Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases
Abstract
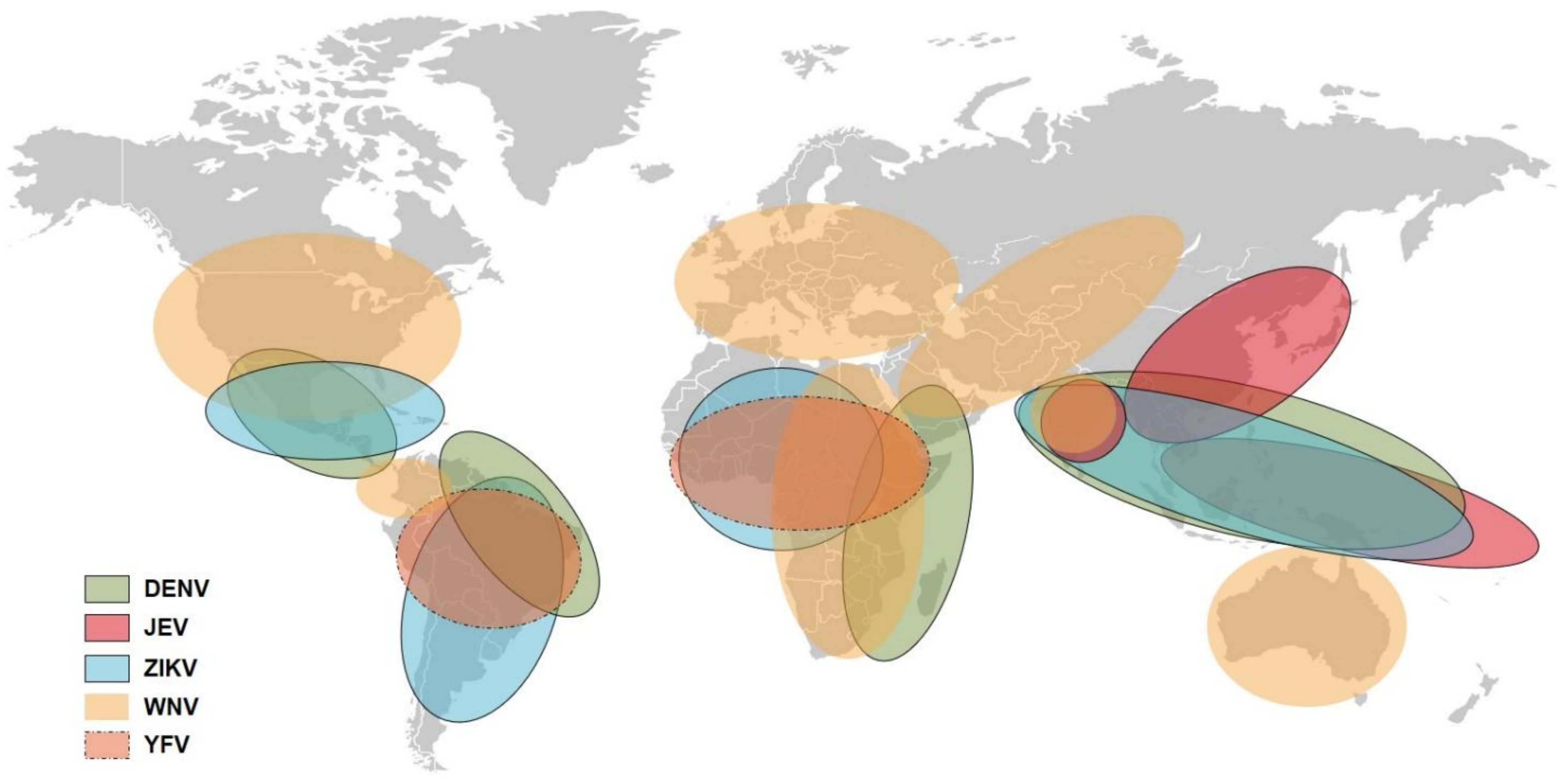
:1. Introduction
2. The Antigenic Relationships among the Five Mosquito-Borne Flaviviruses
3. Cross-Reactive Immunity between DENV and ZIKV
3.1. The Impact of Prior DENV Immunity on ZIKV Infection
- (1)
- In vitro serological experiment
- (2)
- The in vivo mouse model
- (3)
- In vivo non-human primate animal model
- (4)
- Study in humans
3.2. The Impact of Prior ZIKV Immunity on DENV Infection
- (1)
- In vitro serological experiment
- (2)
- In vivo mouse model
4. Cross-Reactive Immunity between YFV and DENV
4.1. The Impact of Prior YFV Immunity on DENV Infection
- (1)
- In vitro serological experiment
- (2)
- In vivo mouse model
- (3)
- Study in humans
4.2. The Impact of Prior DENV Immunity on YFV Infection
5. Cross-Reactive Immunity between JEV and ZIKV
5.1. The Impact of Prior JEV Immunity on ZIKV Infection
- (1)
- In vitro serological experiment
- (2)
- In vivo mouse model
- (3)
- Study in humans
5.2. The Impact of Prior ZIKV Immunity on JEV Infection
6. Cross-Reactive Immunity between JEV and DENV
6.1. The Impact of Prior JEV Immunity on DENV Infection
- (1)
- In vitro serological experiment
- (2)
- In vivo mouse model
- (3)
- Study in humans
6.2. The Impact of Prior DENV Immunity on JEV Infection
7. Cross-Reactive Immunity between JEV and WNV
7.1. The Impact of Prior JEV Immunity on WNV Infection
- (1)
- In vitro experiment
- (2)
- In vivo mouse model
7.2. The Impact of Prior WNV Immunity on JEV Infection
8. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Moreira, M.E.L.; Nielsen-Saines, K.; Brasil, P.; Kerin, T.; Damasceno, L.; Pone, M.; Carvalho, L.M.A.; Pone, S.M.; Vasconcelos, Z.; Ribeiro, I.P.; et al. Neurodevelopment in Infants Exposed to Zika Virus In Utero. N. Engl. J. Med. 2018, 379, 2377–2379. [Google Scholar] [CrossRef]
- Wang, X.; Li, T.; Shu, Y.; Zhang, J.; Shan, X.; Li, D.; Ma, D.; Long, S.; Pan, Y.; Chen, J.; et al. Clinical Characteristics and Risk Factors for Severe Dengue Fever in Xishuangbanna, During the Dengue Outbreak in 2019. Front. Microbiol. 2022, 13, 739970. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Breitbach, M.E.; Newman, C.M.; Dudley, D.M.; Stewart, L.M.; Aliota, M.T.; Koenig, M.R.; Shepherd, P.M.; Yamamoto, K.; Crooks, C.M.; Young, G.; et al. Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLoS Pathog. 2019, 15, e1007766. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Tan, N.W.W.; Chan, K.W.K.; Vasudevan, S.G. Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J. Infect. Dis. 2019, 219, 223–233. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, Y.; Zhao, F.; Tarbe, M.; Zhou, S.; Wang, W.; Zhang, S.; Zhang, W.; Xu, Q.; Shi, L.; et al. The pre-existing cellular immunity to Japanese encephalitis virus heterotypically protects mice from Zika virus infection. Sci. Bull. 2020, 65, 402–409. [Google Scholar] [CrossRef]
