Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19—BNT162b2 mRNA Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Samples Collection
2.3. Analytical Procedures for Evaluation of Immunogenicity
2.4. Statistical Analyses
3. Results
3.1. Demographic and Clinical Characteristics of Lung Transplant Recipients
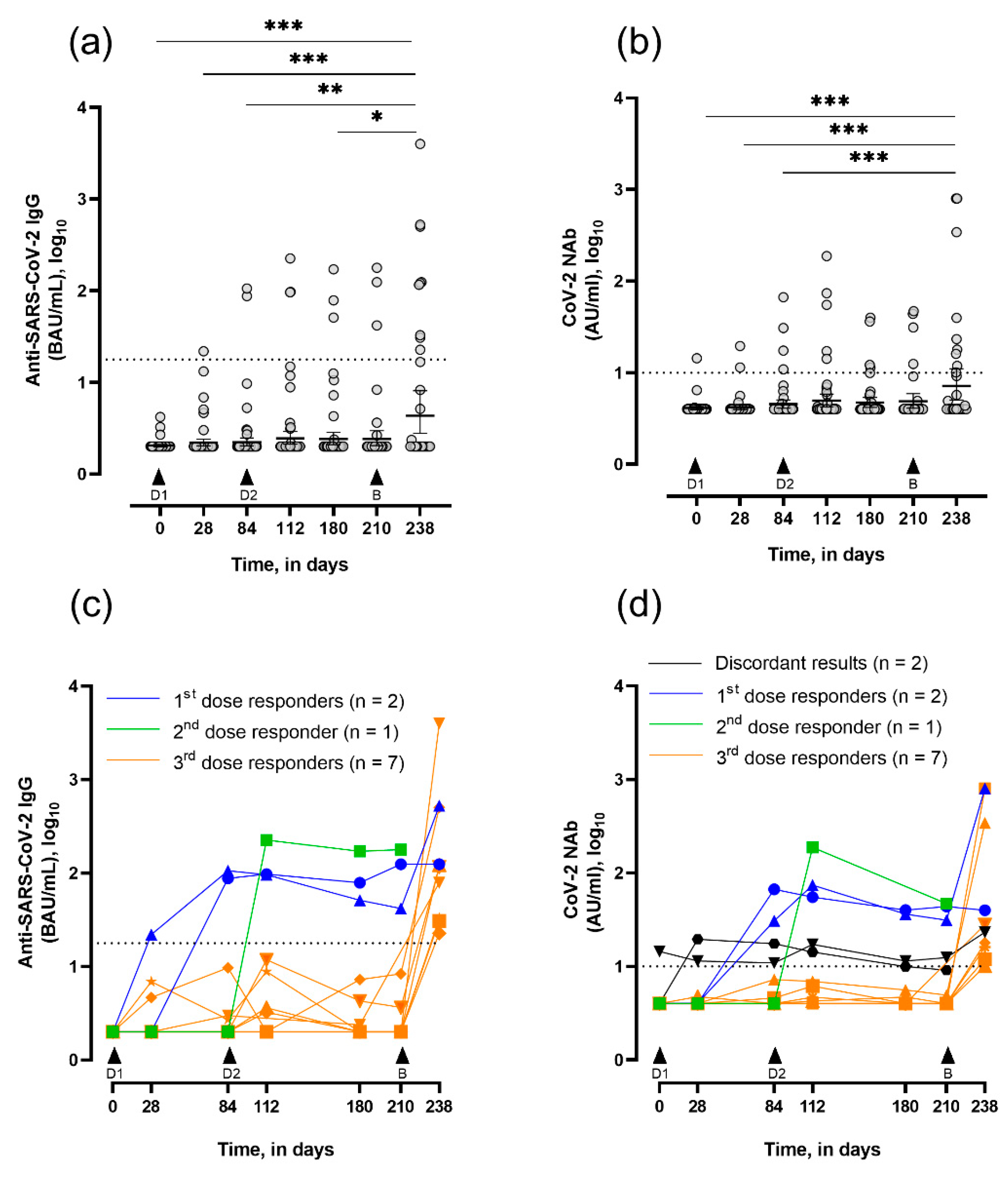
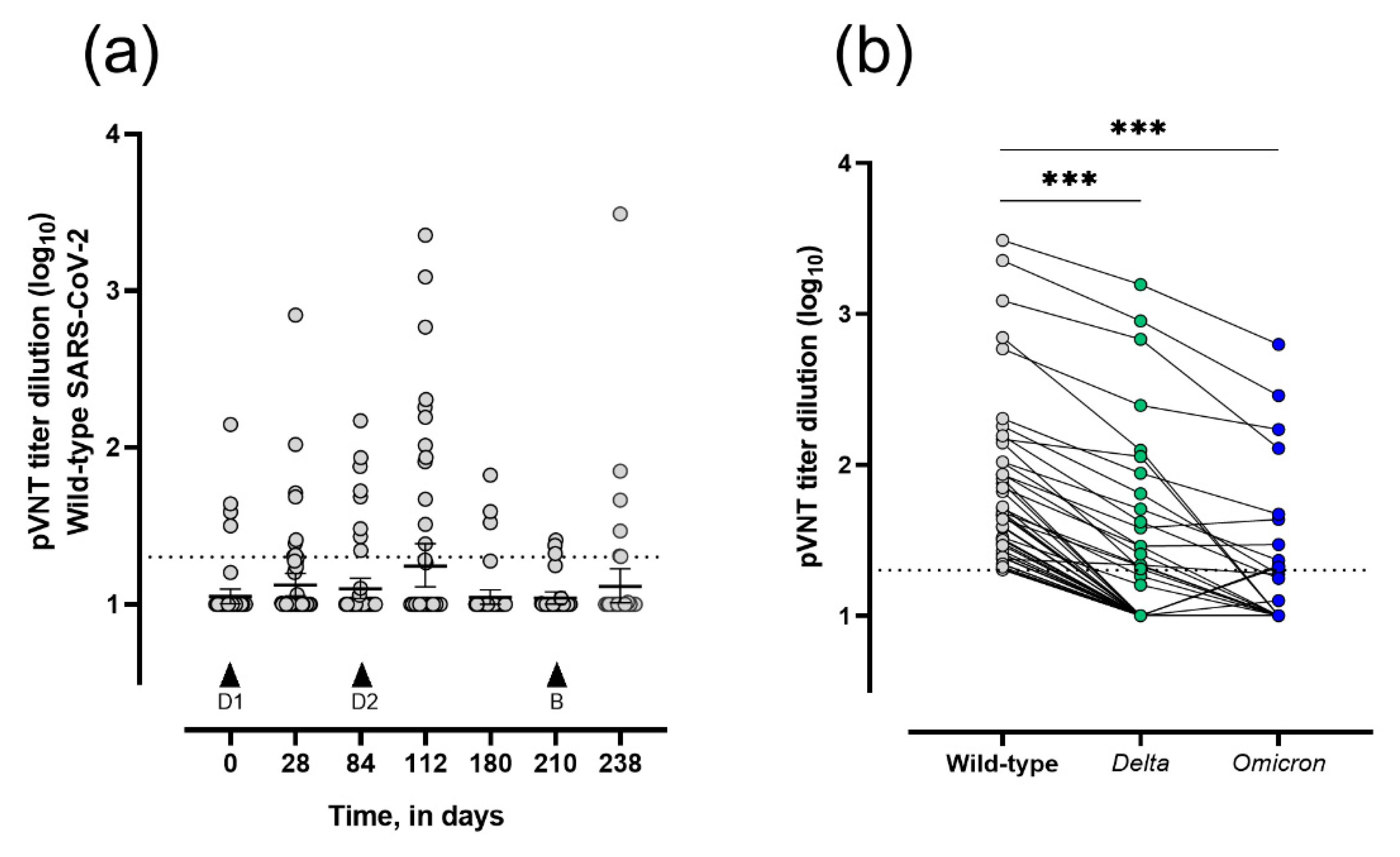
3.2. Seroconversion and Neutralizing Capacity in Vaccinated LTRs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Messika, J.; Eloy, P.; Roux, A.; Hirschi, S.; Nieves, A.; Le Pavec, J.; Senechal, A.; Saint Raymond, C.; Carlier, N.; Demant, X.; et al. COVID-19 in Lung Transplant Recipients. Transplantation 2021, 105, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Myers, C.N.; Scott, J.H.; Criner, G.J.; Cordova, F.C.; Mamary, A.J.; Marchetti, N.; Shenoy, K.V.; Galli, J.A.; Mulhall, P.D.; Brown, J.C.; et al. COVID-19 in lung transplant recipients. Transpl. Infect. Dis. 2020, 22, e13364. [Google Scholar] [CrossRef] [PubMed]
- Scharringa, S.; Hoffman, T.; van Kessel, D.A.; Rijkers, G.T. Vaccination and their importance for lung transplant recipients in a COVID-19 world. Expert Rev. Clin. Pharm. 2021, 14, 1413–1425. [Google Scholar] [CrossRef] [PubMed]
