Abstract
Worldwide, infections by influenza viruses are considered a major public health challenge. In this study, influenza B vaccine mismatches and clinical aspects of Victoria and Yamagata infections in Brazil were assessed. Clinical samples were collected from patients suspected of influenza infection. In addition, sociodemographic, clinical, and epidemiological information were collected by the epidemiological surveillance teams. Influenza B lineages were determined by real-time RT-PCR and/or Sanger sequencing. In addition, putative phylogeny–trait associations were assessed by using the BaTS program after phylogenetic reconstruction by a Bayesian Markov Chain Monte Carlo method (BEAST software package). Over 2010–2020, B/Victoria and B/Yamagata-like lineages co-circulated in almost all seasonal epidemics, with B/Victoria predominance in most years. Vaccine mismatches between circulating viruses and the trivalent vaccine strains occurred in five of the eleven seasons (45.5%). No significant differences were identified in clinical presentation or disease severity caused by both strains, but subjects infected by B/Victoria-like viruses were significantly younger than their B/Yamagata-like counterparts (16.7 vs. 31.4 years, p < 0.001). This study contributes to a better understanding of the circulation patterns and clinical outcomes of B/Victoria- and B/Yamagata-like lineages in Brazil and advocate for the inclusion of a quadrivalent vaccine in the scope of the Brazilian National Immunization Program.
1. Introduction
Globally, influenza infections are a major public health challenge due to morbidity and mortality and have a significant annual economic impact [1,2]. Influenza types A or B are clinically indistinguishable [3,4] and can lead to serious complications and death, especially among children and adults [5,6,7]. In Brazil, the burden of influenza-like illness (ILI) cases was estimated to be over 83 million in 2008 [8].
Annual vaccination plays a key role in influenza control and prevention [9]. Although several countries have regularly used the quadrivalent influenza vaccine (QIV) for some years now, the Brazilian Immunization Program freely provides the trivalent influenza vaccine (TIV), which comprises two strains of influenza A (H1N1 and H3N2) and only one influenza B lineage component-B/Yamagata or B/Victoria-like [10]. Because of the high viral variability, especially in hemagglutinin and neuraminidase proteins, the vaccine composition is annually updated by the World Health Organization (WHO), based on a sample of circulating viruses that are characterized by the global influenza surveillance network [11,12,13,14]. For the TIV, the B/Victoria or B/Yamagata-like strains are chosen according to their prevalence in each Hemisphere based on the prior year [14]. Therefore, the success of the vaccination strategy depends on the concordance between the recommended vaccine strain and their effective prevalence in the population in a given influenza season.
Since 2000, the co-circulation of influenza B lineages has been observed, imposing a challenge for TIV adoption [15]. In many countries, mismatches between vaccine and circulating viruses had been reported in about two-to four year intervals [16]. These events can contribute to additional disease-related burden due to the limited cross-protection between antigens [17,18,19]. Hence, the correct prediction of vaccine strains is pivotal to a successful immunization policy and the reduction in the annual impact of influenza.
In this study, we investigated the distribution and presumed regional patterns of influenza B lineages among 11 influenza seasons (2010–2020) in Brazil. In addition, putative associations between B/Victoria- and B/Yamagata-like viruses and demographic and clinical-epidemiological variables were also explored. This information is critical to tailor public health policies for influenza control and prevention.
2. Materials and Methods
2.1. Population
Nasopharyngeal swabs, aspirates, and/or lung tissue fragments of 920 influenza B laboratory-confirmed cases were investigated. From June 2010 to March 2020, clinical samples were collected from subjects with respiratory influenza-like illness (ILI) or Severe Acute Respiratory Infection (SARI), according to the WHO and Brazilian Ministry of Health (MoH) case definitions [20,21] and sent to our laboratory, a reference laboratory for the MoH and WHO. In addition, the sociodemographic, clinical, and epidemiological information were collected by the local epidemiological surveillance teams using a nationally standardized questionnaire including information on gender, age, symptoms onset, clinical signs and symptoms, hospitalization history and comorbidities, and others.
Among the 920 influenza B positive samples used in the mismatch analysis, 514 samples had complete clinical and epidemiological data and were used to explore putative associations between influenza B lineages and those outcomes. In addition, within the subsample submitted to viral HA sequencing, good quality and complete sequences were obtained in 118 samples.
Clinical severity was defined as the presence of dyspnea, indicative of SARI. Samples were collected in different Brazilian geographical regions. The Northeastern states were represented by Alagoas, Bahia and Sergipe, whereas Southeastern and Southern states by Rio de Janeiro, Espírito Santo, and Minas Gerais, and Rio Grande do Sul, Paraná, and Santa Catarina, respectively. Vaccine mismatch was defined as more than 51% of divergence between the circulating lineage and influenza B vaccine strain in each influenza season.
2.2. Influenza B Molecular Detection, Lineage Determination and Sequencing
Viral RNA was extracted using the QIAmp Viral RNA Mini Kit. Lung tissue fragments were macerated using the Tissue Ruptor Kit and RNA was extracted using a RNeasy Mini Kit (Qiagen, Hilden, Germany). Influenza B detection and lineage determination were performed by real-time RT-PCR using the CDC protocols, as recommended by the WHO [22]. Sanger sequencing of the hemagglutinin gene (HA, 1714bp) was carried out using the CDC primers and protocol. After purification (QIAquick Extraction Kit, Qiagen), both strands were sequenced using the ABI PRISM BigDye Terminator v.3.1 Cycle Sequencing Ready Reaction Kit (Applied Biosystems, Waltham, MA, USA).