- Collins, M.H.; Metz, S.W. Progress and Works in Progress: Update on Flavivirus Vaccine Development. Clin. Ther. 2017, 39, 1519–1536. [Google Scholar] [CrossRef] [Green Version]
- Pierson, T.C.; Diamond, M.S. The continued threat of emerging flaviviruses. Nat. Microbiol. 2020, 5, 796–812. [Google Scholar] [CrossRef]
- Guzman, M.G.; Harris, E. Dengue. Lancet 2015, 385, 453–465. [Google Scholar] [CrossRef]
- Rockstroh, A.; Moges, B.; Berneck, B.S.; Sattler, T.; Revilla-Fernandez, S.; Schmoll, F.; Pacenti, M.; Sinigaglia, A.; Barzon, L.; Schmidt-Chanasit, J.; et al. Specific detection and differentiation of tick-borne encephalitis and West Nile virus induced IgG antibodies in humans and horses. Transbound. Emerg. Dis. 2019, 66, 1701–1708. [Google Scholar] [CrossRef]
- Blazquez, A.B.; Escribano-Romero, E.; Martin-Acebes, M.A.; Petrovic, T.; Saiz, J.C. Limited susceptibility of mice to Usutu virus (USUV) infection and induction of flavivirus cross-protective immunity. Virology 2015, 482, 67–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valderrama, A.; Diaz, Y.; Lopez-Verges, S. Interaction of Flavivirus with their mosquito vectors and their impact on the human health in the Americas. Biochem. Biophys. Res. Commun. 2017, 492, 541–547. [Google Scholar] [CrossRef]
- Hegde, N.R.; Gore, M.M. Japanese encephalitis vaccines: Immunogenicity, protective efficacy, effectiveness, and impact on the burden of disease. Hum. Vacc. Immunother. 2017, 13, 1320–1337. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhang, Y.; Jia, R.; Wang, M.; Yin, Z.; Cheng, A. Structure and function of capsid protein in flavivirus infection and its applications in the development of vaccines and therapeutics. Vet. Res. 2021, 52, 98. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, A.; Marini, V.; di Gennaro, A.; Ronchi, G.F.; Casaccia, C.; Carelli, G.; Passantino, G.; D’Alterio, N.; D’Innocenzo, V.; Savini, G.; et al. Antigenic relationship among zoonotic flaviviruses from Italy. Infect. Genet. Evol. 2019, 68, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Huber, R.G.; Bond, P.J.; Grad, Y.H.; Camerini, D.; Maurer-Stroh, S.; Lipsitch, M. Systematic analysis of protein identity between Zika virus and other arthropod-borne viruses. Bull. World Health Organ. 2017, 95, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Poudyal, S.; Rehman, A.; Hasan, S.S. Structural and biochemical insights into flavivirus proteins. Virus Res. 2021, 296, 198343. [Google Scholar] [CrossRef]
- Hasan, S.S.; Sevvana, M.; Kuhn, R.J.; Rossmann, M.G. Structural biology of Zika virus and other flaviviruses. Nat. Struct. Mol. Biol. 2018, 25, 13–20. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Q.; Qi, J.; Shi, Y.; Yan, J.; Gao, G.F. Molecular basis of antibody-mediated neutralization and protection against flavivirus. IUBMB Life 2016, 68, 783–791. [Google Scholar] [CrossRef]
