Development of a Novel RT-qPCR Detecting Method of Covert Mortality Nodavirus (CMNV) for the National Proficiency Test in Molecular Detection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and RNA Extraction
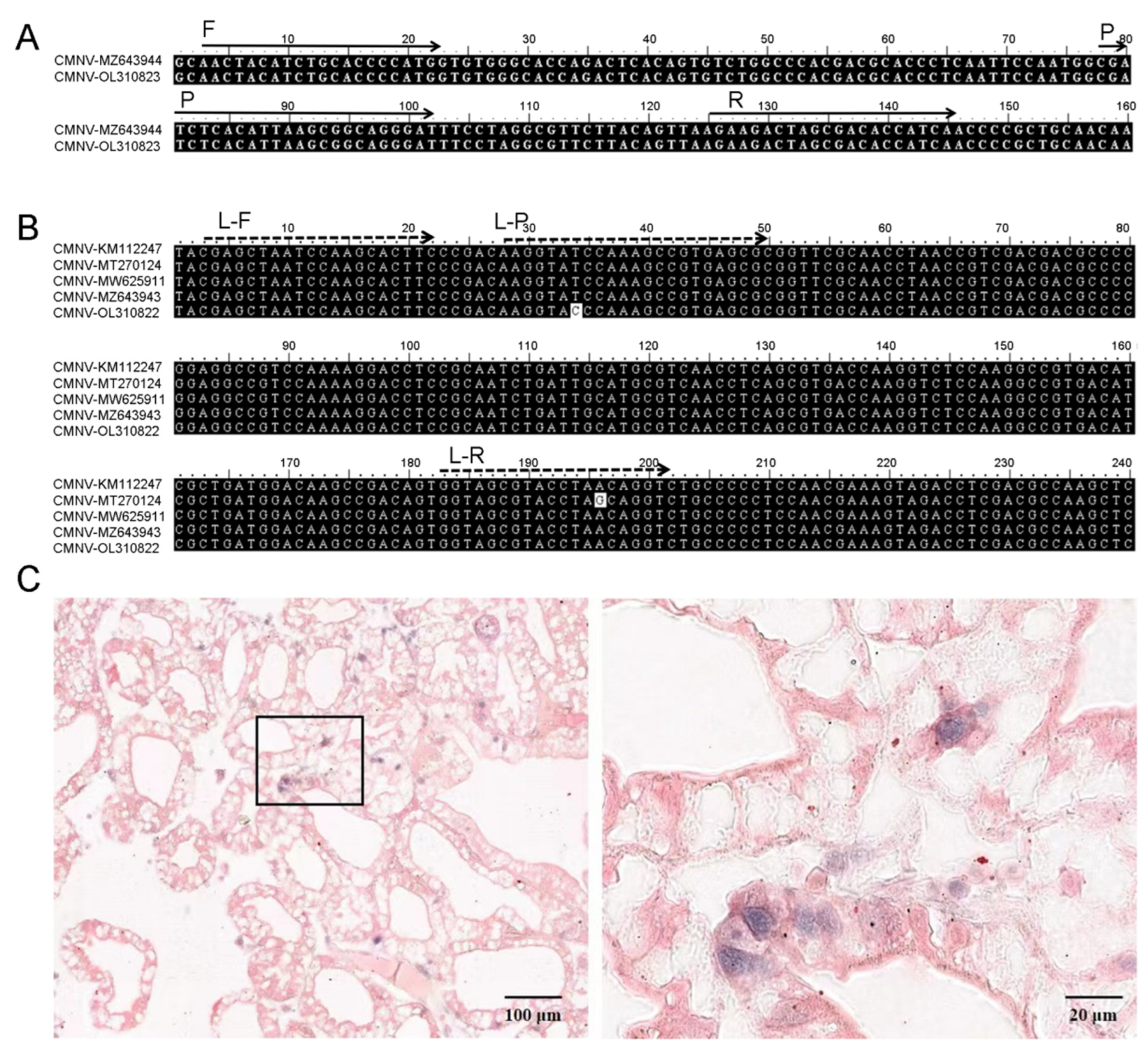
2.2. Primers and Probe
2.3. Preparation of Plasmid Standard and RNA Standard
2.4. TaqMan RT-qPCR Assay and Procedure
2.5. Analysis Specificity
2.6. Standard Curve and Analysis Sensitivity
2.7. Repeatability Test
2.8. Clinical Implement Test
2.9. Probe Preparation and in Situ Hybridization (ISH)
3. Results
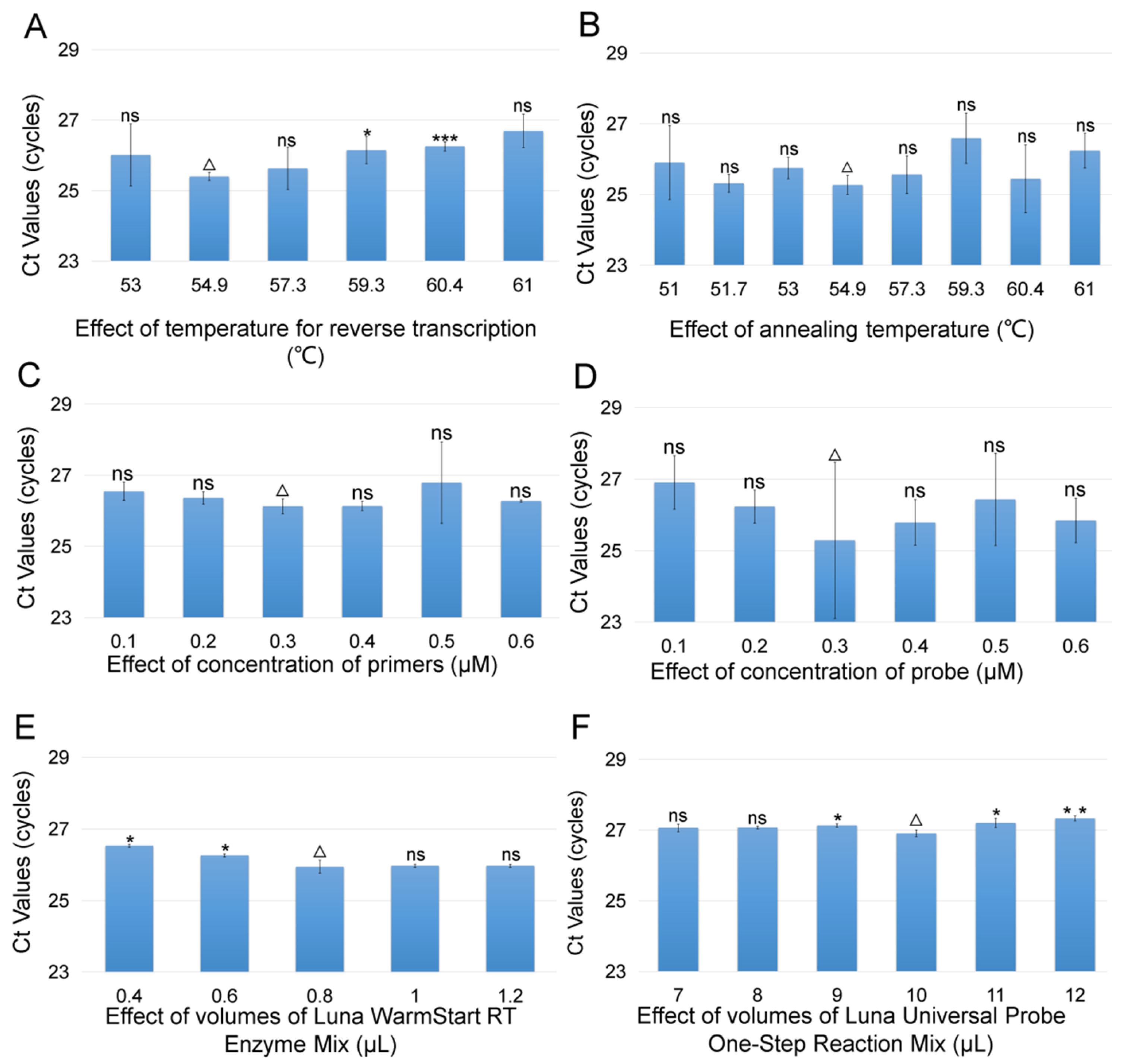
3.1. Optimization of TaqMan RT-qPCR Assay for CMNV
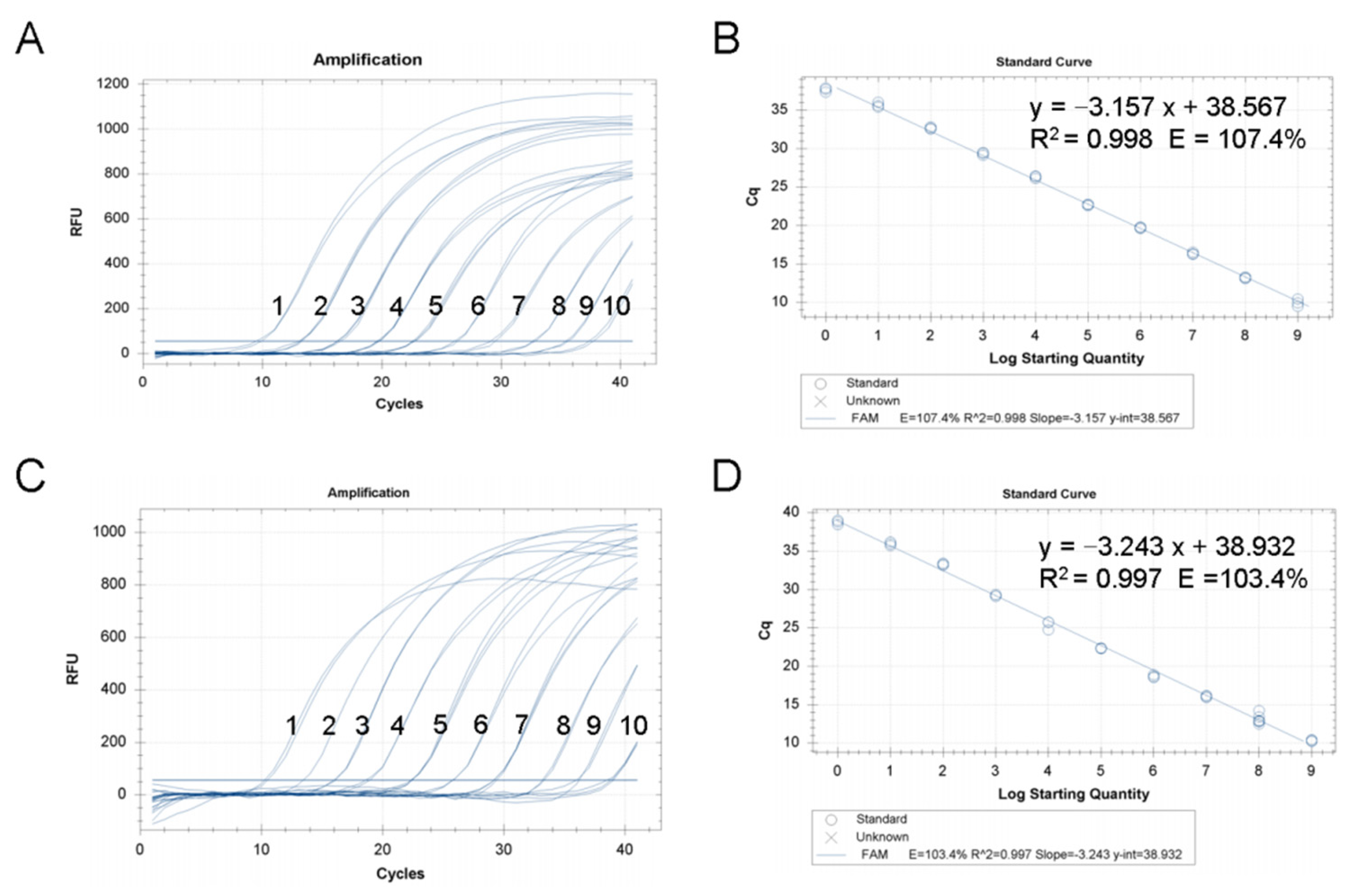
3.2. Analysis Sensitivity and Standard Curve of TaqMan RT-qPCR Assay for CMNV
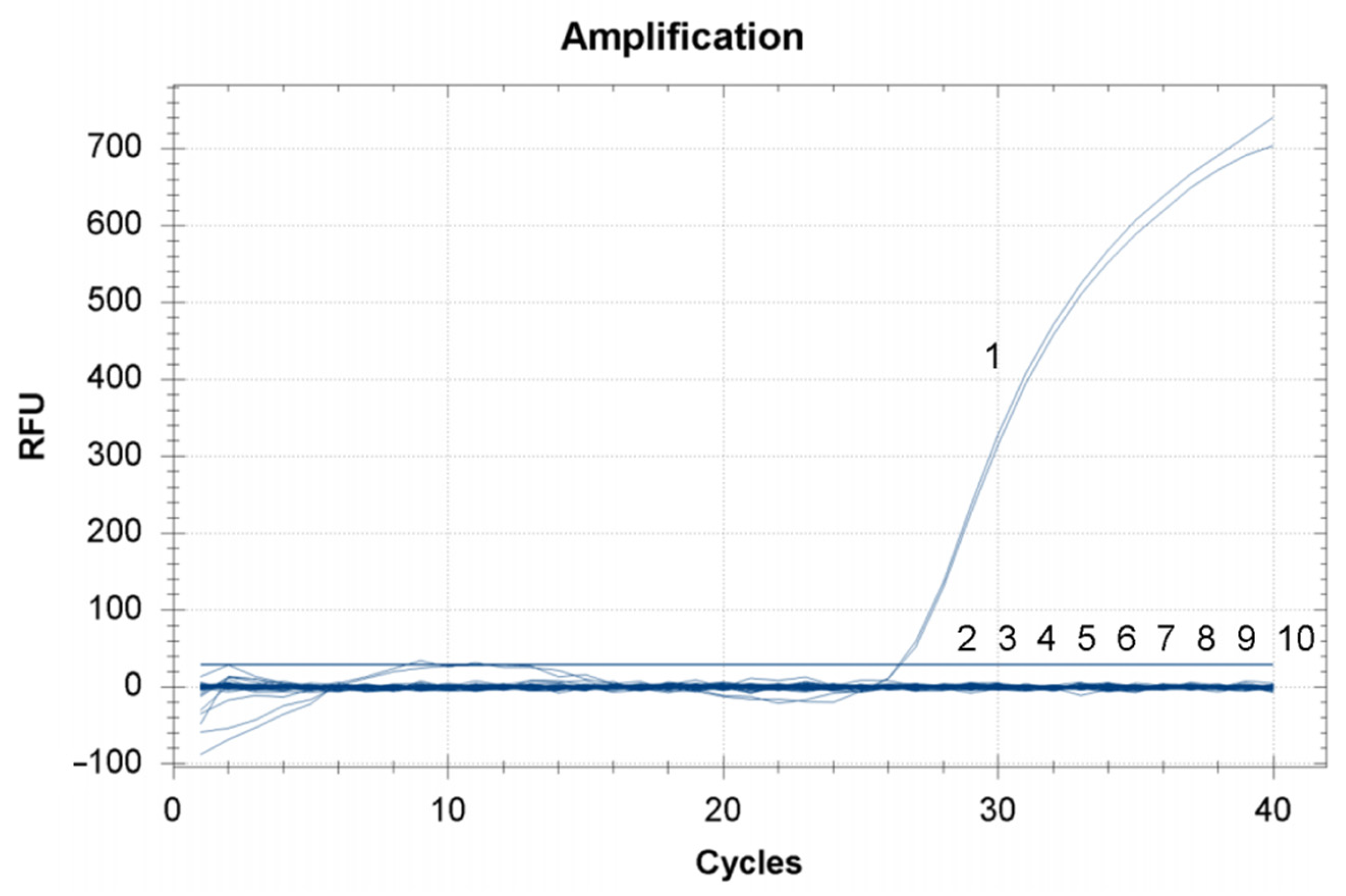
3.3. Analysis Specificity of TaqMan RT-qPCR Assay for CMNV
3.4. Repeatability of TaqMan RT-qPCR Assay for CMNV
3.5. Clinical Application of TaqMan RT-qPCR Assay for CMNV
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Song, S.; Zhuang, S. Measures for control of “bottom death” of Pacific white shrimp. Fish. Sci. 2006, 6, 36–39. [Google Scholar]