- Hallett, A.M.; Greenberg, R.S.; Boyarsky, B.J.; Shah, P.D.; Ou, M.T.; Teles, A.T.; Krach, M.R.; Lopez, J.I.; Werbel, W.A.; Avery, R.K.; et al. SARS-CoV-2 messenger RNA vaccine antibody response and reactogenicity in heart and lung transplant recipients. J. Heart Lung Transpl. 2021, 40, 1579–1588. [Google Scholar] [CrossRef]
- Havlin, J.; Svorcova, M.; Dvorackova, E.; Lastovicka, J.; Lischke, R.; Kalina, T.; Hubacek, P. Immunogenicity of BNT162b2 mRNA COVID-19 vaccine and SARS-CoV-2 infection in lung transplant recipients. J. Heart Lung Transpl. 2021, 40, 754–758. [Google Scholar] [CrossRef]
- Narasimhan, M.; Mahimainathan, L.; Clark, A.E.; Usmani, A.; Cao, J.; Araj, E.; Torres, F.; Sarode, R.; Kaza, V.; Lacelle, C.; et al. Serological Response in Lung Transplant Recipients after Two Doses of SARS-CoV-2 mRNA Vaccines. Vaccines 2021, 9, 708. [Google Scholar] [CrossRef]
- Peled, Y.; Ram, E.; Lavee, J.; Sternik, L.; Segev, A.; Wieder-Finesod, A.; Mandelboim, M.; Indenbaum, V.; Levy, I.; Raanani, E.; et al. BNT162b2 vaccination in heart transplant recipients: Clinical experience and antibody response. J. Heart Lung Transpl. 2021, 40, 759–762. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Schneitler, S.; Reichert, M.C.; Wilkens, H.; Sester, U.; Sester, M.; Mihm, J. Cellular immunity predominates over humoral immunity after homologous and heterologous mRNA and vector-based COVID-19 vaccine regimens in solid organ transplant recipients. Am. J. Transpl. 2021, 21, 3990–4002. [Google Scholar] [CrossRef]
- Pozzetto, B.; Legros, V.; Djebali, S.; Barateau, V.; Guibert, N.; Villard, M.; Peyrot, L.; Allatif, O.; Fassier, J.B.; Massardier-Pilonchery, A.; et al. Immunogenicity and efficacy of heterologous ChAdOx1-BNT162b2 vaccination. Nature 2021, 600, 701–706. [Google Scholar] [CrossRef]
- Liu, X.; Shaw, R.H.; Stuart, A.S.V.; Greenland, M.; Aley, P.K.; Andrews, N.J.; Cameron, J.C.; Charlton, S.; Clutterbuck, E.A.; Collins, A.M.; et al. Safety and immunogenicity of heterologous versus homologous prime-boost schedules with an adenoviral vectored and mRNA COVID-19 vaccine (Com-COV): A single-blind, randomised, non-inferiority trial. Lancet 2021, 398, 856–869. [Google Scholar] [CrossRef]
- European Medicines Agency. Heterologous Primary and Booster COVID-19 Vaccination, Evidence Based Regulatory Considerations (EMA/349565/2021). Issued on 13 December 2021. Available online: https://www.ema.europa.eu/en/documents/report/heterologous-primary-booster-covid-19-vaccination-evidence-based-regulatory-considerations_en.pdf (accessed on 3 March 2022).