2.3. Phylogenetic Analyses
The phylogenetic analyses were composed of 118 complete Brazilian HA sequences (1714bp), for which information on the presence of dyspnea was available. Sequences were edited and contigs were set up using the software Sequencher, v.4.10. After alignment by Muscle [23], HA phylogenetic trees were reconstructed using a maximum likelihood algorithm (PhyML v.3.0) and aLRT SH-like as the fast likelihood-based method [24]. The general time reversible with gamma-distributed rates and invariant sites (GTR + I+G) was employed as the best fit nucleotide substitution model determined by the J Model test, version 2.1.7 [25].
The temporal structure of the dataset was verified using TempEst v. 1.5.1 [26]. Afterward, time–scale phylogenetic trees were reconstructed by a Bayesian Markov Chain Monte Carlo (MCMC) method, accessible in the BEAST software package, v1.10 [27,28]. Time calibration was set based on the year of sample collection, available for all sequences. Beast runs were carried out using the uncorrelated lognormal relaxed molecular clock model and a time-aware Gaussian Markov Random Field (GMRF) Bayesian skyride coalescent tree prior [29,30]. The length of the MCMC chains was established as 80 million, sampled every 8000 steps. Trace files generated through Bayesian phylogenetic inference were visualized and analyzed in Tracer version 1.7.1 [31]. The convergence of parameters was considered in the presence of effective sample size (ESS) values exceeding 200. The target maximum clade credibility (MCC) tree was summarized by TreeAnnotator 1.8.4, with a burn-in corresponding to 10% of states. PhyML and MCC trees were visualized and edited in FigTree, version 1.4.3. (http://tree.bio.ed.ac.uk/software/figtree/ accessed on 31 July 2021).
In order to infer putative phylogeny–trait associations (viral lineages and disease severity), we used 100 replicates for two discrete states (ILI and SARI). Analyses were performed with BaTS program, release 0.9 [32]. Parsimony score statistics (PS), association index (AI), and monophyletic clade (MC) statistics were calculated.
2.4. Statistical Analyses
Descriptive and bivariate analysis (chi-square/Fisher’s exact test for categorical variables and the independent-samples Kruskal–Wallis test for means) were employed to assess the putative associations between the variables of interest and outcomes. Significance was considered when the p value < 0.05. Analyses were performed using SPSS for windows, version 19 (SPSS Inc., Chicago, IL, USA).
2.5. Ethical Statement
This study was approved by the Fiocruz-IOC Ethics Committee (68118417.6.0000.5248). As a National Reference Laboratory for Influenza for the MoH and as a National Influenza Center (NIC) for the WHO, our laboratory continuously receives samples from influenza cases for antigenic and genetic characterization as part of the WHO Influenza Surveillance Network. Clinical samples were collected in the scope of the National Influenza Epidemiological Surveillance Program/MoH, dispensing a formal patient consent.
In accordance with our confidentiality policy, determined in the scope of Quality System (ISO 15189), personal information is confidential, and all analyses remain anonymous as the samples and formularies are coded.
3. Results
The demographic and clinical features of the studied population, according to influenza B viral lineage strata are shown in Table 1. Most of the subjects of the male gender (54.2%), with a median age of 20.5 years (0–99 years), and residents in Southern Brazil (70.2%). About a third of the sample reported any comorbidity (28.5%) and a low frequency of fatal outcomes was observed (3.4%). With concern to clinical symptoms, about 40.0% of cases reported dyspnea, in line with the SARI case classification. The majority of patients presented fever and cough (about 93.0%), sore throat (48.0%), myalgia (26.6%), coryza (21.4%), and arthralgia (4.3%). Individuals infected by B/Victoria-like viruses were significantly younger than their B/Yamagata-like counterparts (16.7 vs. 31.4 years, p < 0.001). No other significant divergence in the demographic or clinical variables could be noted, suggesting a similarity in clinical disease caused by B/Victoria- and B/Yamagata-like infections. These 514 samples were also subtyped and 51.6% of infections were associated with Victoria-like viruses (n = 265), while the remaining 48.4% had Yamagata-like infections (n = 249).

Table 1.
Demographic and clinical features among the 514 Influenza B infected individuals, according to viral lineage in Brazil, 2010–2020.
