Nature 2020, 584, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Lee, J.H.; Ko, G.Y.; Ryu, J.H.; Jang, J.H.; Bae, H.; Yoo, S.H.; Choi, A.R.; Jung, J.; Lee, J.; et al. Quantitative SARS-CoV-2 Spike Antibody Response in COVID-19 Patients Using Three Fully Automated Immunoassays and a Surrogate Virus Neutralization Test. Diagnostics 2021, 11, 1496. [Google Scholar] [CrossRef]
- Yun, S.; Ryu, J.H.; Jang, J.H.; Bae, H.; Yoo, S.H.; Choi, A.R.; Jo, S.J.; Lim, J.; Lee, J.; Ryu, H.; et al. Comparison of SARS-CoV-2 Antibody Responses and Seroconversion in COVID-19 Patients Using Twelve Commercial Immunoassays. Ann. Lab. Med. 2021, 41, 577–587. [Google Scholar] [CrossRef]
- Valcourt, E.J.; Manguiat, K.; Robinson, A.; Chen, J.C.; Dimitrova, K.; Philipson, C.; Lamoureux, L.; McLachlan, E.; Schiffman, Z.; Drebot, M.A.; et al. Evaluation of a commercially-available surrogate virus neutralization test for severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). Diagn. Microbiol. Infect. Dis. 2021, 99, 115294. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]



| AZ/AZ (n = 15) | BNT/BNT (n = 15) | ||||||
|---|---|---|---|---|---|---|---|
| Sampling Time | SARS-CoV-2 Antibody Assays | Median | (IQR) | Median | (IQR) | p-value * | |
| 1 month after two-dose vaccination | PRNT | PRNT50 (titer) | 1:10 | (1:10– 1:50) | 1:113 | (1:44–1:160) | 0.002 |
| sVNT | GenScript (% inhibition) | 90.3 | (86.4–92.9) | 96.0 | (92.3–97.0) | 0.036 | |
| GenBody (% inhibition) | 91.2 | (86.4–95.2) | 98.6 | (97.7–99.3) | <0.001 | ||
| CLIA | Roche (U/mL) | 1236.0 | (849.0–1473.5) | 1622.0 | (1086.5–2329.8) | 0.054 | |
| Roche (BAU/mL) | 1270.6 | (872.8–1514.8) | 1667.4 | (1116.9–2395.0) | 0.054 | ||
| Siemens (U/mL) | 14.6 | (9.5–21.7) | 121.9 | (91.0–152.9) | <0.001 | ||
| Siemens (BAU/mL) | 318.5 | (208.1–472.4) | 2658.2 | (1984.8–3333.4) | <0.001 | ||
| EIA | LG RBD (ratio) | 3.47 | (2.08–4.70) | 7.42 | (7.18–7.49) | <0.001 | |
| LG S1 (ratio) | 5.78 | (5.47–5.99) | 7.57 | (7.45–7.67) | <0.001 | ||
| 3 months after two–dose vaccination | PRNT | PRNT50 (titer) | 1:10 | (1:10–1:10) | 1:50 | (1:10–1:131.3) | 0.003 |
| sVNT | GenScript (% inhibition) | 61.4 | (50.9–79.0) | 93.8 | (86.1–95.6) | <0.001 | |
| GenBody (% inhibition) | 71.9 | (58.3–82.9) | 91.8 | (90.0–97.4) | <0.001 | ||
| CLIA | Roche (U/mL) | 528.0 | (369.3–803.3) | 846.0 | (601.5–1190.8) | 0.021 | |
| Roche (BAU/mL) | 542.8 | (379.6–825.7) | 869.7 | (618.3–1224.1) | 0.021 | ||
| Siemens (U/mL) | 4.9 | (3.3–10.0) | 23.7 | (17.9–57.8) | <0.001 | ||
| Siemens (BAU/mL) | 106.4 | (72.4–218.4) | 516.3 | (390.8–1260.9) | <0.001 | ||
| EIA | LG RBD (ratio) | 2.69 | (2.44–3.41) | 4.96 | (4.17–5.45) | <0.001 | |
| LG S1 (ratio) | 4.58 | (4.23–5.06) | 5.88 | (5.84–6.04) | <0.001 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Jung, J.; Lee, J.H.; Lee, D.-G.; Kim, Y.B.; Oh, E.-J. Comparison of Six Serological Immunoassays for the Detection of SARS-CoV-2 Neutralizing Antibody Levels in the Vaccinated Population. Viruses 2022, 14, 946. https://doi.org/10.3390/v14050946
Lee H-J, Jung J, Lee JH, Lee D-G, Kim YB, Oh E-J. Comparison of Six Serological Immunoassays for the Detection of SARS-CoV-2 Neutralizing Antibody Levels in the Vaccinated Population. Viruses. 2022; 14(5):946. https://doi.org/10.3390/v14050946
Chicago/Turabian StyleLee, Hee-Jung, Jin Jung, Ji Hyun Lee, Dong-Gun Lee, Young Bong Kim, and Eun-Jee Oh. 2022. "Comparison of Six Serological Immunoassays for the Detection of SARS-CoV-2 Neutralizing Antibody Levels in the Vaccinated Population" Viruses 14, no. 5: 946. https://doi.org/10.3390/v14050946
APA StyleLee, H.-J., Jung, J., Lee, J. H., Lee, D.-G., Kim, Y. B., & Oh, E.-J. (2022). Comparison of Six Serological Immunoassays for the Detection of SARS-CoV-2 Neutralizing Antibody Levels in the Vaccinated Population. Viruses, 14(5), 946. https://doi.org/10.3390/v14050946