- Favresse, J.; Bayart, J.L.; Mullier, F.; Dogne, J.M.; Closset, M.; Douxfils, J. Early antibody response in health-care professionals after two doses of SARS-CoV-2 mRNA vaccine (BNT162b2). Clin. Microbiol. Infect. 2021, 27, 1351.e5–1351.e7. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Gillot, C.; Di Chiaro, L.; Eucher, C.; Elsen, M.; Van Eeckhoudt, S.; David, C.; Morimont, L.; Dogne, J.M.; Douxfils, J. Neutralizing Antibodies in COVID-19 Patients and Vaccine Recipients after Two Doses of BNT162b2. Viruses 2021, 13, 1364. [Google Scholar] [CrossRef]
- Gillot, C.; Favresse, J.; Maloteau, V.; Dogne, J.M.; Douxfils, J. Identification of SARS-CoV-2 neutralizing antibody with pseudotyped virus-based test on HEK293T hACE2 cells. Bio-Protocol 2022, 12, e4377. [Google Scholar] [CrossRef] [PubMed]
- Gillot, C.; Favresse, J.; Maloteau, V.; Dogne, J.M.; Douxfils, J. Dynamics of Neutralizing Antibody Responses Following Natural SARS-CoV-2 Infection and Correlation with Commercial Serologic Tests. A Reappraisal and Indirect Comparison with Vaccinated Subjects. Viruses 2021, 13, 2329. [Google Scholar] [CrossRef] [PubMed]
- Boyarsky, B.J.; Werbel, W.A.; Avery, R.K.; Tobian, A.A.R.; Massie, A.B.; Segev, D.L.; Garonzik-Wang, J.M. Antibody Response to 2-Dose SARS-CoV-2 mRNA Vaccine Series in Solid Organ Transplant Recipients. JAMA 2021, 325, 2204–2206. [Google Scholar] [CrossRef]
- Voysey, M.; Clemens, S.A.C.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: An interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021, 397, 99–111. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Douxfils, J. Reply to Schulte-Pelkum, J. Comment on “Favresse et al. Persistence of Anti-SARS-CoV-2 Antibodies Depends on the Analytical Kit: A Report for Up to 10 Months after Infection. Microorganisms 2021, 9, 556”. Microorganisms 2021, 9, 1849. [Google Scholar] [CrossRef]
- Bayart, J.L.; Morimont, L.; Closset, M.; Wieers, G.; Roy, T.; Gerin, V.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Ausselet, N.; et al. Confounding Factors Influencing the Kinetics and Magnitude of Serological Response Following Administration of BNT162b2. Microorganisms 2021, 9, 1340. [Google Scholar] [CrossRef]
- Favresse, J.; Bayart, J.L.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Wieers, G.; Laurent, C.; Dogne, J.M.; et al. Antibody titres decline 3-month post-vaccination with BNT162b2. Emerg. Microbes Infect. 2021, 10, 1495–1498. [Google Scholar] [CrossRef]
- Peled, Y.; Ram, E.; Lavee, J.; Segev, A.; Matezki, S.; Wieder-Finesod, A.; Halperin, R.; Mandelboim, M.; Indenbaum, V.; Levy, I.; et al. Third dose of the BNT162b2 vaccine in heart transplant recipients: Immunogenicity and clinical experience. J. Heart Lung Transpl. 2021, 41, 148–157. [Google Scholar] [CrossRef]
- Aslam, S.; Danziger-Isakov, L.; Mehra, M.R. COVID-19 vaccination immune paresis in heart and lung transplantation. J. Heart Lung Transpl. 2021, 40, 763–766. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. Issued on 28 December 2021. Available online: https://www.cdc.gov/vaccines/covid-19/downloads/summary-interim-clinical-considerations.pdf (accessed on 3 March 2022).