- Zhang, Q.; Liu, Q.; Liu, S.; Yang, H.; Liu, S.; Zhu, L.; Yang, B.; Jin, J.; Ding, L.; Wang, X. A new nodavirus is associated with covert mortality disease of shrimp. J. Gen. Virol. 2014, 95, 2700–2709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Xu, T.; Wan, X.; Liu, S.; Wang, X.; Li, X.; Dong, X.; Yang, B.; Huang, J. Prevalence and distribution of covert mortality nodavirus (CMNV) in cultured crustacean. Virus Res. 2017, 233, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Thitamadee, S.; Prachumwat, A.; Srisala, J.; Jaroenlak, P.; Salachan, P.V.; Sritunyalucksana, K.; Flegel, T.W.; Itsathitphaisarn, O. Review of current disease threats for cultivated penaeid shrimp in Asia. Aquaculture 2016, 452, 69–87. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Xu, T.; Li, X.; Du, L.; Zhang, Q. Vectors and reservoir hosts of covert mortality nodavirus (CMNV) in shrimp ponds. J. Invertebr. Pathol. 2018, 154, 29–36. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Li, X.; Hao, J.; Tang, K.F.J.; Zhang, Q. Infection of covert mortality nodavirus in Japanese flounder reveals host jump of the emerging alphanodavirus. J. Gen. Virol. 2019, 100, 166–175. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Tang, K.F.J.; Zhang, Q. Natural infection of covert mortality nodavirus affects Zebrafish (Danio rerio). J. Fish Dis. 2021, 44, 1315–1324. [Google Scholar] [CrossRef]
- Wang, C.; Liu, S.; Xu, T.; Li, X.; Li, J.; Zhang, Q. Pathogenicity study of covert mortality nodavirus (CMNV) infection in zebrafish model. Aquaculture 2022, 546, 737378. [Google Scholar] [CrossRef]