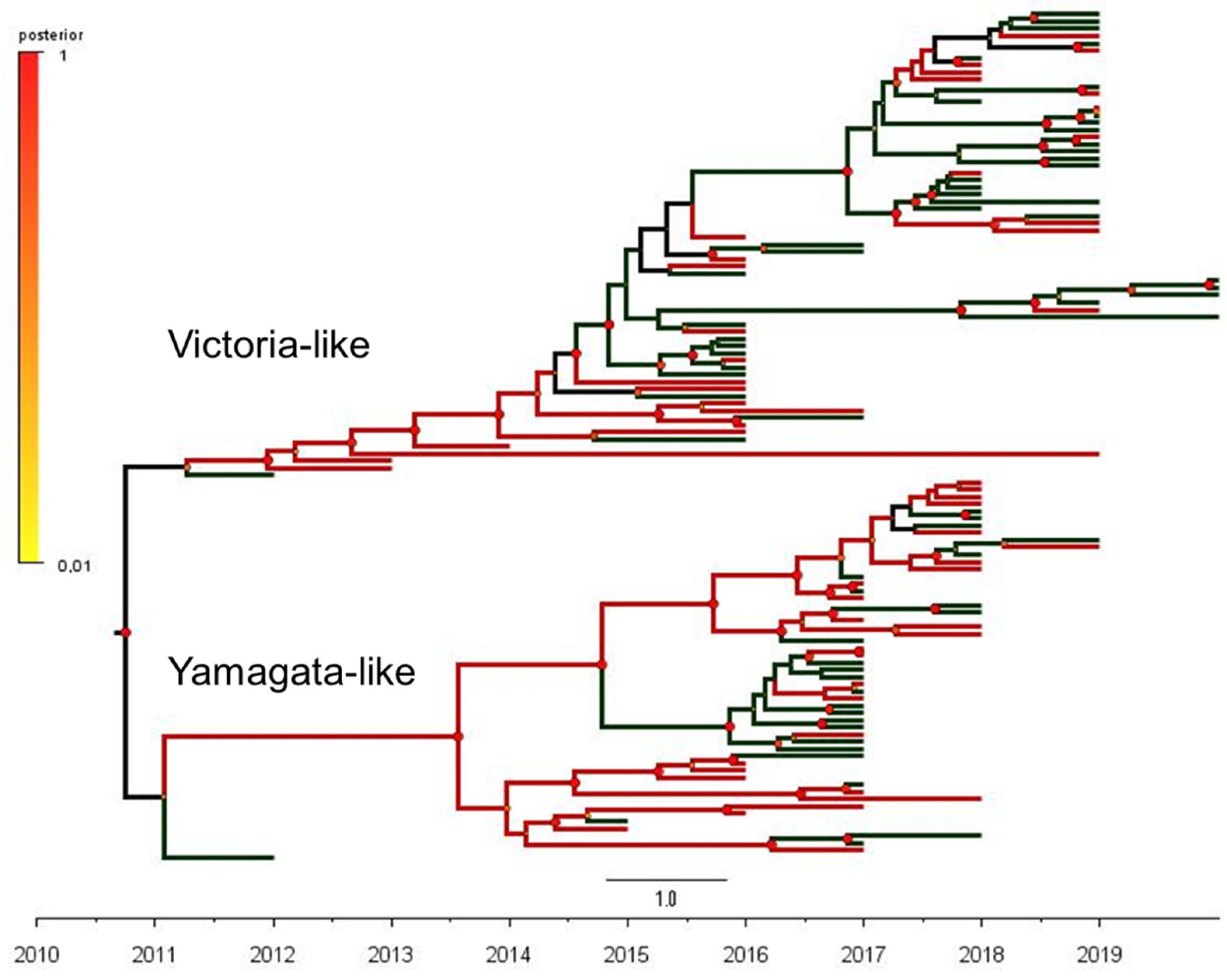
Full HA sequences and clinical information was available for a subsample of 118 positives (66 sequences of B/Victoria and 52 sequences of B/Yamagata-like), which were further classified as ILI or SARI-associated cases (Table S1). The maximum clade credibility (MCC) phylogenetic tree of the influenza B hemagglutinin gene (1714 bp) from B/Victoria- and B/Yamagata-like lineages circulating in Brazil and the outcomes of the phylogeny–trait analysis are shown in Figure 1. In order to effectively describe the putative correlations between traits (clinical severity, ILI or SARI) and phylogeny, we used a Bayesian tip-association significance test (Table 2). In these assessments, low PS scores and AI values represent strong phylogeny–trait association. In addition, MC values will be correlated with the strength of the phylogeny–trait association [32]. The comparison between the AI and PS values obtained in our subsample and the null mean values corresponded to 6.4 (95%CI 5.5–7.2) vs. 6.3 (95%CI 5.1–7.4), p = 0.550 and 37.8 (95% CI 36.0–40.0) vs. 38.9 (95% CI 34.9–43.1), p = 0.370), respectively. These outcomes revealed a weak phylogeny–trait association. In addition, the monophyletic clade statistics (MC) for SARI was 2.9 (95% CI 2.0–5.0) vs. 3.4 (95% CI 2.6–4.5), p = 0.810) and for ILI, it was 3.7 (95% CI 3.0–5.0) vs 4.4 (95% CI 3.1–6.2, p = 0.569), showing that the SARI and ILI traits were randomly distributed among the B/Victoria and B/Yamagata groups. Altogether, these results support the view that infections caused by B/Victoria-like and B/Yamagata-like viruses have similar clinical severity, independent of the hypothesis testing approach.
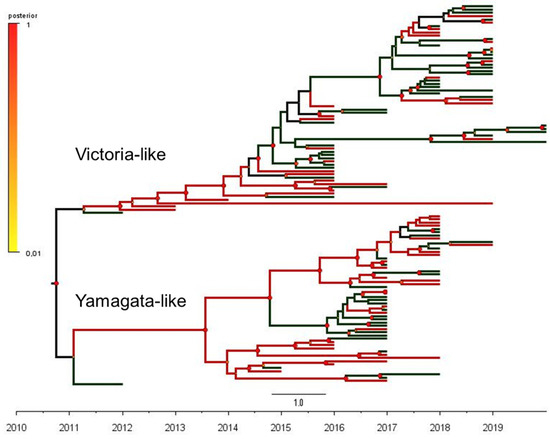
Figure 1.
The phylogeny–trait analysis based on the maximum clade credibility (MCC) phylogenetic tree of 118 complete sequences of the influenza B hemagglutinin gene (1714 bp) from B/Victoria-like (66 sequences) and B/Yamagata-like (52 sequences) antigenic lineages circulating in Brazil, 2010–2020.

Table 2.
Results of phylogeny trait association tests for viral lineages (B/Victoria and B/Yamagata) and disease severity (SARI and ILI).