- Centre Fédéral d’Expertise des Soins de Santé (KCE). Rapid Review of the Evidence on a COVID-19 Booster Dose after a Primary Vaccination Schedule, Report for the TASK Force Vaccination. Issued on 17 August 2021. Available online: https://kce.fgov.be/sites/default/files/atoms/files/Third%20Covid-19%20vaccination_Report_DUTCH.pdf (accessed on 3 March 2022).
- Del Bello, A.; Abravanel, F.; Marion, O.; Couat, C.; Esposito, L.; Lavayssiere, L.; Izopet, J.; Kamar, N. Efficiency of a boost with a third dose of anti-SARS-CoV-2 messenger RNA-based vaccines in solid organ transplant recipients. Am. J. Transpl. 2021, 22, 322–323. [Google Scholar] [CrossRef] [PubMed]
- Werbel, W.A.; Boyarsky, B.J.; Ou, M.T.; Massie, A.B.; Tobian, A.A.R.; Garonzik-Wang, J.M.; Segev, D.L. Safety and Immunogenicity of a Third Dose of SARS-CoV-2 Vaccine in Solid Organ Transplant Recipients: A Case Series. Ann. Intern. Med. 2021, 174, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Benotmane, I.; Gautier, G.; Perrin, P.; Olagne, J.; Cognard, N.; Fafi-Kremer, S.; Caillard, S. Antibody Response After a Third Dose of the mRNA-1273 SARS-CoV-2 Vaccine in Kidney Transplant Recipients With Minimal Serologic Response to 2 Doses. JAMA 2021, 326, 1063–1065. [Google Scholar] [CrossRef] [PubMed]
- Massa, F.; Cremoni, M.; Gerard, A.; Grabsi, H.; Rogier, L.; Blois, M.; Couzin, C.; Hassen, N.B.; Rouleau, M.; Barbosa, S.; et al. Safety and cross-variant immunogenicity of a three-dose COVID-19 mRNA vaccine regimen in kidney transplant recipients. EBioMedicine 2021, 73, 103679. [Google Scholar] [CrossRef]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef]
- Bayart, J.L.; Douxfils, J.; Gillot, C.; David, C.; Mullier, F.; Elsen, M.; Eucher, C.; Van Eeckhoudt, S.; Roy, T.; Gerin, V.; et al. Waning of IgG, Total and Neutralizing Antibodies 6 Months Post-Vaccination with BNT162b2 in Healthcare Workers. Vaccines 2021, 9, 1092. [Google Scholar] [CrossRef]
- Favresse, J.; Eucher, C.; Elsen, M.; Gillot, C.; Van Eeckhoudt, S.; Dogne, J.M.; Douxfils, J. Persistence of Anti-SARS-CoV-2 Antibodies Depends on the Analytical Kit: A Report for Up to 10 Months after Infection. Microorganisms 2021, 9, 556. [Google Scholar] [CrossRef]
- Barrett, J.R.; Belij-Rammerstorfer, S.; Dold, C.; Ewer, K.J.; Folegatti, P.M.; Gilbride, C.; Halkerston, R.; Hill, J.; Jenkin, D.; Stockdale, L.; et al. Phase 1/2 trial of SARS-CoV-2 vaccine ChAdOx1 nCoV-19 with a booster dose induces multifunctional antibody responses. Nat. Med. 2021, 27, 279–288. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Ramasamy, M.N.; Minassian, A.M.; Ewer, K.J.; Flaxman, A.L.; Folegatti, P.M.; Owens, D.R.; Voysey, M.; Aley, P.K.; Angus, B.; Babbage, G.; et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021, 396, 1979–1993. [Google Scholar] [CrossRef]