- Xu, T.; Liu, S.; Li, X.; Zhang, Q. Genomic characterization of covert mortality nodavirus from farming shrimp: Evidence for a new species within the family Nodaviridae. Virus Res. 2020, 286, 198092. [Google Scholar] [CrossRef]
- Wang, C.; Wang, W.; Yao Sang, S.; Li, C.; Zhang, Q. Histopathological study of covert mortality nodavirus infection in sea cucumber (Apostichopus japonicus). Aquaculture 2021, 545, 737161. [Google Scholar] [CrossRef]
- Pooljun, C.; Direkbusarakom, S.; Chotipuntu, P.; Hirono, I.; Wuthisuthimethaveea, S. Development of a TaqMan real-time RT-PCR assay for detection of covert mortality nodavirus (CMNV) in penaeid shrimp. Aquaculture 2016, 464, 445–450. [Google Scholar] [CrossRef]
- Varela, A. Pathologies of the hepatopancreas in marine shrimp cultivated in America and its differential diagnosis by histopathology. Aqua TIC Mag. 2018, 50, 13–30. [Google Scholar]
- Páez-Osuna, F. The environmental impact of shrimp aquaculture: A global perspective. Environ. Pollut. 2001, 112, 229–231. [Google Scholar] [CrossRef]
- Stentiford, G.D.; Neil, D.M.; Peeler, E.J.; Shields, J.D.; Small, H.J.; Flegel, T.W.; Vlak, J.; Jones, B.; Morado, F.; Moss, S.; et al. Disease will limit future food supply from the global crustacean fishery and aquaculture sectors. J. Invertebr. Pathol. 2012, 110, 141–157. [Google Scholar] [CrossRef] [Green Version]