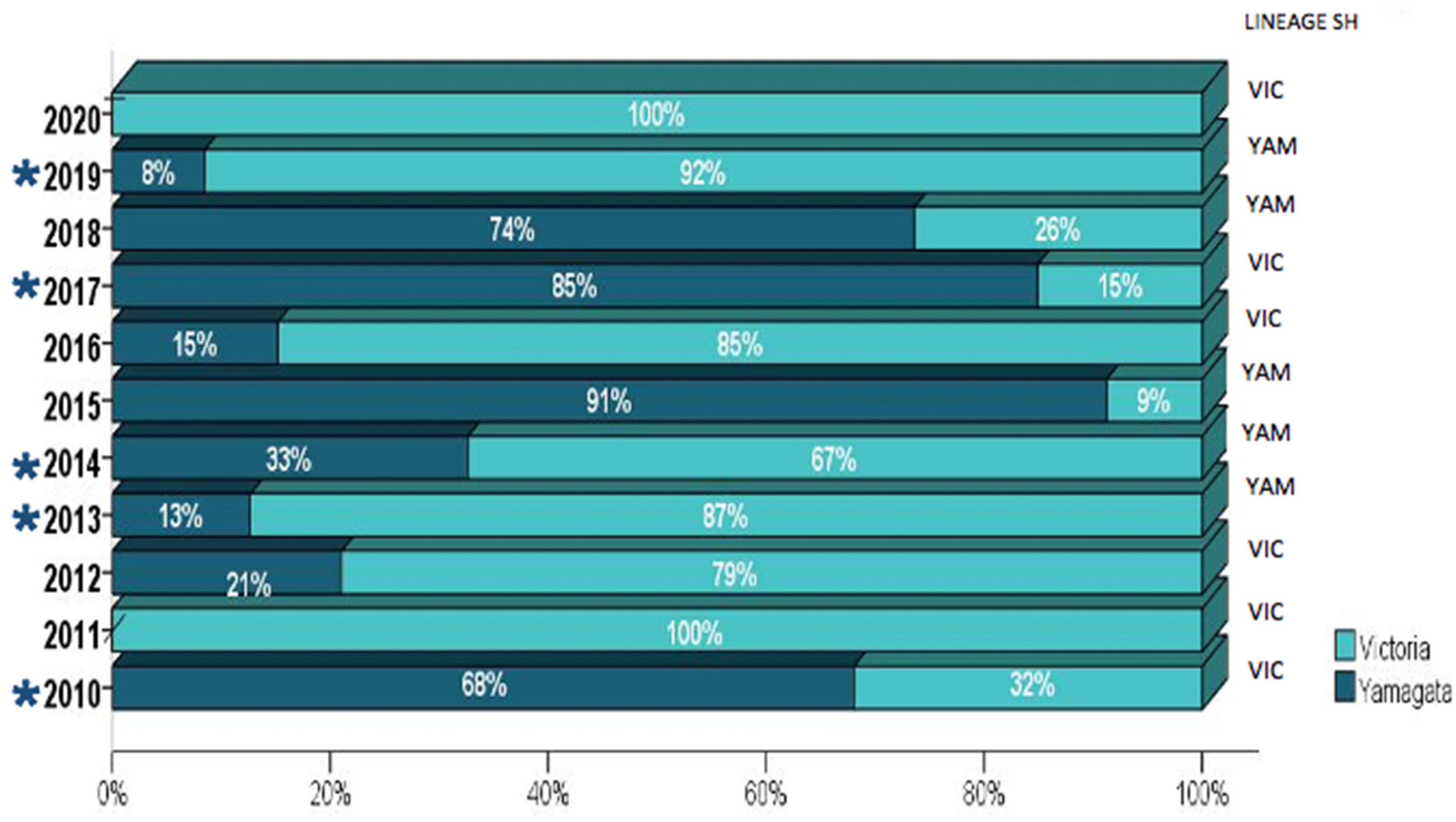
The distribution of viral lineages according to the year of sample collection and the presence of vaccine mismatches is shown in Figure 2. Both influenza B lineages co-circulated through the studied decade, with a higher prevalence of B/Victoria-like viruses in most years. Mismatches between vaccine strains (Table 3) and circulating lineages (B/Victoria and B/Yamagata) were observed in 45.4% (5/11) of the investigated seasons (2010, 2013, 2014, 2017, and 2019).
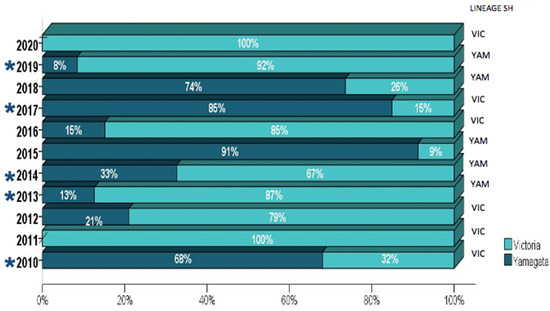
Figure 2.
The distribution of influenza B lineages among the 920 Brazilian samples collected from 2010–2020. The number of tested samples for each year is further detailed in Table S2. The years where mismatches between the vaccine and circulating viruses were observed are indicated by (*).

Table 3.
WHO recommended influenza vaccine composition in the respective influenza seasons.
4. Discussion
In this study, the demographic and clinical aspects of influenza B/Victoria and B/Yamagata infections, and vaccine mismatches over a decade of influenza seasons were explored.
Independent of the adopted hypothesis testing approach—if based on statistical analyses on the main influenza signs and symptoms or on phylogeny–trait analyses—our results revealed that infections caused by B/Victoria-like and B/Yamagata-like viruses presented similar clinical outcomes/severity. These findings are in line with previous reports [33,34,35,36]. Nonetheless, observations from Tan et al. suggest that the B/Victoria Guangzhou clade 2 lineage infected patients showed fewer upper respiratory tract infections than their B/Victoria Guangzhou clade 1 counterparts [37].