- Swanstrom, J.A.; Plante, J.A.; Plante, K.S.; Young, E.F.; McGowan, E.; Gallichotte, E.N.; Widman, D.G.; Heise, M.T.; de Silva, A.M.; Baric, R.S. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. mBio 2016, 7, e1116–e1123. [Google Scholar] [CrossRef] [Green Version]
- Dejnirattisai, W.; Supasa, P.; Wongwiwat, W.; Rouvinski, A.; Barba-Spaeth, G.; Duangchinda, T.; Sakuntabhai, A.; Cao-Lormeau, V.; Malasit, P.; Rey, F.A.; et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat. Immunol. 2016, 17, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Bardina, S.V.; Bunduc, P.; Tripathi, S.; Duehr, J.; Frere, J.J.; Brown, J.A.; Nachbagauer, R.; Foster, G.A.; Krysztof, D.; Tortorella, D.; et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 2017, 356, 175–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, P.; Narvekar, P.; Montoya, M.; Michlmayr, D.; Balmaseda, A.; Coloma, J.; Harris, E. Primary and Secondary Dengue Virus Infections Elicit Similar Memory B-Cell Responses, but Breadth to Other Serotypes and Cross-Reactivity to Zika Virus is Higher in Secondary Dengue. J. Infect. Dis. 2020, 222, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Collazo, C.; Perez-Guzman, E.X.; Pantoja, P.; Hassert, M.A.; Rodriguez, I.V.; Giavedoni, L.; Hodara, V.; Parodi, L.; Cruz, L.; Arana, T.; et al. Effective control of early Zika virus replication by Dengue immunity is associated to the length of time between the 2 infections but not mediated by antibodies. PLoS Negl. Trop. Dis. 2020, 14, e8285. [Google Scholar] [CrossRef]
- Wen, J.; Elong, N.A.; Regla-Nava, J.A.; Kim, K.; Gorman, M.J.; Diamond, M.S.; Shresta, S. Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat. Commun. 2017, 8, 1459. [Google Scholar] [CrossRef] [Green Version]
- Wen, J.; Wang, Y.T.; Valentine, K.M.; Dos, S.A.R.; Xu, Z.; Regla-Nava, J.A.; Ngono, A.E.; Young, M.P.; Ferreira, L.; Shresta, S. CD4(+) T Cells Cross-Reactive with Dengue and Zika Viruses Protect against Zika Virus Infection. Cell Rep. 2020, 31, 107566. [Google Scholar] [CrossRef]
- Regla-Nava, J.A.; Ngono, A.E.; Viramontes, K.M.; Huynh, A.; Wang, Y.; Nguyen, A.T.; Salgado, R.; Mamidi, A.; Kim, K.; Diamond, M.S.; et al. Cross-reactive Dengue virus-specific CD8+ T cells protect against Zika virus during pregnancy. Nat. Commun. 2018, 9, 3042. [Google Scholar] [CrossRef] [Green Version]
- Pantoja, P.; Pérez-Guzmán, E.X.; Rodríguez, I.V.; White, L.J.; González, O.; Serrano, C.; Giavedoni, L.; Hodara, V.; Cruz, L.; Arana, T.; et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat. Commun. 2017, 8, 15674. [Google Scholar] [CrossRef]
- Larocca, R.A.; Abbink, P.; Ventura, J.D.; Chandrashekar, A.; Mercado, N.; Li, Z.; Borducchi, E.; de la Barrera, R.A.; Eckels, K.H.; Modjarrad, K.; et al. Impact of prior Dengue immunity on Zika vaccine protection in rhesus macaques and mice. PLoS Pathog. 2021, 17, e1009673. [Google Scholar] [CrossRef]