- Schmidt, T.; Klemis, V.; Schub, D.; Mihm, J.; Hielscher, F.; Marx, S.; Abu-Omar, A.; Ziegler, L.; Guckelmus, C.; Urschel, R.; et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat. Med. 2021, 27, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Moss, P. The T cell immune response against SARS-CoV-2. Nat. Immunol. 2022, 23, 186–193. [Google Scholar] [CrossRef]
- Havlin, J.; Skotnicova, A.; Dvorackova, E.; Hubacek, P.; Svorcova, M.; Lastovicka, J.; Sediva, A.; Kalina, T.; Lischke, R. Impaired Humoral Response to Third Dose of BNT162b2 mRNA COVID-19 Vaccine Despite Detectable Spike Protein-specific T cells in Lung Transplant Recipients. Transplantation 2022, 106, e183–e184. [Google Scholar] [CrossRef]
- Dudreuilh, C.; Basu, S.; Scottà, C.; Dorling, A.; Lombardi, G. Potential Application of T-Follicular Regulatory Cell Therapy in Transplantation. Front. Immunol. 2021, 11, 612848. [Google Scholar] [CrossRef]
- Yan, L.; de Leur, K.; Hendriks, R.W.; van der Laan, L.J.W.; Shi, Y.; Wang, L.; Baan, C.C. T Follicular Helper Cells As a New Target for Immunosuppressive Therapies. Front. Immunol. 2017, 8, 1510. [Google Scholar] [CrossRef] [Green Version]
- Cui, D.; Tang, Y.; Jiang, Q.; Jiang, D.; Zhang, Y.; Lv, Y.; Xu, D.; Wu, J.; Xie, J.; Wen, C.; et al. Follicular Helper T Cells in the Immunopathogenesis of SARS-CoV-2 Infection. Front. Immunol. 2021, 12, 3806. [Google Scholar] [CrossRef]

| Participant Characteristics | LTRs (n = 49) |
|---|---|
| Age, years (median ± IQR) | 64 (60.5–68) |
| Female sex, n (%) | 24 (48.9) |
| White, n (%) | 47 (95.9) |
| Non-white, n (%) | 2 (4.1) |
| Initial respiratory disease, n (%) | |
| Chronic obstructive pulmonary disease | 34 (69.4) |
| Pulmonary fibrosis | 7 (14.3) |
| Other | 8 (16.3) |
| Types of lung transplantation | |
| Bi-pulmonary transplantation | 47 (95.9) |
| Mono-pulmonary transplantation | 2 (4.1) |
| Years since transplant, years (median ± IQR) | 3.8 (1.8–6.1) |
| Immunosuppressive regimen | |
| Corticosteroids (methylprednisolone), n (%) | |
| 2–6 mg/day | 41 (83.7) |
| ≥8 mg/day | 8 (16.3) |
| Calcineurin inhibitors, n (%) | |
| Tacrolimus | 43 (87.7) |
| Cyclosporine | 6 (12.2) |
| Others, n (%) | |
| Mycophenolate mofetil | 25 (51.0) |
| Azathioprine | 11 (22.4) |
| Everolimus | 8 (16.3) |
| Type of vaccines, n (%) | |
| Two-dose ChAdOx1 nCoV-19 vaccine | 49 (100) |
| BNT162b2 mRNA COVID-19 vaccine (3rd dose) | 32 (65.3) |
| LTR Participants | |||||
|---|---|---|---|---|---|
| Day of Collection | Anti-SARS-CoV-2 IgG (BAU/mL) | IgG-seropositive LTRs, n (%) | NAbs (AU/mL) | NAbs-seropositive LTRs, n (%) | IgG and/or NAbs Seropositive LTRs, n (%) |
| Day 0 (n = 49) | 2.1 (2.0–2.2) | 0 (0.0) | 4.3 (3.8–4.7) | 1 (2.0) | 1 (2.0) |
| Day 28 (n = 47) | 2.7 (1.9–3.8) | 1 (2.1) | 4.2 (3.9–4.5) | 2 (4.2) | 3 (6.4) |
| Day 84 (n = 49) | 6.1 (0.7–11.6) | 2 (4.0) | 6.2 (3.3–9.0) | 4 (8.2) | 4 (8.2) |