- Doyle, R.W. Inbreeding and disease in tropical shrimp aquaculture: A reappraisal and caution. Aquac. Res. 2016, 47, 21–35. [Google Scholar] [CrossRef]
- Lee, Y.K.; Chew, P.F.; Soh, B.S.; Tham, L.Y. Enhancing phagocytic activity of hemocytes and disease resistance in the prawn Penaeus merguiensis by feeding Spirulina platensis. J. Appl. Phycol. 2003, 15, 279–287. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Aweya, J.J.; Yao, D.; Zheng, Z.H.; Tran, N.T.; Li, S.K.; Zhang, Y.L. Ubiquitination as an important host-immune response strategy in penaeid shrimp: Inferences from other species. Front. Immunol. 2021, 12, 2052. [Google Scholar] [CrossRef]
- Li, X.; Wan, X.; Xu, T.; Huang, J.; Zhang, Q. Development and validation of a TaqMan RT-qPCR for the detection of convert mortality nodavirus (CMNV). J. Virol. Methods 2018, 262, 65–71. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, S.; Yang, H.; Zhu, L.; Wan, X.; Li, X.; Huang, J. Reverse transcription loop-mediated isothermal amplification for rapid and quantitative assay of covert mortality nodavirus in shrimp. J. Invertebr. Pathol. 2017, 150, 130–135. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Nolan, T.; Pfaffl, M.W. Quantitative real-time RT-PCR–A perspective. J. Mol. Endocrinol. 