- Reynolds, C.J.; Watber, P.; Santos, C.N.O.; Ribeiro, D.R.; Alves, J.C.; Fonseca, A.B.L.; Bispo, A.J.B.; Porto, R.L.S.; Bokea, K.; de Jesus, A.M.R.; et al. Strong CD4 T Cell Responses to Zika Virus Antigens in a Cohort of Dengue Virus Immune Mothers of Congenital Zika Virus Syndrome Infants. Front. Immunol. 2020, 11, 185. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, M.S.; Freitas, L.P.; Cruz, O.G.; Brasil, P.; Bastos, L.S. Association of past dengue fever epidemics with the risk of Zika microcephaly at the population level in Brazil. Sci. Rep. 2020, 10, 1752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pedroso, C.; Fischer, C.; Feldmann, M.; Sarno, M.; Luz, E.; Moreira-Soto, A.; Cabral, R.; Netto, E.M.; Brites, C.; Kümmerer, B.M.; et al. Cross-Protection of Dengue Virus Infection against Congenital Zika Syndrome, Northeastern Brazil. Emerg. Infect. Dis. 2019, 25, 1485–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hattakam, S.; Elong, N.A.; McCauley, M.; Shresta, S.; Yamabhai, M. Repeated exposure to dengue virus elicits robust cross neutralizing antibodies against Zika virus in residents of Northeastern Thailand. Sci. Rep. 2021, 11, 9634. [Google Scholar] [CrossRef] [PubMed]
- Kawiecki, A.B.; Christofferson, R.C. Zika Virus–Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication In Vitro. J. Infect. Dis. 2016, 214, 1357–1360. [Google Scholar] [CrossRef] [Green Version]
- Katzelnick, L.C.; Zambrana, J.V.; Elizondo, D.; Collado, D.; Garcia, N.; Arguello, S.; Mercado, J.C.; Miranda, T.; Ampie, O.; Mercado, B.L.; et al. Dengue and Zika virus infections in children elicit cross-reactive protective and enhancing antibodies that persist long term. Sci. Transl. Med. 2021, 13, g9478. [Google Scholar] [CrossRef] [PubMed]
- Langerak, T.; Kasbergen, L.; Chandler, F.; Brinkman, T.; Faerber, Z.; Phalai, K.; Ulbert, S.; Rockstroh, A.; Bruin, E.; Koopmans, M.; et al. Zika Virus Antibody Titers Three Years after Confirmed Infection. Viruses 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Stettler, K.; Beltramello, M.; Espinosa, D.A.; Graham, V.; Cassotta, A.; Bianchi, S.; Vanzetta, F.; Minola, A. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science 2016, 353, 823–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saron, W.; Rathore, A.; Ting, L.; Ooi, E.E.; Low, J.; Abraham, S.N.; St, J.A. Flavivirus serocomplex cross-reactive immunity is protective by activating heterologous memory CD4 T cells. Sci. Adv. 2018, 4, r4297. [Google Scholar] [CrossRef] [Green Version]
- Luppe, M.J.; Verro, A.T.; Barbosa, A.S.; Nogueira, M.L.; Undurraga, E.A.; da Silva, N.S. Yellow fever (YF) vaccination does not increase dengue severity: A retrospective study based on 11,448 dengue notifications in a YF and dengue endemic region. Travel Med. Infect. Dis. 2019, 30, 25–31. [Google Scholar] [CrossRef]