| Day 112 (n = 44) | 12.1 (0.4–23.8) | 3 (6.8) | 11.7 (2.3–21.0) | 5 (11.4) | 5 (11.4) |
| Day 180 (n = 42) a | 9.7 (0.6–18.7) | 3 (7.2) | 6.3 (3.8–8.8) | 4 (10.0) | 5 (11.9) |
| Day 210 (n = 32) b | 12.8 (0.0–26.4) | 3 (9.3) | 8.0 (3.7–12.2) | 4 (12.9) | 4 (12.5) |
| Day 238 (n = 28) | 197.8 (0.0–491.4) * | 9 (32.2) | 76.6 (0–159.6) # | 9 (32.2) | 9 (32.2) |
| LTRs, (n) | ||||||
|---|---|---|---|---|---|---|
| Seropositive with Neutralization Capacities (sVNT), (9) (%) | Seronegative, (19) (%) | p-Value | With WT Neutralization Capacities, (24) (%) | With Delta Neutralization Capacities, (13) (%) | With Omicron Neutralization Capacities, (9) (%) | |
| Age, years | ||||||
| 18–39 | 0 (0.0) | 1 (5.3) | ns | 1 (4.2) | 0 (0.0) | 1 (11.1) |
| 40–59 | 2 (22.2) | 2 (10.5) | 3 (12.5) | 1 (7.7) | 1 (11.1) | |
| ≥60 | 7 (77.8) | 16 (84.2) | 20 (83.3) | 12 (92.3) | 7 (77.8) | |
| Sex | ||||||
| Female | 6 (66.7) | 6 (31.6) | ns | 11 (45.8) | 5 (38.5) | 4 (44.4) |
| Male | 3 (33.3) | 13 (68.4) | 13 (54.2) | 8 (61.5) | 5 (55.6) | |
| Time since transplant, years | ||||||
| <1 | 0 (0.0) | 4 (21.0) | ns | 3 (12.5) | 1 (7.7) | 1 (11.1) |
| >1 | 9 (100) | 15 (79.0) | 21 (87.5) | 12 (92.3) | 8 (88.9) | |
| Initial respiratory disease | ||||||
| COPD | 8 (88.9) | 13 (68.4) | ns | 20 (83.4) | 11 (84.6) | 6 (66.7) |
| Pulmonary fibrosis | 0 (0.0) | 3 (15.8) | 2 (8.3) | 1 (7.7) | 1 (11.1) | |
| Other | 1 (11.1) | 3 (15.8) | 2 (8.3) | 1 (7.7) | 2 (22.2) | |
| Corticosteroids | ||||||
| 2–6 mg/day | 9 (100) | 15 (79.0) | ns | 23 (95.8) | 13 (100) | 9 (100) |
| ≥8 mg/day | 0 (0.0) | 4 (21.0) | 1 (4.2) | 0 (0.0) | 0 (0.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catry, E.; Favresse, J.; Gillot, C.; Bayart, J.-L.; Frérotte, D.; Dumonceaux, M.; Evrard, P.; Mullier, F.; Douxfils, J.; Carlier, F.M.; et al. Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19—BNT162b2 mRNA Vaccination. Viruses 2022, 14, 1470. https://doi.org/10.3390/v14071470
Catry E, Favresse J, Gillot C, Bayart J-L, Frérotte D, Dumonceaux M, Evrard P, Mullier F, Douxfils J, Carlier FM, et al. Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19—BNT162b2 mRNA Vaccination. Viruses. 2022; 14(7):1470. https://doi.org/10.3390/v14071470
Chicago/Turabian StyleCatry, Emilie, Julien Favresse, Constant Gillot, Jean-Louis Bayart, Damien Frérotte, Michel Dumonceaux, Patrick Evrard, François Mullier, Jonathan Douxfils, François M. Carlier, and et al. 2022. "Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19—BNT162b2 mRNA Vaccination" Viruses 14, no. 7: 1470. https://doi.org/10.3390/v14071470
APA StyleCatry, E., Favresse, J., Gillot, C., Bayart, J.-L., Frérotte, D., Dumonceaux, M., Evrard, P., Mullier, F., Douxfils, J., Carlier, F. M., & Closset, M. (2022). Lung Transplant Recipients Immunogenicity after Heterologous ChAdOx1 nCoV-19—BNT162b2 mRNA Vaccination. Viruses, 14(7), 1470. https://doi.org/10.3390/v14071470