Of note, subjects infected by B/Victoria-like viruses were significantly younger than those infected by B/Yamagata-like viruses, corroborating data from epidemiological studies conducted in Brazil and elsewhere (Slovenia, Australia, New Zealand, and South Africa) [34,38,39,40,41]. In addition, previous analyses on demographic data (2008 to 2019) had shown a younger profile among B/Victoria (median age of 13 y) when compared to the B/Yamagata cases (median age of 32.5y)—the latter showing a bimodal age distribution with peaks within pediatric and adult age groups, respectively [42,43]. According to Vijaykrishna et al., this outcome could be partially explained by subtle differences in the prevalence of α-2,3 and α-2,6 linked glycans on respiratory tract cells from young children, in contrast to those found among adults [39], in addition to pre-exposure to infection or immunization combined with pre-existing population immunity. Moreover, the existence of an immunological impression induced by a first B/Yamagata infection could act on more conserved epitopes than those neutralized by antibodies induced by B/Victoria-like viruses [39,44,45]—a phenomenon already described for influenza A viruses [46,47].
In our analysis, no special geographical patterns in the distribution of viral lineages were found. Despite a profound imbalance in the demographic density between Brazilian regions [48], the reduced number of available sequences from the Northeastern states is noteworthy (Table 1), reinforcing the need to improve epidemiological and genomic surveillance, in order to have a representative sample of all regions. This is pivotal information to effectively evaluate the putative geographical patterns of viral distribution and to better guide vaccination strategies. It is important to emphasize that all of these findings should be interpreted in light of a small and non-representative sample, which could have introduced biases in the present analyses.
The presence of mismatches between the circulating influenza B lineages and vaccine strains in the 2010–2020 influenza seasons was also explored. The re-emergence of B/Victoria-like viruses and the cocirculation of both influenza B strains since 2000–2002 [49,50,51] including the sample assessed in this study imposed a challenge to a correct prediction of the TIV influenza B component. Our figures pointed to a vaccine mismatch in 45.5% of the studied seasons (2010, 2013, 2014, 2017, and 2019), in line with the previous Brazilian information [52,53,54,55]. Luna et al. described similar findings (46.0%) over seven influenza seasons (2010–2016) [38,55], and Barros et al. found a significant vaccine mismatch in 2013, both for Brazil (Vic 91.4%) and for South America (Vic 52%) [54]. A study carried out in Europe and in the United States showed vaccine mismatches in about half of the 2001 to 2011 influenza seasons [16]. It is relevant to mention that global sampling on the influenza surveillance program is not representative at all, and is based on the sample collection of sentinel health services [14,21]. Moreover, the effective impact of this event on influenza morbidity and mortality need to be further addressed.
Altogether, these results highlight the difficulty to accurately predict the influenza B component of the annual trivalent vaccines, despite the WHO global efforts to monitor and characterize circulating viruses in the Northern and Southern Hemispheres. In addition, influenza vaccine effectiveness may be suboptimal in mismatched seasons, potentially increasing the disease burden [56]. In order to reduce the impact of influenza B vaccine mismatch, the WHO has recommended the inclusion of both influenza B lineages in the vaccine composition since 2013 [57]. However, the TIV is under current use by the MoH Immunization Program to vaccinate influenza target groups [10]. The impact of replacing the TIV by QIV in a pediatric group has been estimated. When the dynamic epidemiological model was applied to the Brazilian context, QIV adoption would be able to avoid 406,600 symptomatic cases, 11,300 hospitalizations, and almost 400 deaths per influenza season, reinforcing the cost-effectiveness of QIV and its respective public health benefits [58,59,60,61].
5. Conclusions
Despite annual vaccination campaigns, seasonal influenza remains responsible for a relevant morbidity and mortality and economic burden in Brazil and worldwide. Overall, our findings advocate for the inclusion of the QIV in the context of the Brazilian National Immunization Program, in order to improve the health promotion and economic benefits of influenza vaccination.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v14071477/s1, Table S1: Sequences from GISAID’s EpiFlu™ Database, Table S2: Distribution of Influenza B lineages among 920 Brazilian samples collected from 2010–2020. The number of tested samples for each year and lineages are detailed.
Author Contributions
M.d.L.A.-O. conceived the study, performed the real-time RT-PCR, viral sequencing, phylogenetic, and statistical analyses and prepared the original draft. J.C.d.C. performed the real-time RT-PCR, viral sequencing, and prepared the original draft; J.O.L. and B.C.d.C. performed the real-time RT-PCR; B.C.d.C. performed the influenza B sequencing; E.L.G. helped with the sequence datasets; D.B. contributed with the text; and M.M.S. supervised the study and contributed with the text. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Oswaldo Cruz Foundation, Ministry of Health (MoH), The Health Surveillance Secretariat, SVS, MoH, the National Council for Scientific and Technological Development, CNPQ, grant number 402457/2020-0, and the Research Support Foundation of The State of Rio de Janeiro, FAPERJ, grant number E-26/210.196/2020.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and by Fiocruz-IOC Ethics Committee (68118417.6.0000.5248), 21-12-2012.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are grateful to the technical team of the Laboratory of Respiratory Viruses, IOC, Fiocruz for their collaboration.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Keech, M.; Beardsworth, P. The Impact of Influenza on Working Days Lost: A Review of the Literature. Pharmacoeconomics 2008, 26, 911–924. [Google Scholar] [CrossRef] [PubMed]