- Grifoni, A.; Voic, H.; Dhanda, S.K.; Kidd, C.K.; Brien, J.D.; Buus, S.; Stryhn, A.; Durbin, A.P.; Whitehead, S.; Diehl, S.A.; et al. T Cell Responses Induced by Attenuated Flavivirus Vaccination Are Specific and Show Limited Cross-Reactivity with Other Flavivirus Species. J. Virol. 2020, 94, e00089-20. [Google Scholar] [CrossRef]
- Izurieta, R.O.; Macaluso, M.; Watts, D.M.; Tesh, R.B.; Guerra, B.; Cruz, L.M.; Galwankar, S.; Vermund, S.H. Anamnestic immune response to dengue and decreased severity of yellow Fever. J. Glob. Infect. Dis. 2009, 1, 111–116. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Lang, X.; Yu, J.; Zhu, L.; Qin, Z.; Liu, X.; Chen, P.; Dai, C.; Chen, T.; Li, X.; et al. The effects of Japanese encephalitis virus antibodies on Zika virus infection. Med. Microbiol. Immun. 2020, 209, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Duan, Z.; Zhou, W.; Zou, W.; Jin, S.; Li, D.; Chen, X.; Zhou, Y.; Yang, L.; Zhang, Y.; et al. Japanese encephalitis virus-primed CD8+ T cells prevent antibody-dependent enhancement of Zika virus pathogenesis. J. Exp. Med. 2020, 217, e20192152. [Google Scholar] [CrossRef] [PubMed]
- Tarbe, M.; Dong, W.; Hu, G.; Xu, Y.; Sun, J.; Grayo, S.; Chen, X.; Qin, C.; Zhao, J.; Liu, L.; et al. Japanese Encephalitis Virus Vaccination Elicits Cross-Reactive HLA-Class I-Restricted CD8 T Cell Response Against Zika Virus Infection. Front. Immunol. 2020, 11, 577546. [Google Scholar] [CrossRef]
- Wang, R.; Zhen, Z.; Turtle, L.; Hou, B.; Li, Y.; Wu, N.; Gao, N.; Fan, D.; Chen, H.; An, J. T cell immunity rather than antibody mediates cross-protection against Zika virus infection conferred by a live attenuated Japanese encephalitis SA14-14-2 vaccine. Appl. Microbiol. Biotechnol. 2020, 104, 6779–6789. [Google Scholar] [CrossRef]
- Lima, N.S.; Moon, D.; Darko, S.; De La Barrera, R.A.; Lin, L.; Koren, M.A.; Jarman, R.G.; Eckels, K.H.; Thomas, S.J.; Michael, N.L.; et al. Pre-existing Immunity to Japanese Encephalitis Virus Alters CD4 T Cell Responses to Zika Virus Inactivated Vaccine. Front. Immunol. 2021, 12, 640190. [Google Scholar] [CrossRef]
- Saito, Y.; Moi, M.L.; Takeshita, N.; Lim, C.; Shiba, H.; Hosono, K.; Saijo, M.; Kurane, I.; Takasaki, T. Japanese encephalitis vaccine-facilitated dengue virus infection-enhancement antibody in adults. BMC Infect. Dis. 2016, 16, 578. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gao, N.; Fan, D.; Chen, H.; Sheng, Z.; Fu, S.; Liang, G.; An, J. Cross-protection induced by Japanese encephalitis vaccines against different genotypes of Dengue viruses in mice. Sci. Rep. 2016, 6, 19953. [Google Scholar] [CrossRef]