2005, 34, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Zhang, Y.; Cui, P.; Wang, C.; Zeng, X.; Deng, G.; Wang, X. Development of a duplex TaqMan real-time RT-PCR assay for simultaneous detection of newly emerged H5N6 influenza viruses. Virol. J. 2019, 16, 119. [Google Scholar] [CrossRef] [PubMed]
- Mester, P.; Witte, A.K.; Rossmanith, P. Sample Preparation for qPCR Detection of Listeria from Food. Methods Mol. Biol. 2021, 2220, 31–40. [Google Scholar] [PubMed]
- World Organisation for Animal Health (OIE). Manual of Diagnostic Tests for Aquatic Animals; World Organisation for Animal Health (OIE): Paris, France, 2019. [Google Scholar]
- Pope, E.C.; Powell, A.; Roberts, E.C.; Shields, R.J.; Wardle, R.; Rowley, A.F. Enhanced cellular immunity in shrimp (Litopenaeus vannamei) after ‘vaccination’. PLoS ONE 2011, 6, e20960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.Z.J.; Ye, Q.Z. RNA Virus Replication Complexes. PLoS Pathog. 2010, 6, e1000943. [Google Scholar] [CrossRef] [Green Version]
- Moeller, N.H.; Shi, K.; Demir, Ö.; Belica, C.; Banerjee, S.; Yin, L.; Durfee, C.; Amaro, R.E.; Aihara, H. Structure and dynamics of SARS-CoV-2 proofreading exoribonuclease ExoN. Proc. Natl. Acad. Sci. USA 2022, 119, e2106379119. [Google Scholar] [CrossRef]
- Rose, T.M.; Henikoff, J.G.; Henikoff, S. CODEHOP (COnsensus-DEgenerate hybrid oligonucleotide primer) PCR primer design. Nucleic Acids Res. 2003, 31, 3763–3766. [Google Scholar] [CrossRef]