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global Burden of Respiratory Infections Due to Seasonal Influenza in Young Children: A Systematic Review and Meta-Analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef]
- Irving, S.A.; Patel, D.C.; Kieke, B.A.; Donahue, J.G.; Vandermause, M.F.; Shay, D.K.; Belongia, E.A. Comparison of Clinical Features and Outcomes of Medically Attended Influenza A and Influenza B in a Defined Population over Four Seasons: 2004–2005 through 2007–2008. Influenza Other Respir Viruses 2012, 6, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hong, K.-W.; Cheong, H.J.; Song, J.Y.; Noh, J.Y.; Yang, T.U.; Kim, W.J. Clinical Manifestations of Influenza A and B in Children and Adults at a Tertiary Hospital in Korea during the 2011–2012 Season. Jpn. J. Infect. Dis. 2015, 68, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.R.; Heffernan, R.T.; Paladini, M.; Konty, K.; Weiss, D.; Mostashari, F. Monitoring the Impact of Influenza by Age: Emergency Department Fever and Respiratory Complaint Surveillance in New York City. PLoS Med. 2007, 4, e247. [Google Scholar] [CrossRef]
- McCullers, J.A.; Hayden, F.G. Fatal Influenza B Infections: Time to Reexamine Influenza Research Priorities. J. Infect. Dis. 2012, 205, 870–872. [Google Scholar] [CrossRef]
- Paul Glezen, W.; Schmier, J.K.; Kuehn, C.M.; Ryan, K.J.; Oxford, J. The Burden of Influenza B: A Structured Literature Review. Am. J. Public Health 2013, 103, e43–e51. [Google Scholar] [CrossRef]
- Savy, V.; Ciapponi, A.; Bardach, A.; Glujovsky, D.; Aruj, P.; Mazzoni, A.; Gibbons, L.; Ortega-Barría, E.; Colindres, R.E. Burden of Influenza in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. Influenza Other Respir. Viruses 2013, 7, 1017–1032. [Google Scholar] [CrossRef]
- WHO. Vaccines against Influenza WHO Position Paper—November 2012 = Note de Synthèse de l’OMS Concernant Les Vaccins Antigrippaux—Novembre 2012. Wkly. Epidemiol. Rec. 2012, 87, 461–476. [Google Scholar]
- Brazil Informe Técnico 24a Campanha Nacional de Vacinação Contra a Influenza Brasília. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/c/calendario-nacional-de-vacinacao/arquivos/informe-da-24a-campanha-nacional-de-vacinacao-contra-a-influenza.pdf (accessed on 7 May 2022).
- Cox, N.J.; Subbarao, K. Global Epidemiology of Influenza: Past and Present. Annu. Rev. Med. 2000, 51, 407–421. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Masaro, C.; Kwindt, T.L.; Mak, A.; Petric, M.; Li, Y.; Sebastian, R.; Chong, M.; Tam, T.; De Serres, G. Estimating Vaccine Effectiveness against Laboratory-Confirmed Influenza Using a Sentinel Physician Network: Results from the 2005-2006 Season of Dual A and B Vaccine Mismatch in Canada. Vaccine 2007, 25, 2842–2851. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; De Serres, G.; Dickinson, J.; Petric, M.; Mak, A.; Fonseca, K.; Kwindt, T.L.; Chan, T.; Bastien, N.; Charest, H.; et al. Component-Specific Effectiveness of Trivalent Influenza Vaccine as Monitored through a Sentinel Surveillance Network in Canada, 2006–2007. J. Infect. Dis. 2009, 199, 168–179. [Google Scholar] [CrossRef]
- WHO. Global Influenza Surveillance and Response System (GISRS). Available online: https://www.who.int/initiatives/global-influenza-surveillance-and-response-system (accessed on 31 March 2022).
- Belshe, R.B.; Coelingh, K.; Ambrose, C.S.; Woo, J.C.; Wu, X. Efficacy of Live Attenuated Influenza Vaccine in Children against Influenza B Viruses by Lineage and Antigenic Similarity. Vaccine 2010, 28, 2149–2156. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, C.S.; Levin, M.J. The Rationale for Quadrivalent Influenza Vaccines. Hum. Vaccin Immunother. 2012, 8, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Tricco, A.C.; Chit, A.; Soobiah, C.; Hallett, D.; Meier, G.; Chen, M.H.; Tashkandi, M.; Bauch, C.T.; Loeb, M. Comparing Influenza Vaccine Efficacy against Mismatched and Matched Strains: A Systematic Review and Meta-Analysis. BMC Med. 2013, 11, 153. [Google Scholar] [CrossRef]
- Heikkinen, T.; Ikonen, N.; Ziegler, T. Impact of Influenza B Lineage-Level Mismatch between Trivalent Seasonal Influenza Vaccines and Circulating Viruses, 1999–2012. Clin. Infect. Dis. 2014, 59, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Caini, S.; Kusznierz, G.; Garate, V.V.; Wangchuk, S.; Thapa, B.; de Paula Júnior, F.J.; Ferreira de Almeida, W.A.; Njouom, R.; Fasce, R.A.; Bustos, P.; et al. The Epidemiological Signature of Influenza B Virus and Its B/Victoria and B/Yamagata Lineages in the 21st Century. PLoS ONE 2019, 14, e0222381. [Google Scholar] [CrossRef]
- WHO. Surveillance Case Definitions for ILI and SARI. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/lymphatic-filariasis/morbidity-management-and-disability-prevention/global-influenza-programme (accessed on 31 March 2022).
- Brazil. Ministry of Health. Guia de Vigilância em Saúde; 2019; p. 741. Brasília. Available online: https://bvsms.saude.gov.br/bvs/publicacoes/guia_vigilancia_saude_3ed.pdf (accessed on 7 May 2022).
- World Health Organization. WHO Information for Molecular Diagnosis of Influenza Virus—Update. Available online: http://www.who.int/influenza/gisrs_laboratory/molecular_diagnosis/en/ (accessed on 31 July 2020).