- Komiya, T.; Honda-Okubo, Y.; Baldwin, J.; Petrovsky, N. An Advax-Adjuvanted Inactivated Cell-Culture Derived Japanese Encephalitis Vaccine Induces Broadly Neutralising Anti-Flavivirus Antibodies, Robust Cellular Immunity and Provides Single Dose Protection. Vaccines 2021, 9, 1235. [Google Scholar] [CrossRef]
- Anderson, K.B.; Gibbons, R.V.; Thomas, S.J.; Rothman, A.L.; Nisalak, A.; Berkelman, R.L.; Libraty, D.H.; Endy, T.P.; Singh, S.K. Preexisting Japanese encephalitis virus neutralizing antibodies and increased symptomatic dengue illness in a school-based cohort in Thailand. PLoS Negl. Trop. Dis. 2011, 5, e1311. [Google Scholar] [CrossRef]
- Verma, A.; Jain, A.; Kumar, C.; Agarwal, M.; Kumar, R. Effect of prior dengue infection on severity and outcome of Japanese encephalitis. Eur. J. Clin. Microbiol. 2018, 37, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Yamshchikov, G.; Borisevich, V.; Kwok, C.W.; Nistler, R.; Kohlmeier, J.; Seregin, A.; Chaporgina, E.; Benedict, S.; Yamshchikov, V. The suitability of yellow fever and Japanese encephalitis vaccines for immunization against West Nile virus. Vaccine 2005, 23, 4785–4792. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Zhang, J.; Liu, W.; Zhao, Q.; Zhang, F.; Wu, X.; Yang, H.; Ly, H.; Cao, W. Short Report: Failure of Japanese Encephalitis Vaccine and Infection in Inducing Neutralizing Antibodies against West Nile Virus, People’s Republic of China. Am. J. Trop. Med. Hyg. 2008, 78, 999–1001. [Google Scholar] [CrossRef]
- Li, S.; Li, X.; Zhao, H.; Jiang, T.; Deng, Y.; Yu, X.; Zhu, Q.; Qin, E.; Qin, C. Cross protection against lethal West Nile virus challenge in mice immunized with recombinant E protein domain III of Japanese encephalitis virus. Immunol. Lett. 2011, 138, 156–160. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, Z.; Zhang, Y.; Liu, J.; Deng, C.; Shi, P.; Yuan, Z.; Ye, H.; Zhang, B. A replication-defective Japanese encephalitis virus (JEV) vaccine candidate with NS1 deletion confers dual protection against JEV and West Nile virus in mice. npj Vaccines 2020, 5, 73. [Google Scholar] [CrossRef]
- Tesh, R.B.; Travassos, D.R.A.; Guzman, H.; Araujo, T.P.; Xiao, S.Y. Immunization with heterologous flaviviruses protective against fatal West Nile encephalitis. Emerg. Infect. Dis. 2002, 8, 245–251. [Google Scholar] [CrossRef] [Green Version]
- Lim, C.; Takasaki, T.; Kotaki, A.; Kurane, I. Vero cell-derived inactivated West Nile (WN) vaccine induces protective immunity against lethal WN virus infection in mice and shows a facilitated neutralizing antibody response in mice previously immunized with Japanese encephalitis vaccine. Virology 2008, 374, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Takasaki, T.; Yabe, S.; Nerome, R.; Ito, M.; Yamada, K.; Kurane, I. Partial protective effect of inactivated Japanese encephalitis vaccine on lethal West Nile virus infection in mice. Vaccine 2003, 21, 4514–4518. [Google Scholar] [CrossRef]