- Zlateva, K.T.; Crusio, K.M.; Leontovich, A.M.; Lauber, C.; Claas, E.; Kravchenko, A.A.; Spaan, W.J.; Gorbalenya, A.E. Design and validation of consensus-degenerate hybrid oligonucleotide primers for broad and sensitive detection of corona-and toroviruses. J. Virol. Methods 2011, 177, 174–183. [Google Scholar] [CrossRef]
| Dilution of Plasmid | Intra-Assay Ct | INTER-Assay Ct | ||||
|---|---|---|---|---|---|---|
| (Copies/Reaction) | Mean | SD | CV (%) | Mean | SD | CV (%) |
| 2.15 × 109 | 10.34 | 0.11 | 1.07 | 9.98 | 0.27 | 2.72 |
| 2.15 × 108 | 12.87 | 0.07 | 0.56 | 13.25 | 0.30 | 2.23 |
| 2.15 × 107 | 16.08 | 0.13 | 0.81 | 16.26 | 0.18 | 1.09 |
| 2.15 × 106 | 18.71 | 0.18 | 0.98 | 19.34 | 0.69 | 3.55 |
| 2.15 × 105 | 22.35 | 0.05 | 0.21 | 22.44 | 0.28 | 1.26 |
| 2.15 × 104 | 25.42 | 0.58 | 2.27 | 25.61 | 0.81 | 3.17 |
| 2.15 × 103 | 29.22 | 0.12 | 0.43 | 29.48 | 0.37 | 1.24 |
| 2.15 × 102 | 33.29 | 0.13 | 0.39 | 32.87 | 0.36 | 1.11 |
| 2.15 × 101 | 35.95 | 0.24 | 0.67 | 35.68 | 0.56 | 1.57 |
| 2.15 × 100 | 38.75 | 0.28 | 0.73 | 38.27 | 0.49 | 1.28 |
| Dilution of Plasmid (Copies/Reaction) | Inter-Assay Ct | |
|---|---|---|
| F Value | p Value | |
| 2.15 × 109 | 4.409 | 0.310 |
| 2.15 × 108 | 0.020 | 0.213 |
| 2.15 × 107 | 2.896 | 0.109 |