- Edgar, R.C. MUSCLE: Multiple Sequence Alignment with High Accuracy and High Throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New Algorithms and Methods to Estimate Maximum-Likelihood Phylogenies: Assessing the Performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Darriba, D.; Taboada, G.L.; Doallo, R.; Posada, D. JModelTest 2: More Models, New Heuristics and Parallel Computing. Nat. Methods 2012, 9, 772. [Google Scholar] [CrossRef]
- Rambaut, A.; Lam, T.T.; Max Carvalho, L.; Pybus, O.G. Exploring the Temporal Structure of Heterochronous Sequences Using TempEst (Formerly Path-O-Gen). Virus Evol. 2016, 2, vew007. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian Evolutionary Analysis by Sampling Trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Rambaut, A. Many-Core Algorithms for Statistical Phylogenetics. Bioinformatics 2009, 25, 1370–1376. [Google Scholar] [CrossRef]
- Drummond, A.J.; Nicholls, G.K.; Rodrigo, A.G.; Solomon, W. Estimating Mutation Parameters, Population History and Genealogy Simultaneously from Temporally Spaced Sequence Data. Genetics 2002, 161, 1307–1320. [Google Scholar] [CrossRef] [PubMed]
- Minin, V.N.; Bloomquist, E.W.; Suchard, M.A. Smooth Skyride through a Rough Skyline: Bayesian Coalescent-Based Inference of Population Dynamics. Mol. Biol. Evol. 2008, 25, 1459–1471. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.; Rambaut, A.; Pybus, O.G. Correlating Viral Phenotypes with Phylogeny: Accounting for Phylogenetic Uncertainty. Infect. Genet. Evol. 2008, 8, 239–246. [Google Scholar] [CrossRef]
- Yap, J.; Tan, C.H.; Cook, A.R.; Loh, J.P.; Tambyah, P.A.; Tan, B.H.; Lee, V.J. Differing Clinical Characteristics between Influenza Strains among Young Healthy Adults in the Tropics. BMC Infect. Dis. 2012, 12, 12. [Google Scholar] [CrossRef]
- Sočan, M.; Prosenc, K.; Učakar, V.; Berginc, N. A Comparison of the Demographic and Clinical Characteristics of Laboratory-Confirmed Influenza B Yamagata and Victoria Lineage Infection. J. Clin. Virol. 2014, 61, 156–160. [Google Scholar] [CrossRef]
- Orsi, A.; Colomba, G.M.E.; Pojero, F.; Calamusa, G.; Alicino, C.; Trucchi, C.; Canepa, P.; Ansaldi, F.; Vitale, F.; Tramuto, F. Trends of Influenza B during the 2010-2016 Seasons in 2 Regions of North and South Italy: The Impact of the Vaccine Mismatch on Influenza Immunisation Strategy. Hum. Vaccine Immunother. 2018, 14, 523–531. [Google Scholar] [CrossRef]
- Hönemann, M.; Martin, D.; Pietsch, C.; Maier, M.; Bergs, S.; Bieck, E.; Liebert, U.G. Influenza B Virus Infections in Western Saxony, Germany in Three Consecutive Seasons between 2015 and 2018: Analysis of Molecular and Clinical Features. Vaccine 2019, 37, 6550–6557. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Guan, W.; Lam, T.T.-Y.; Pan, S.; Wu, S.; Zhan, Y.; Viboud, C.; Holmes, E.C.; Yang, Z. Differing Epidemiological Dynamics of Influenza B Virus Lineages in Guangzhou, Southern China, 2009–2010. J. Virol. 2013, 87, 12447–12456. [Google Scholar] [CrossRef] [PubMed]
- Perosa, A.H.; Granato, C.; Bellei, N. Detection of Influenza B Lineages from 2001 to 2013 in a Tertiary Hospital in the City of São Paulo, Brazil. Mem. Inst. Oswaldo Cruz. 2015, 110, 606–610. [Google Scholar] [CrossRef]
- Vijaykrishna, D.; Holmes, E.C.; Joseph, U.; Fourment, M.; Su, Y.C.F.; Halpin, R.; Lee, R.T.C.; Deng, Y.-M.; Gunalan, V.; Lin, X.; et al. The Contrasting Phylodynamics of Human Influenza B Viruses. Elife 2015, 4, e05055. [Google Scholar] [CrossRef] [PubMed]
- Barr, I.G.; Vijaykrishna, D.; Sullivan, S.G. Differential Age Susceptibility to Influenza B/Victoria Lineage Viruses in the 2015 Australian Influenza Season. Euro Surveill 2016, 21, 30118. [Google Scholar] [CrossRef]
- Seleka, M.; Treurnicht, F.K.; Tempia, S.; Hellferscee, O.; Mtshali, S.; Cohen, A.L.; Buys, A.; McAnerney, J.M.; Besselaar, T.G.; Pretorius, M.; et al. Epidemiology of Influenza B/Yamagata and B/Victoria Lineages in South Africa, 2005–2014. PLoS ONE 2017, 12, e0177655. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.-L.; Dickinson, J.A.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H.; et al. Age-Related Differences in Influenza B Infection by Lineage in a Community-Based Sentinel System, 2010–2011 to 2015–2016, Canada. J. Infect. Dis. 2017, 216, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Virk, R.K.; Jayakumar, J.; Mendenhall, I.H.; Moorthy, M.; Lam, P.; Linster, M.; Lim, J.; Lin, C.; Oon, L.L.E.; Lee, H.K.; et al. Divergent Evolutionary Trajectories of Influenza B Viruses Underlie Their Contemporaneous Epidemic Activity. Proc. Natl. Acad. Sci. USA 2020, 117, 619–628. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Hottes, T.S.; De Serres, G.; Ward, B.J.; Janjua, N.Z.; Sabaiduc, S.; Chan, T.; Petric, M. Influenza B/Victoria Antigen Induces Strong Recall of B/Yamagata But Lower B/Victoria Response in Children Primed With Two Doses of B/Yamagata. Pediatric Infect. Dis. J. 2011, 30, 833–839. [Google Scholar] [CrossRef]
- Vieira, M.C.; Donato, C.M.; Arevalo, P.; Rimmelzwaan, G.F.; Wood, T.; Lopez, L.; Huang, Q.S.; Dhanasekaran, V.; Koelle, K.; Cobey, S. Lineage-Specific Protection and Immune Imprinting Shape the Age Distributions of Influenza B Cases. Nat. Commun. 2021, 12, 4313. [Google Scholar] [CrossRef]
- Gostic, K.M.; Bridge, R.; Brady, S.; Viboud, C.; Worobey, M.; Lloyd-Smith, J.O. Childhood Immune Imprinting to Influenza A Shapes Birth Year-Specific Risk during Seasonal H1N1 and H3N2 Epidemics. PLoS Pathog. 2019, 15, e1008109. [Google Scholar] [CrossRef] [PubMed]
- Bedford, T.; Suchard, M.A.; Lemey, P.; Dudas, G.; Gregory, V.; Hay, A.J.; McCauley, J.W.; Russell, C.A.; Smith, D.J.; Rambaut, A. Integrating Influenza Antigenic Dynamics with Molecular Evolution. eLife 2014, 3, e01914. [Google Scholar] [CrossRef] [PubMed]
- Brazil. IBGE The Brazilian Institute of Geography and Statistics. IBGE. Available online: https://www.ibge.gov.br/apps/populacao/projecao/index.html (accessed on 30 March 2022).