- Petrovsky, N.; Larena, M.; Siddharthan, V.; Prow, N.A.; Hall, R.A.; Lobigs, M.; Morrey, J. An Inactivated Cell Culture Japanese Encephalitis Vaccine (JE-ADVAX) Formulated with Delta Inulin Adjuvant Provides Robust Heterologous Protection against West Nile Encephalitis via Cross-Protective Memory B Cells and Neutralizing Antibody. J. Virol. 2013, 87, 10324–10333. [Google Scholar] [CrossRef] [Green Version]
- Kaufusi, P.H.; Tseng, A.C.; Kelley, J.F.; Nerurkar, V.R. Selective Reactivity of Anti-Japanese Encephalitis Virus NS4B Antibody Towards Different Flaviviruses. Viruses 2020, 12, 212. [Google Scholar] [CrossRef] [Green Version]
- Martina, B.E.; Koraka, P.; van den Doel, P.; van Amerongen, G.; Rimmelzwaan, G.F.; Osterhaus, A.D.M.E. Immunization with West Nile virus envelope domain III protects mice against lethal infection with homologous and heterologous virus. Vaccine 2008, 26, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Zhao, J.; Yang, T.; Xu, Q.; Qin, Y.; Wang, W.; Wei, P.; Wu, D. Antibodies generated by immunization with the NS1 protein of West Nile virus confer partial protection against lethal Japanese encephalitis virus challenge. Vet. Microbiol. 2013, 166, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Kayesh, M.; Tsukiyama-Kohara, K. Mammalian animal models for dengue virus infection: A recent overview. Arch. Virol. 2022, 167, 31–44. [Google Scholar] [CrossRef]
- Lai, S.; Huang, Z.; Zhou, H.; Anders, K.L.; Perkins, T.A.; Yin, W.; Li, Y.; Mu, D.; Chen, Q.; Zhang, Z.; et al. The changing epidemiology of dengue in China, 1990–2014: A descriptive analysis of 25 years of nationwide surveillance data. BMC Med. 2015, 13, 100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.C.; Zhao, H.; Li, L.H.; Jiang, T.; Hong, W.X.; Wang, J.; Zhao, L.Z.; Yang, H.Q.; Ma, D.H.; Bai, C.H.; et al. Severe dengue outbreak in Yunnan, China, 2013. Int. J. Infect. Dis. 2014, 27, 4–6. [Google Scholar] [CrossRef] [Green Version]
- Gao, N.; Li, J.; Sheng, Z.; Chen, H.; Fan, D.; Wang, P.; An, J. Japanese encephalitis virus prM-E antigen immunization conferred protection against challenge by four different serotypes of Dengue viruses in mice. Appl. Microbiol. Biotechnol. 2019, 103, 4977–4986. [Google Scholar] [CrossRef]
- Dai, L.; Xu, K.; Li, J.; Huang, Q.; Song, J.; Han, Y.; Zheng, T.; Gao, P.; Lu, X.; Yang, H.; et al. Protective Zika vaccines engineered to eliminate enhancement of dengue infection via immunodominance switch. Nat. Immunol. 2021, 22, 958–968. [Google Scholar] [CrossRef]
- Shukla, R.; Beesetti, H.; Brown, J.A.; Ahuja, R.; Ramasamy, V.; Shanmugam, R.K.; Poddar, A.; Batra, G.; Krammer, F.; Lim, J.K.; et al. Dengue and Zika virus infections are enhanced by live attenuated dengue vaccine but not by recombinant DSV4 vaccine candidate in mouse models. eBioMedicine 2020, 60, 102991. [Google Scholar] [CrossRef]