| 2.15 × 106 | 3.423 | 0.460 |
| 2.15 × 105 | 3.347 | 0.297 |
| 2.15 × 104 | 5.614 | 0.435 |
| 2.15 × 103 | 2.523 | 0.291 |
| 2.15 × 102 | 0.048 | 0.777 |
| 2.15 × 101 | 0.378 | 0.460 |
| 2.15 × 100 | 8.520 | 0.171 |
| Number of Reference Samples | ||
|---|---|---|
| Positive Samples, 79 | Negative Samples, 221 | |
| Detected positive | 76 | 12 |
| Detected negative | 3 | 209 |
| Calculation | DSe = 76/79 × 100% = 96.20% | DSp = 209/221 × 100% = 94.57% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Liu, S.; Yao, L.; Xia, J.; Xu, T.; Wang, C.; Li, C.; Zhang, Q. Development of a Novel RT-qPCR Detecting Method of Covert Mortality Nodavirus (CMNV) for the National Proficiency Test in Molecular Detection. Viruses 2022, 14, 1475. https://doi.org/10.3390/v14071475
Wang W, Liu S, Yao L, Xia J, Xu T, Wang C, Li C, Zhang Q. Development of a Novel RT-qPCR Detecting Method of Covert Mortality Nodavirus (CMNV) for the National Proficiency Test in Molecular Detection. Viruses. 2022; 14(7):1475. https://doi.org/10.3390/v14071475
Chicago/Turabian StyleWang, Wei, Shuang Liu, Liang Yao, Jitao Xia, Tingting Xu, Chong Wang, Chen Li, and Qingli Zhang. 2022. "Development of a Novel RT-qPCR Detecting Method of Covert Mortality Nodavirus (CMNV) for the National Proficiency Test in Molecular Detection" Viruses 14, no. 7: 1475. https://doi.org/10.3390/v14071475