- McCullers, J.A.; Saito, T.; Iverson, A.R. Multiple Genotypes of Influenza B Virus Circulated between 1979 and 2003. J. Virol. 2004, 78, 12817–12828. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Holmes, E.C. The Evolutionary Dynamics of Human Influenza B Virus. J. Mol. Evol. 2008, 66, 655–663. [Google Scholar] [CrossRef]
- Langat, P.; Raghwani, J.; Dudas, G.; Bowden, T.A.; Edwards, S.; Gall, A.; Bedford, T.; Rambaut, A.; Daniels, R.S.; Russell, C.A.; et al. Genome-Wide Evolutionary Dynamics of Influenza B Viruses on a Global Scale. PLoS Pathog. 2017, 13, e1006749. [Google Scholar] [CrossRef]
- Motta, F.C.; Siqueira, M.M.; Lugon, A.K.; Straliotto, S.M.; Fernandes, S.B.; Krawczuk, M.M. The Reappearance of Victoria Lineage Influenza B Virus in Brazil, Antigenic and Molecular Analysis. J. Clin. Virol. 2006, 36, 208–214. [Google Scholar] [CrossRef]
- Paiva, T.M.; Benega, M.A.; Silva, D.B.B.; Santos, K.C.O.; Cruz, A.S.; Hortenci, M.F.; Barbieri, M.T.; Monteiro, M.M.; Barbosa, H.A.; Carvalhanas, T.R.M.P. Evolutionary Pattern of Reemerging Influenza B/Victoria Lineage Viruses in São Paulo, Brazil, 1996–2012: Implications for Vaccine Composition Strategy. J. Med. Virol. 2013, 85, 1983–1989. [Google Scholar] [CrossRef]
- de Barros, E.N.C.; Cintra, O.; Rossetto, E.; Freitas, L.; Colindres, R. Patterns of Influenza B Circulation in Brazil and Its Relevance to Seasonal Vaccine Composition. Braz. J. Infect. Dis. 2016, 20, 81–90. [Google Scholar] [CrossRef][Green Version]
- Lapinscki, B.; Pereira, L.A.; Nogueira, M.B.; Vidal, L.R.; Riediger, I.; Debur, M.C.; Presibella, M.; Raboni, S.M. Molecular Epidemiology of Influenza B Virus and Implications in Immunization Strategy, Southern Brazil. Vaccine 2018, 36, 107–113. [Google Scholar] [CrossRef]
- Ray, R.; Dos Santos, G.; Buck, P.O.; Claeys, C.; Matias, G.; Innis, B.L.; Bekkat-Berkani, R. A Review of the Value of Quadrivalent Influenza Vaccines and Their Potential Contribution to Influenza Control. Hum. Vaccine Immunother. 2017, 13, 1640–1652. [Google Scholar] [CrossRef]
- WHO. Recommended Composition of Influenza Virus Vaccines for Use in the 2013 Influenza Season. Available online: https://www.who.int/influenza/vaccines/virus/recommendations/201209_recommendation.pdf?ua=1 (accessed on 31 March 2022).
- Crépey, P.; Boiron, L.; Araujo, R.R.; Lopez, J.G.; Petitjean, A.; de Albuquerque Luna, E.J. Impact of Quadrivalent Influenza Vaccines in Brazil: A Cost-Effectiveness Analysis Using an Influenza Transmission Model. BMC Public Health 2020, 20, 1374. [Google Scholar] [CrossRef] [PubMed]
- Tisa, V.; Barberis, I.; Faccio, V.; Paganino, C.; Trucchi, C.; Martini, M.; Ansaldi, F. Quadrivalent Influenza Vaccine: A New Opportunity to Reduce the Influenza Burden. J. Prev. Med. Hyg. 2016, 57, E28–E33. [Google Scholar] [PubMed]
- Jamotte, A.; Chong, C.F.; Manton, A.; Macabeo, B.; Toumi, M. Impact of Quadrivalent Influenza Vaccine on Public Health and Influenza-Related Costs in Australia. BMC Public Health 2016, 16, 630. [Google Scholar] [CrossRef] [PubMed]
- de Boer, P.T.; van Maanen, B.M.; Damm, O.; Ultsch, B.; Dolk, F.C.K.; Crépey, P.; Pitman, R.; Wilschut, J.C.; Postma, M.J. A Systematic Review of the Health Economic Consequences of Quadrivalent Influenza Vaccination. Expert Rev. Pharm. Outcomes Res. 2017, 17, 249–265. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).