| WNV (%) | ZIKV (%) | YFV (%) | DENV1 (%) | DENV2 (%) | DENV3 (%) | DENV4 (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PP | E | PP | E | PP | E | PP | E | PP | E | PP | E | PP | E | |
| JEV | 77 | 79 | 56 | 56 | 45 | 44 | 51 | 51 | 51 | 48 | 51 | 49 | 51 | 48 |
| WNV | 57 | 56 | 45 | 44 | 51 | 52 | 52 | 49 | 51 | 48 | 52 | 50 | ||
| ZIKV | 46 | 43 | 55 | 58 | 55 | 54 | 56 | 58 | 56 | 56 | ||||
| YFV | 45 | 43 | 45 | 44 | 45 | 42 | 45 | 40 | ||||||
| DENV1 | 72 | 69 | 78 | 78 | 69 | 64 | ||||||||
| DENV2 | 72 | 69 | 70 | 64 | ||||||||||
| DENV3 | 70 | 63 | ||||||||||||
| Model | Effect | Probable Mechanism | |
|---|---|---|---|
| DENV–ZIKV | mouse | protection [5] | cross-reactive sera: no effect/enhancement [22] cross-reactive T cells: protection [25,26] |
| human | multiple exposures: protective effect for newborns [32] | high titers of cross-reactive NAbs [33] | |
| ZIKV–DENV | mouse | previous infection: protection [5] INV vaccination: enhancement [5] | cross-reactive sera: enhancement [5] cross-reactive T cells: deserve more research |
| YFV–DENV | mouse | not observed [38] | cross-reactive sera: no cross-neutralizing ability [38] cross-reactive T cells: fail to protect [38] |
| human | no effect on the clinical symptoms [39] | cross-reactive CD4+ and CD8+ T cells: limited [40] | |
| JEV–ZIKV | mouse | protection [6,45] | cross-reactive sera: enhancement [42,43] cross-reactive T cells: protective, but depended on the immune conditions [6,43,45] |
| ZIKV–JEV | mouse | depend on more investigations | cross-reactive sera: enhancement [43] cross-reactive T cells: protection [43] |
| JEV–DENV | mouse | protection [38,48] | cooperation of cross-reactive antibodies and T cells [38] |
| human | increase the probability of symptomatic infection [50] | JEV antibody is associated with occurence of symptom [50]. | |
| DENV–JEV | human | dengue IgG-positive patients have a significantly better outcome [51] | not reported |
| JEV–WNV | mouse | previous infection: protection [56] INV or protein vaccination: protection [57,58,59] | cross-reactive CD8+ T cells: little effect [59] cross-reactive B or CD4+ T cells: protection [59] |
| WNV–JEV | mouse | INV or protein vaccination: protection [61,62] | not reported |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, B.; Chen, H.; Gao, N.; An, J. Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases. Viruses 2022, 14, 1213. https://doi.org/10.3390/v14061213
Hou B, Chen H, Gao N, An J. Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases. Viruses. 2022; 14(6):1213. https://doi.org/10.3390/v14061213
Chicago/Turabian StyleHou, Baohua, Hui Chen, Na Gao, and Jing An. 2022. "Cross-Reactive Immunity among Five Medically Important Mosquito-Borne Flaviviruses Related to Human Diseases" Viruses 14, no. 6: 1213. https://doi.org/10.3390/v14061213






